Back to Journals » Infection and Drug Resistance » Volume 13
The First Case of Community-Acquired Pneumonia Due to Capsular Genotype K2-ST86 Hypervirulent Klebsiella pneumoniae in Okinawa, Japan: A Case Report and Literature Review
Authors Hirai J, Sakanashi D, Kinjo T, Haranaga S, Fujita J
Received 15 March 2020
Accepted for publication 24 June 2020
Published 10 July 2020 Volume 2020:13 Pages 2237—2243
DOI https://doi.org/10.2147/IDR.S252637
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sahil Khanna
Jun Hirai,1 Daisuke Sakanashi,2 Takeshi Kinjo,1 Shusaku Haranaga,3 Jiro Fujita2
1Department of Infectious, Respiratory and Digestive Medicine, Graduate School of Medicine, University of the Ryukyus, Okinawa 903-0215, Japan; 2Department of Infection Control and Prevention, Aichi Medical University Hospital, Aichi 480-1195, Japan; 3Comprehensive Health Professions Education Center, University of the Ryukyus Hospital, Okinawa 903-0215, Japan
Correspondence: Jun Hirai Email [email protected]
Abstract: Hypervirulent Klebsiella pneumoniae (HV-KP) typically causes pyogenic liver abscess and bacteremia with metastatic infections. Community-acquired pneumonia (CAP) due to HV-KP is uncommon and details of its clinical and microbiological features are limited. We report the first case of CAP due to capsular genotype K2-ST86 HV-KP in Okinawa, Japan and review infections caused by the K2-ST86 strain. A 79-year-old woman presenting with fever and productive cough persisting for the past three days was admitted to hospital. Her vital signs indicated septic shock. Lung examination by auscultation revealed holo-crackle and lobar pneumonia in chest radiography, and Streptococcus pneumoniae was suspected. However, sputum and blood cultures revealed Gram-negative coccus identified as K. pneumoniae. Genetic analysis identified the isolated strain as the K2 serotype harboring rmpA, iutA, entB, and mrkD. Therefore, we identified the isolated strain as hypervirulent. The isolate belonged to ST86 as determined by multilocus sequence typing. The case was not complicated by predisposing factors such as diabetes mellitus and malignancy related to HV-KP infection; thus, this CAP-causing HV-KP strain may differ from the typical HV-KP strain that induces liver abscess. A literature review identified only nine cases with CAP due to HV-KP. In all cases, the disease mainly occurred in older males with diabetes mellitus, which makes the present case unusual, and had high rates of septic shock and death. No case, including ours, was complicated by metastatic infection, suggesting that CAP due to HV-KP poses little distant metastasis risk, even in patients with bloodstream infection. In our review, consistent with our case, K2-ST86 was the most common strain of HV-KP in patients with CAP. Therefore, studies are needed to elucidate the clinical and microbiological features of HV-KP CAP, with a focus on the K2-ST86 strain. Physicians should always consider K. pneumoniae in cases of sepsis CAP with lobar pneumonia.
Keywords: hypervirulent Klebsiella pneumoniae, community-acquired pneumonia, serotype K2, sequence type 86, lobar pneumonia, Streptococcus pneumoniae
Introduction
Similar to pneumococcus, Klebsiella pneumoniae classically produces lobar pneumonia, particularly in alcoholics, and is known to cause hospital-acquired and ventilator-associated pneumonia, urinary tract infections, and bacteremia in immunocompromised or frequently healthcare-exposed patients.1,2 The frequency of K. pneumoniae as a cause of community-acquired pneumonia (CAP) in adults varies with location, with higher frequencies in Taiwan and China (the isolation rate is 5–14% and 5–10.3%, respectively, in each country).3 It is an infrequent cause of CAP in the United States, Europe, and Japan (0%, 0.3–5.0%, 0–1%, respectively).3–6
Recently, hypervirulent K. pneumoniae (HV-KP), which was first reported in 1986 in Taiwan, has received increased attention as a cause of severe infections.2,7 This strain typically causes community-acquired pyogenic liver abscess, bacteremia, and meningitis in healthy individuals and also tends to cause metastatic infections, with the most common sites being the eyes and central nervous system.2 The definition of HV-KP is controversial; however, several recent studies have reported that possessing iutA (aerobactin receptor-encoding gene) in addition to rmpA (for regulation of mucoid phenotype A via increased capsule production) is a defining HV-KP trait.8–10 Although increasing rates of HVKP infection have been reported all over the world,11 CAP due to HV-KP is uncommon.2
In addition, although studies have investigated the clinical and microbiological features and risk factors for HV-KP infections, none focused on HV-KP CAP.2,11 Therefore, details, including the capsular genotype of the HV-KP strain that causes CAP, are limited.
Herein, we present an unusual case of CAP due to the capsular genotype K2-ST86 HV-KP strain in a patient who did not have complicating risk factors for HV-KP infection. We also briefly reviewed previous case reports, combined with our case, to elucidate the clinical manifestations and microbiological characteristics, including serotype and sequence type (ST) of the HV-KP strain associated with CAP. In addition, we reviewed the types of infections caused by the K2-ST86 strain.
Case Report
A 79-year-old Japanese non-drinker woman with a history of dementia and primary aldosteronism was admitted to our hospital presenting with fever and a productive cough persisting for the past three days and respiratory distress. Despite the advanced age, the score of performance status was 2 (ambulatory and capable of all self-care but unable to carry out any work activities; up and about more than 50% of waking hours). She had never been a resident in a nursing home or an extended-care ward. She also did not receive antimicrobial agents and was not hospitalized in the preceding 90 days, and had no history of overseas travel. All these factors were indicative of her infection being community acquired.
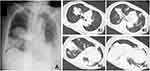
Upon admission (day 1), her general condition was severe, and vital signs indicated sepsis; blood pressure was 89/60 mmHg; heart rate, 130 beats/min; body temperature, 38.5 °C; respiratory rate, 25 breaths/min; and oxygen saturation, 92% in room air. A physical examination revealed crackles on both lateral lung fields upon auscultation. Blood tests showed elevated white blood cell count (16,400 cells/μL; neutrophil 88%) and C-reactive protein (42.81 mg/dL). A chest X-ray revealed lobar pneumonia on both lung fields, and computed tomography (CT) revealed multiple consolidations with air-bronchogram, nodules, and ground-glass opacities, particularly in both lower lobes (Figure 1). Although, radiological findings indicated pneumonia due to S. pneumoniae, Gram staining of sputum samples revealed no predominant microorganism. A commercially available urine antigen test for detecting S. pneumoniae and Legionella pneumophila and the rapid influenza virus test were also negative. The case was diagnosed as severe CAP with septic shock, and fluid resuscitation for septic shock and administration of meropenem (MEPM) were started immediately. The following day, both sputum and two sets of blood cultures confirmed only Gram-negative coccus. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry revealed the isolate as K. pneumoniae. Colonies on sheep blood agar demonstrated hypermucoviscosity, and the string test—a semiquantitative phenotypic estimation that determines hypermucoviscosity by stretching a bacterial colony on an agar plate using an inoculation needle—was positive (the string reached over 5 mm in length, which is consistent with the characteristics of HV-KP). A biochemical test confirmed that the isolated organism was K. pneumoniae. Multiplex PCR (magA, iutA, allS, kfu, rmpA, entB, mrkD, and ybtS) and multilocus sequence typing (MLST) were performed according to previously described methods.12,13 Genetic analysis identified this strain as the K2 serotype harboring rmpA, iutA, entB, and mrkD (Figure 2). Therefore, we identified the isolated strain as hypervirulent. The isolate belonged to ST 86 (ST86) as determined by MLST.
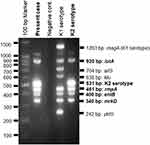 |
Figure 2 Multiplex PCR. The present strain harbored iutA, rmpA, entB, and mrkD genes. |
Additionally, urinalysis, urine culture, lumbar puncture, ultrasonography, and CT of the abdomen and head were performed to determine if the patient had complicating disseminated HV-KP infection, because treatment duration depends on the site and extent of infection. Metastatic infections, such as liver abscess, meningitis, brain abscess, or ocular infection, were all absent. The isolate was susceptible to all routinely tested antibiotics except ampicillin. MEPM was stopped and the patient was placed on ceftriaxone (CTRX) as a definitive therapy, because the usual dose of CTRX is given once a day, which could avoid intravascular over volume in the elderly. The patient received 2 weeks of intravenous antibiotics, recovered gradually, without recurrence, and was discharged on day 20.
Discussion
We describe here a case of CAP due to HV-KP strain K2-ST86. Although CAP was complicated with bacteremic septic shock, the patient recovered without other metastatic infections. To the best of our knowledge, this is the first report describing CAP due to K2-ST86 strain in Okinawa, Japan. Although K. pneumoniae causes lobar pneumonia and septic shock, which is generally caused by gram-negative bacteria, we did not initially consider its involvement in this case because K. pneumoniae is not a common cause of CAP in Japan (0–1%).3
Diabetes mellitus is considered as a significant risk factor for acquiring an HV-KP infection,2 and this bacterial infection affects predominantly 55–60 year old males.11 A recent study that assessed CAP due to K. pneumoniae (isolated HV-KP accounted for 54 of 68 patients; 79.4%) also reported chronic kidney disease (66.2%) and malignancy (38.2%) as being common co-morbidities.14 However, the present case was not complicated by other conditions, and the patient was an elderly woman, suggesting that the HV-KP strain causing CAP might have different clinical characteristics from those of the typical HV-KP strain that causes liver abscess, meningitis, and endophthalmitis.2
In this case, the patient had bacteremia in addition to septic shock and respiratory failure in the initial presentation. Importantly, when compared with S. pneumoniae, which is the leading cause of CAP globally, bacteremic CAP due to HV-KP has statistically higher mortality (55.1% vs 27.3%, p = 0.007) and respiratory failure (61.2% vs 31.8%, p = 0.005), and a greater likelihood of initial presentation with septic shock (51% vs 31.8%, p = 0.061) than bacteremic CAP due to S. pneumoniae.15 Therefore, prompt diagnosis and appropriate treatment are vital for this lethal infection. One of the important distinguishing factor between bacteremic CAP due to HV-KP versus bacteremic CAP due to S. pneumonia is that the former has a higher prevalence of bilateral involvement on chest radiography than that of the latter (65.3% vs 22.7%, p < 0.001),15 which was consistent with our case. Therefore, we should seriously consider K. pneumoniae as a potentially causative pathogen of sepsis CAP when a lobar pneumonia patient has septic shock, and sputum Gram-stain and urine antigen test for S. pneumoniae are negative, as was the case with our patient.
Generally, HV-KP tends to cause metastatic infection.2 However, Yi-Tsung reported that none of the 49 cases of bacteremic CAP due to HV-KP had concurrent liver abscess or other metastatic infection.15 This finding suggests that CAP due to HV-KP carries little risk for distant metastasis, even in patients with bloodstream infection.
The patient in our case was treated for 14 days, mainly with CTRX because third-generation cephalosporins are recommended as empiric antibiotic treatment options for HV-KP pneumonia2 and management of HV-KP infections requires usual treatment durations ranging from two to six weeks depending on the site and extent of infection.11,16 However, there have been no trials estimating which antimicrobial agents are best suited for treating HV-KP infections.2 Further investigations are needed to elucidate the appropriate treatment course for HV-KP pneumonia.
Although data are limited, we found other HV-KP CAP cases in past reports.17–20 Including our case, we analyzed ten adult patients with CAP due to HV-KP (Table 1). In our review, the average age was 66.3 (range, 39–90) and the prevalence of CAP due to HV-KP was slightly higher in males (70%). Four out of ten patients (40%) were complicated with diabetes mellitus as an underlying disease, which was lower than the rate in non-CAP HV-KP infections such as liver abscess and meningitis (61 of 80; 76.3%).8 Bacteremia and septic shock occurred in 5 (50%) and 7 patients (70%), respectively; however, no patient had an occurrence of metastatic infection. The most-prescribed antimicrobial agent for treatment was third-generation cephalosporins (70%). Two of five patients with bacteremia died, and five of seven patients with septic shock died (71.4%). The overall mortality rate was 50%, which was higher than patients with HV-KP invasive diseases such as liver abscess (a mortality rate ranging from 3 to 31%).2 The most striking detection in this review was that K2-ST86 K. pneumoniae was the most common strain (60%), which was consistent with our case.
 |
Table 1 The Characteristics of Nine Adult Cases with Community-Acquired Pneumonia Due to Hypervirulent Klebsiella pneumoniae Including the Present Case [14–16] |
We also researched the type of infections caused by the K2-ST86 strain. Although detailed clinical information was not included in all studies (n = 33), we found that this strain was mainly associated with pneumonia (especially CAP) and other infections are described as follows; CAP (n = 9), hospital-acquired pneumonia (n = 4), bacteremic pneumonia (n = 3, the types of pneumonia are unknown), bacteremia (n = 3, infectious origin is unknown), liver abscess (n = 3), meningitis (n = 2), urinary tract infection (n = 2), wound (n = 2), ventilator-associated pneumonia (n = 1), aspiration pneumoniae (n = 1), abscess (n = 1, origin is unknown), endocarditis (n = 1), and biliary tract infection (n = 1).17–30
Our study has certain limitations. Firstly, this is a single-case report presenting minimal data. However, no other studies have precisely described an infectious case due to K2-ST86 HV-KP. Second, we did not perform whole-genome sequencing to characterize the genome of the isolated strain. However, we performed PCR and MLST analysis, and revealed the presence of virulence genes, such as rmpA, iutA, entB, and mrkD, in addition to ST. Third, although the size of the cohort (n = 10) precludes any definitive conclusion, our literature review indicated that the K2-ST86 HV-KP strain was highly likely to cause CAP. More research is needed to reveal the relationship between the K2-ST86 strain and CAP because reports of invasive infections caused by the K2-ST86 strain are scarce.
In summary, we have reported a case of CAP due to K2-ST86 HV-KP in a patient without risk factors for HV-KP infection. The take-home messages are (1) physicians should suspect involvement of K. pneumoniae in cases of sepsis CAP with lobar pneumonia, although K. pneumoniae is an uncommon cause of CAP in some Asian countries, and (2) patients with CAP due to HV-KP should be treated promptly and appropriately owing to the high mortality rate of this infection. Our findings suggest that ST86 HV-KP should be investigated more deeply to elucidate the etiology, epidemiology, risk factors, serotype, and ST of CAP due to HV-KP.
Abbreviations
CAP, community-acquired pneumonia; CT, computed tomography; CTRX, ceftriaxone; HV-KP, hypervirulent Klebsiella pneumoniae; MEPM, meropenem; MLST, multilocus sequence typing; ST, sequence type.
Ethics and Consent
Written informed consent to have the case details and any accompanying images published has been provided by the patient. The ethics committee approved the waiver in this case report, based on the Japanese ethical guidelines for clinical research to publish the case details.
Acknowledgment
We would like to thank Editage for English language editing.
Disclosure
The authors declare no conflicts of interests.
References
1. Ishiguro T, Yoshii Y, Kanauchi T, et al. Re-evaluation of the etiology and clinical and radiological features of community-acquired lobar pneumonia in adults. J Infect Chemother. 2018;24(6):463–469. doi:10.1016/j.jiac.2018.02.001.
2. Choby JE, Howard-Anderson J, Weiss DS. Hypervirulent Klebsiella pneumoniae - clinical and molecular perspectives. J Intern Med. 2019. doi:10.1111/joim.13007.
3. Peto L, Nadjm B, Horby P, et al. The bacterial aetiology of adult community-acquired pneumonia in Asia: a systematic review. Trans R Soc Trop Med Hyg. 2014;108(6):326–337. doi:10.1093/trstmh/tru058.
4. Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. 2015;373(5):415–427. doi:10.1056/NEJMoa1500245.
5. Henig O, Kaye KS. Bacterial pneumonia in older adults. Infect Dis Clin North Am. 2017;31(4):689–713. doi:10.1016/j.idc.2017.07.015.
6. Torres A, Blasi F, Peetermans WE, Viegi G, Welte T. The aetiology and antibiotic management of community-acquired pneumonia in adults in Europe: a literature review. Eur J Clin Microbiol Infect Dis. 2014;33(7):1065–1079. doi:10.1007/s10096-014-2067-1.
7. Liu YC, Cheng DL, Lin CL. Klebsiella pneumoniae liver abscess associated with septic endophthalmitis. Arch Intern Med. 1986;146:1913–1916. doi:10.1001/archinte.1986.00360220057011
8. Liu C, Guo J. Hypervirulent Klebsiella pneumoniae (hypermucoviscous and aerobactin positive) infection over 6 years in the elderly in China: antimicrobial resistance patterns, molecular epidemiology and risk factor. Ann Clin Microbiol Antimicrob. 2019;18:4. doi:10.1186/s12941-018-0302-9.
9. Russo TA, Olson R, Fang C-T, et al. Identification of biomarkers for differentiation of hypervirulent Klebsiella pneumoniae from classical K. pneumoniae. J Clin Microbiol. 2018;56:e00776–18. doi:10.1128/JCM.00776-18.
10. Russo TA, Olson R, MacDonald U, Beanan J, Davidson BA. Aerobactin, but not yersiniabactin, salmochelin, or enterobactin, enables the growth/survival of hypervirulent (hypermucoviscous) Klebsiella pneumoniae ex vivo and in vivo. Infect Immun. 2015;83(8):3325–3333. doi:10.1128/IAI.00430-15.
11. Siu LK, Yeh KM, Lin JC, Fung CP, Chang FY. Klebsiella pneumoniae liver abscess: a new invasive syndrome. Lancet Infect Dis. 2012;12:881–887. doi:10.1016/S1473-3099(12)70205-0.
12. Compain F, Babosan A, Brisse S, et al. Multiplex PCR for detection of seven virulence factors and K1/K2 capsular serotypes of Klebsiella pneumoniae. J Clin Microbiol. 2014;52:4377–4380. doi:10.1128/JCM.02316-14.
13. Diancourt L, Passet V, Verhoef J, Grimont PA, Brisse S. Multilocus sequence typing of Klebsiella pneumoniae nosocomial isolates. J Clin Microbiol. 2005;43:4178–4182. doi:10.1128/JCM.43.8.4178-4182.2005.
14. Juan CH, Fang SY, Chou CH, Tsai TY, Lin YT. Clinical characteristics of patients with pneumonia caused by Klebsiella pneumoniae in Taiwan and prevalence of antimicrobial-resistant and hypervirulent strains: a retrospective study. Antimicrob Resist Infect Control. 2020;9:4. doi:10.1186/s13756-019-0660-x.
15. Lin YT, Jeng YY, Chen TL, Fung CP. Bacteremic community-acquired pneumonia due to Klebsiella pneumoniae: clinical and microbiological characteristics in Taiwan, 2001–2008. BMC Infect Dis. 2010;10:307. doi:10.1186/1471-2334-10-307.
16. Shon AS, Bajwa RP, Russo TA. Hypervirulent (hypermucoviscous) Klebsiella pneumoniae: a new and dangerous breed. Virulence. 2013;4:107–118. doi:10.4161/viru.22718.
17. Zhang Y, Zeng J, Liu W, et al. Emergence of a hypervirulent carbapenem-resistant Klebsiella pneumoniae isolate from clinical infections in China. J Infect. 2015;71:553–560. doi:10.1016/j.jinf.2015.07.010.
18. Harada S, Aoki K, Yamamoto S, et al. Clinical and molecular characteristics of Klebsiella pneumoniae isolates causing bloodstream infections in Japan: occurrence of hypervirulent infections in health care. J Clin Microbiol. 2019;57:
19. Rafat C, Messika J, Barnaud G, et al. Hypervirulent Klebsiella pneumoniae, a 5-year study in a French ICU. J Med Microbiol. 2018;67:1083–1089. doi:10.1099/jmm.0.000788.
20. Yamamoto H, Iijima A, Kawamura K, Matsuzawa Y, Suzuki M, Arakawa Y. Fatal fulminant community-acquired pneumonia caused by hypervirulent Klebsiella pneumoniae K2-ST86: case report. Medicine. 2020;99(21):e20360. doi:10.1097/MD.0000000000020360.
21. Liu Y, Long D, Xiang TX, et al. Whole genome assembly and functional portrait of hypervirulent extensively drug-resistant NDM-1 and KPC-2 co-producing Klebsiella pneumoniae of capsular serotype K2 and ST86. J Antimicrob Chemother. 2019;74:1233–1240. doi:10.1093/jac/dkz023.
22. Lev AI, Astashkin EI, Kislichkina AA, et al. Comparative analysis of Klebsiella pneumoniae strains isolated in 2012–2016 that differ by antibiotic resistance genes and virulence genes profiles. Pathog Glob Health. 2018;112:142–151. doi:10.1080/20477724.2018.1460949.
23. Shi Q, Lan P, Huang D, et al. Diversity of virulence level phenotype of hypervirulent Klebsiella pneumoniae from different sequence type lineage. BMC Microbiol. 2018;18:94. doi:10.1186/s12866-018-1236-2.
24. Cubero M, Grau I, Tubau F, et al. Hypervirulent Klebsiella pneumoniae clones causing bacteraemia in adults in a teaching hospital in Barcelona, Spain (2007–2013). Clin Microbiol Infect. 2016;22:154–160. doi:10.1016/j.cmi.2015.09.025.
25. Yan Q, Zhou M, Zou M, Liu WE. Hypervirulent Klebsiella pneumoniae induced ventilator-associated pneumonia in mechanically ventilated patients in China. Eur J Clin Microbiol Infect Dis. 2016;35:387–396. doi:10.1007/s10096-015-2551-2.
26. Melot B, Brisse S, Breurec S, et al. Community-acquired meningitis caused by a CG86 hypervirulent Klebsiella pneumoniae strain: first case report in the Caribbean. BMC Infect Dis. 2016;16:736. doi:10.1186/s12879-016-2065-2.
27. Zhang Y, Sun J, Mi C, et al. First report of two rapid-onset fatal infections caused by a newly emerging hypervirulent K. Pneumonia ST86 strain of serotype K2 in China. Front Microbiol. 2015;6:721. doi:10.3389/fmicb.2015.00721.
28. Guo S, Xu J, Wei Y, Xu J, Li Y, Xue R. Clinical and molecular characteristics of Klebsiella pneumoniae ventilator-associated pneumonia in mainland China. BMC Infect Dis. 2016;16:608. doi:10.1186/s12879-016-1942-z.
29. Liao CH, Huang YT, Chang CY, Hsu HS, Hsueh PR. Capsular serotypes and multilocus sequence types of bacteremic Klebsiella pneumoniae isolates associated with different types of infections. Eur J Clin Microbiol Infect Dis. 2014;33:365–369. doi:10.1007/s10096-013-1964-z.
30. Decré D, Verdet C, Emirian A, et al. Emerging severe and fatal infections due to Klebsiella pneumoniae in two university hospitals in France. J Clin Microbiol. 2011;49:3012–3014. doi:10.1128/JCM.00676-11.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.