Back to Journals » Pediatric Health, Medicine and Therapeutics » Volume 15
The Feasibility of C-Reactive Protein Point-of-Care Testing to Reduce Overuse of Antibiotics in Children with Acute Respiratory Tract Infections in Rural Kyrgyzstan: A Pilot Study
Authors Isaeva E , Akylbekov A, Bloch J, Poulsen A, Kurtzhals J , Siersma V, Sooronbaev T, Aabenhus RM, Kjærgaard J
Received 6 July 2023
Accepted for publication 30 December 2023
Published 13 February 2024 Volume 2024:15 Pages 67—76
DOI https://doi.org/10.2147/PHMT.S425095
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Laurens Holmes, Jr
Elvira Isaeva,1 Azamat Akylbekov,2 Joakim Bloch,3 Anja Poulsen,3 Jørgen Kurtzhals,4,5 Volkert Siersma,6 Talant Sooronbaev,2 Rune Munck Aabenhus,6 Jesper Kjærgaard3
1Allergology Department, National Centre of Maternity and Childhood Care (NCMCC), Bishkek, Kyrgyzstan; 2Pulmonology Department, National Centre of Cardiology and Internal Medicine Named After Academician M. Mirrakhimov, Bishkek, Kyrgyzstan; 3Department of Paediatrics and Adolescent Medicine, Copenhagen University Hospital, Rigshospitalet, Copenhagen, Denmark; 4Department of Clinical Microbiology, Copenhagen University Hospital, Rigshospitalet, Copenhagen, Denmark; 5Centre for Medical Parasitology, Department of Immunology and Microbiology, University of Copenhagen, Copenhagen, Denmark; 6Research Unit for General Practice and Section of General Practice, Department of Public Health, University of Copenhagen, Copenhagen, Denmark
Correspondence: Elvira Isaeva, Allergology Department, National Centre of Maternity and Childhood Care (NCMCC), Postal Address: 720038, 190, Akhunbaev St, Bishkek, Kyrgyzstan, Tel +996 (709) 795 979, Email [email protected]
Background: In Kyrgyzstan, the morbidity prevalence of and morbidity from acute respiratory tract infections (ARTI) in children is high. Local healthcare workers (HCW) often prescribe antibiotics that are not indicative due to a mix of professional and societal factors. It is suggested to precede with a decision on antibiotics by a point-of-care test (POCT) on the appropriateness of the treatment, eg, a measurement of C-reactive protein (CRP). CRP-guided antibiotic stewardship in children with ARTI has not previously been studied in Central Asia.
Purpose: This pilot study was conducted to examine the feasibility of the methods and procedures to be used in the upcoming randomised controlled COORDINATE clinical trial (NCT05195866) and in daily clinical practice in primary care.
Patients and methods: HCWs from three selected rural healthcare facilities were trained in the CRP POCT and in interpretation of results. Children aged 6 months to 12 years attending the primary healthcare facilities with respiratory symptoms were randomly assigned to CRP-guided management or standard care, guided by clinical findings only. Children were followed up for 14 days by scheduled telephone calls to caregivers.
Results: Eighty-one children participated in this pilot study. The CRP POCT and the trial procedures were acceptable to the target group as well as to the HCWs. Children from both groups recovered equally well, with an observed significant lower use of antibiotics in the CRP group. HCWs generally adhered to the CRP guidelines, and only once was an antibiotic prescribed despite low CRP results. No safety concerns were observed. Four parents provided wrong phone numbers impeding follow-up. We will collect all mobile phone numbers in the household for the main trial.
Conclusion: The pilot provided satisfactory results, suggesting that the COORDINATE trial of CRP POCT is effective, feasible with minor adjustments and without apparent safety concerns for the participants.
Keywords: paediatrics, family medicine, respiratory medicine, C-reactive protein, feasibility
Introduction
Acute respiratory tract infections (ARTI) are a serious problem worldwide,1 especially in children under 5, costing the lives of almost 1 million children annually. In Central Asian countries, such as Kyrgyzstan, the prevalence and mortality from this group of diseases are disproportionately high.2 ARTI are the most common cause of death among children under 5 globally,3 costing the lives of almost 1 million children annually.4 The annual incidence of influenza and acute respiratory viral infection (ARVI) in Kyrgyzstan exceeds the total incidence of all other infections: their share averages 66.6% of all registered cases of infectious disease, reaching 78% in some years.5,6
Healthcare workers (HCW) in primary care in Kyrgyzstan use the Integrated Management of Childhood Illness (IMCI) approach, as there are only a limited range of diagnostic methods in these often remote rural settings, eg, blood count (haemoglobin, red blood cells, and white blood cells), stethoscope, peak flow meter, and scales.7 In addition, the medical staff, typically represented by feldshers and family nurses, most often prescribe antibiotics unsupervised.8 In some cases, HCWs, regardless of educational background, may end up prescribing antibiotics that are not needed9 due to a mix of professional and societal factors, and patient expectations.10
A high rate of ARTI together with an overuse of antibiotics contributes to increasing antimicrobial resistance levels.11 In low- and middle-income countries (LMICs), antibiotic consumption increased 114% between 2000 and 2015.12 An analysis of the consumption of antibiotics in Kyrgyzstan showed that 46.8% of the population uses antibiotics as self-medication. The main driver for self-medication was the sale of antibiotics without a prescription.13 In Kyrgyzstan, patients take, on average, over two courses of antibiotics per year and harbour many misconceptions about the usefulness of and indications for antibiotics, such misconceptions are also present among HCWs.10
In this rapidly evolving landscape of antibacterial resistance, urgent actions are needed as we are moving towards a post-antibiotic era where common infections may once again become life-threatening.
One approach to reduce unnecessary antibiotic use is to assist HCWs in identifying patients who likely have a viral or self-limiting bacterial infection in order to withhold antibiotics in such patients.
C-reactive protein (CRP) is an acute inflammatory protein that increases in cases of infection or inflammation.14,15 It is an established biomarker for assessing inflammation and its severity in patients presenting to a healthcare facility16 and can be used as a point-of-care test (POCT) to guide antibiotic prescribing decisions.17 A Cochrane review concluded that CRP testing for ARTI in primary care reduces the number of antibiotic prescriptions with 23% without apparent safety concerns.18 There are many studies all over the world on the effectiveness and acceptability of CRP POCT testing in primary healthcare settings in LMICs,19–24 but few in children and none from Central Asia.
Objectives
This pilot study was conducted to examine the feasibility and possible barriers of the methods and procedures intended to be used in the upcoming randomised controlled clinical (RCT) COORDINATE trial.25 We were specifically interested in understanding issues related to the study protocol, randomisation, inclusion and exclusion criteria, enrolment, implementation of the intervention, data capture, loss to follow-up and study withdrawal rate. The general feasibility was assessed through the willingness of HCWs to carry out and follow the CRP POCT algorithm.
Materials and Methods
Design
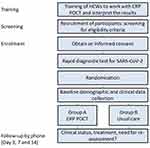
An individually randomised trial (ISCRNT No. NCT05195866) with 14 days blinded follow-up was conducted as a pilot study. HCWs from three selected healthcare centres from different villages of the Chui region in northern Kyrgyzstan were trained to perform and interpret the results of CRP POCT in the clinical assessment of children with ARTI. It was emphasized that CRP levels below 10 mg/L strongly indicated a viral or non-severe bacterial cause for the disease, making antibiotics generally unnecessary. Intermediate CRP levels ranging from 10 to 50 mg/L suggested that antibiotics might be considered. When CRP levels exceeded 50 mg/L, antibiotics were recommended. The training also covered information about CRP kinetics and scenarios where a low CRP result needed to be interpreted cautiously, such as in cases where the patient had a fever lasting less than 24 hours. The main researcher and research assistants (RA) collected data at the index consultation. Follow-up phone calls (days 3, 7, and 14) were done by another group of RAs in the general study unit in Bishkek, blinded to individuals’ group allocation. Two family doctors and two nurses were trained in the family medicine centre (FMC) in Sokuluk village, two doctors and one nurse in the family physicians group (FPG) in Kyzyl-Tuu village, and one feldscher in the feldscher-obstetrics point (FOP) in Shalta village.
Study Population
All children aged 6 months to 12 years attending the selected primary care health clinics during normal business hours with symptoms of ARTI were screened for eligibility, in the two-week period November 24th to December 7th 2021. Inclusion criteria were age between 6 months and 12 years; parents/caregivers able and willing to comply with all study requirements; parents/caregivers able and willing to give informed consent; the patient had at least one of the following focal symptoms lasting for less than 2 weeks: cough, fast/difficult breathing, sore throat, shortness of breath, wheezing.25 The study excluded very ill children where CRP POCT measurement would delay care, children with known immunosuppression or severe chronic disease, children with a positive SARS-CoV-2 rapid test, and children who had taken antibiotics within 24 hours before the consultation.25
Data Collection
The data collection consisted of four stages:
Inclusion
Screening of patients took place in the waiting areas of the three participating medical facilities. A screening form was used to identify eligible patients according to inclusion and exclusion criteria. The screening form was in Russian (which is the official language of interethnic communication along with Kyrgyz, which is the state language in Kyrgyzstan). If the child’s caregiver desired, the researcher could translate the question into Kyrgyz to help if language difficulties arose. After verbal and written information, the informed consent was signed. All included children underwent a rapid test for SARS-CoV-2. If the test was positive, the child was withdrawn from the study and managed according to the present Kyrgyz guidelines for COVID-19.
Randomisation
If the test was negative, the child was randomised to intervention or control using the SealedEnvelope™ programme.26 Children allocated to the intervention had a CRP POCT done during their consultation with a HCW and the result used as guidance for initiating or withholding antibiotic treatment. The randomised children fell into two groups: the intervention group – management based on the results of the CRP test, and the control group – usual care, as in daily practice before the intervention (ie, the doctor prescribes medicines without knowledge of CRP results, but based on the clinical picture).
Data Collection
The RA completed a case report form (CRF) for all included children during the consultation in collaboration with a HCW in their office. The CRF contained items on the child’s demographics, symptoms at the time of the consultation, physical examination results, treatment prescribed, and diagnosis. In addition, at the end of the CRF there were several questions to the HCWs and the caregivers of the child about their experience of accepting and working with the CRP POCT method, which was necessary for examining the feasibility. At the conclusion of the pilot study, a dedicated feasibility survey (as part of the CRF) was administered to all HCWs involved in the research. Additionally, caregivers also provided unsolicited feedback in this survey.
Follow-Up 14 Days
Follow-up was carried out through phone calls to the caregivers. Both groups were assessed on the 3rd, 7th, and 14th day by phone calls using follow-up questionnaires including questions on recovery and symptoms as well as complications or hospitalisations.
The study procedure is shown in Figure 1.
Data Analysis
This feasibility trial was not powered to detect statistically significant differences between groups. However, we analysed and calculated proportions of patient inclusion, antibiotic use, and follow-up rates to assist in optimal planning of the main trial.
Results
Pilot results
A total of 192 children underwent screening during the pilot study period, of which 81 children were enrolled in the study. The last follow-up phone call was conducted on 31st of December 2021. One hundred and eleven children had one or more exclusion criteria with “taken an antibiotic 24 hours before the consultation” being the most common reason (n = 54).
The enrolment is shown in Figure 2 at three different healthcare facilities. Forty children were randomised to intervention group and 41 children to control group. Six children (4 from intervention group and 2 from control group) were referred for hospitalisation during the index consultation, after the randomisation procedure, after being examined by a HCW (by decision of a local HCW). Baseline characteristics of included children, the commonest primary complaint as stated by caregivers, HCWs clinical diagnosis, CRP values of patients from the intervention group and antibiotics prescriptions in both groups are presented in Table 1.
 |
Table 1 Baseline Characteristics, Group Allocation, CRP Values, and Antibiotics Prescriptions |
The commonest primary complaint as stated by caregivers was cough, while an unspecific viral infection. ARVI was the most frequent diagnosis by the HCWs. The CRP guidance reduced antibiotic prescriptions markedly (17% in intervention group vs 59% in control group).
Most children had low CRP levels <10 mg/mL (29/40) and only 1/40 had a value above 50 mg/mL. In general, HCWs followed the protocol and prescribed antibiotics according to CRP levels in the intervention group. When the level of CRP <10, almost all HCWs (97.3%) did not prescribe antibiotics; however, there was still one HCW who prescribed antibiotics despite CRP - 8.9 (1 case).
Follow-up and recovery: children were considered recovered when caregivers indicated in the follow-up questionnaire that their child was feeling better, well, or excellent (see Figure 3). The majority presented with infections that recovered well during the first week of follow-up. Children from both groups recovered successfully and equally in general well-being.
In terms of the data completeness, 71 children had all baseline assessment data and all follow-up data recorded (full data); 4 children had baseline data but no follow-up data. In hospitalised children allocated to the intervention group, the CRP values were 5.5, 20, 37, and 46. But at the decision of the research team, follow-up calls were also conducted to them. Follow-up assessments were done for 95% (n = 77) of children; for 4 children a baseline assessment had been conducted, but no follow-up data were obtained as caregivers had provided wrong phone numbers.
Feasibility Results
Based on a feasibility study conducted among all HCWs involved in the study at the conclusion of the pilot project, as well as among the parents or caregivers of children participating in the study, key opinions regarding the CRP POCT method were identified. When HCWs were asked about their general feelings about the use of the test, the majority responded very positively. Regarding the question about the convenience and practicality of implementing this test in their clinical practice, a majority answered “Definitely yes”. Similarly, when asked if they would like to use CRP POCT in their future practice, the majority once again responded “Definitely yes”. Only one out of eight HCWs answered “Do not know” to all three questions.
Regarding the survey for parents and caregivers, there were only two questions: how they reacted to the use of CRP POCT to diagnose their children in general, and whether they consider it acceptable to use this test to diagnose children. The vast majority of responses were positive.
Discussion
Feasibility Findings
HCWs viewed CRP as a simple and quick aid in diagnosis, and normal CRP levels were a strong argument for them to refrain from prescribing antibiotics. Local HCWs fully supported the introduction of this method into routine clinical practice based on their first experience with it and were positive about its use in the future. As for the attitude of caregivers to the CRP POCT method in relation to their children, their high willingness to accept this method in the daily clinical practice of health workers was noted, suggesting a high acceptability of this method could be expected in the main trial. With regard to their attitude towards the participation of their children in the study, in general, no incidents and opposition were noticed. A qualitative study in Lebanon on children’s participation in clinical trials found that Lebanese caregivers have similar perceptions and attitudes towards children’s participation in clinical trials as those reported in industrialised countries.27 One qualitative study targeting the acceptability of the test among general practitioners reported that CRP POCT was acceptable to clinicians who believed in its usefulness and that CRP POCT, when used as an adjunct to clinical assessment, could be used to support efforts to counselling patients and improving decisions about prescribing antibiotics for ARTI.28
The logistics of data collection worked as expected in the pilot. The completeness of data collection at the stage of follow-up calls was high. Four caregivers provided incorrect or no longer serviced phone numbers, leading to loss of follow-up. There are five mobile phone operators in Kyrgyzstan,29 and many people have SIM cards from different operators; it may be wise to collect all phone numbers from the child’s caregiver and household. We estimate that the research model is quite suitable for a larger sample size in the main trial and will likely improve data completeness.
Pilot Study Findings
The pilot study showed that we could enrol roughly half of the screened patients; 81 children were enrolled in approximately two weeks (data collection in fields were conducted in period from 24th of November to 7th of December and the last follow-up phone call was conducted on 31st of December). In the upcoming main study, we plan to enrol 1204 children at 14 primary healthcare centres. According to the archival data of local medical institutions, the largest number of children with respiratory syndromes is observed in winter (December to March). It follows that the main study should also be done in winter to get the most patients in the least amount of time. The second finding is that children in the CRP POCT group were prescribed less antibiotics (17% as opposed to 59% in the control group). Given the small sample size of the pilot, the estimate must be interpreted with caution. Regardless, the finding is in line with a published meta-analysis summarising current data from 13 studies on the association between CRP POCT testing and antibiotic prescribing for ARTIs in general practice. CRP POCT testing has been found to significantly reduce antibiotic prescribing at the initial consultation.30 This may generally speak in favour of confirming the effectiveness of this method, especially in conditions of low resources, when there are no other methods of laboratory or instrumental diagnostics at hand.30–36
In terms of the primary outcomes, the recovery based on caregivers’ phone responses on Days 3, 7, and 14, overall recovery was similar in both groups. Naturally, we take into account that caregivers are not clinicians to assess the clinical or laboratory recovery of children, but they seem to do well in reporting subjective or objective indicators of recovery (general well-being of the child, body temperature, cough, shortness of breath, runny nose, etc).37
It is worth paying attention to the fact that in both groups (A and B) a large proportion of the diagnosis of ARVI was made (40% in intervention group and 24.4% in control group, as well as laryngotracheitis 29.3% in control group, which can also be attributed to the manifestation of ARVI). That is, clinicians were aware that they diagnosed an infection of predominantly viral nature, but prescribed an antibiotic nevertheless. It is interesting that pneumonia was not particularly common and that most diagnoses recorded would normally not warrant antibiotic therapy. The low levels of CRP measured in the pilot support the notion that most cases of ARVI were likely self-limiting viral infections. These preliminary pilot data suggest that local HCWs are not entirely sure about the correctness of prescribing antibiotics for children with ARVI, highlighting the need for education and practical tools to assist in a more rational antibiotic use for children with ARVI. Furthermore, the study is likely safe to conduct in children, as no adverse events occurred during the pilot period.
Conclusion
This study was a pilot model before running a full-scale RCT. The pilot provided satisfactory results regarding both the effectiveness of the CRP algorithm as the CRP intervention group was prescribed markedly less antibiotic treatments as well as the feasibility of the study set up and associated procedures. Important issues to consider in the upcoming main trial include logistics and follow-up data from caregivers. Overall, we found both caregivers HCW to be interested in and comply with study requirements.
Abbreviations
ARTI, Acute respiratory tract infections; ARVI, Acute respiratory viral infections; COVID-19, coronavirus disease 2019; CRF, clinical report form; CRP, C-reactive protein; FMC, family medicine centre; FOP, feldscher-obstetrics point; FPG, family physicians group; HCW, healthcare workers; IMCI, integrated management of childhood illness; ISCRNT, international standard randomised controlled trial number; KAP, knowledge, attitudes and practices; LMIC, low- and middle-income countries; POCT, point-of-care test; RCT, randomised controlled clinical trial; SARS-CoV-2, severe acute respiratory syndrome-related coronavirus 2.
Ethics
The Ethical Committee of the National Centre of Maternity and Childhood Care of the Kyrgyzstan has approved the Study Protocol (Ref. no. 1. Date: 18/06/2021). The study participants are children living in the Kyrgyzstan, who are covered by the protection of citizens of the Kyrgyzstan. The trial will comply fully with all regulatory authorities and will be executed in accordance with Good Clinical Practice. Children in the CRP POCT arm are subjected to a finger prick causing minor transient pain.
Acknowledgments
The authors thank the University of Copenhagen library and authors of the included studies for providing full-text articles. The authors express their deep gratitude to the parents of the children who were sympathetic to the study and with great enthusiasm agreed to the participation of their children, as well as to the participants themselves for their courage and willingness to give their finger for analysis. Special thanks to the RAs, who travelled to rural medical facilities and collected data so carefully and diligently and who did all follow-up calls (Azat Bolotbek uulu, Nargiza Osmonbaeva, Aigerim Tilebalieva, Uuljan Bekbolsunova, Aichurok Alymbekova, Aiperi Muratova, Mohisitora Zhalalova, Zhamilya Zhumadilova).
Funding
This research was funded by the International Centre for Antimicrobial Resistance Solutions (ICARS) (grant number 100008) Copenhagen, Denmark, and Global Health Unit, Department of Paediatrics and Adolescent Health, Copenhagen University Hospital, Rigshospitalet, Copenhagen, Denmark.
Disclosure
The authors report no conflicts of interest in this work.
References
1. van Gageldonk-Lafeber AB, van der Sande MAB, Heijnen MLA, Peeters MF, Bartelds AIM, Wilbrink B. Risk factors for acute respiratory tract infections in general practitioner patients in The Netherlands: a case-control study. BMC Infect Dis. 2007;7:1–8. doi:10.1186/1471-2334-7-35
2. Shalabayeva BS, Asheraliyev MY, Kabylova ET, Cherikchiyeva AB. Standardized approaches in the diagnosis and therapy of children with acute diseases of the respiratory and gastrointestinal tract in conditions of short-stay departments. Med J West Kazakhstan. 2018;3(59):34–39.
3. Hug L, Sharrow D, You DL. Trends in child mortality. New York; 2017. Available from: http://www.childmortality.org/files_v21/download/IGMEreport.2017.child.mortality.final.pdf.
4. Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of child mortality in 2000 – 13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2015;385:430–440. doi:10.1016/S0140-6736(14)61698-6
5. Nurmatov ZS. Assessment of the costs associated with outpatient and inpatient treatment of ARVI and influenza and its complications. Epidemiol Vaccinal Prev. 2015;14(5):26–30. doi:10.31631/2073-3046-2015-14-5-26-30
6. Nurmatov Z. Influenza and ARVI in Kyrgyzstan. Russ J Infect Immun. 2015;4:365–374. doi:10.15789/2220-7619-2014-4-365-374
7. Ibraimova A, Akkazieva B, Ibraimov A, Manzhieva E, Rechel B. Kyrgyzstan: health system review. Health Syst Transit. 2011;13:3.
8. World Health Organization. Regional Office for Europe and Policies, Health care systems in transition: Kyrgyzstan. Copenhagen PP - Copenhagen: World Health Organization. Regional Office for Europe; 2000. Available from: https://apps.who.int/iris/handle/10665/108323.
9. Østergaard MS, Kaplan A. Recurrent lower respiratory illnesses among young children in rural Kyrgyzstan: overuse of antibiotics and possible under-diagnosis of asthma. A qualitative FRESH AIR study. NPJ Prim Care Respir Med. 2018;28:1. doi:10.1038/s41533-018-0081-y
10. Smith B. Promoting Rational Use of Antibiotics in the Kyrgyz Republic. In: Marquez LR, editor. Improv Heal. Care Low- Middle-Income Ctries. A Case B. Springer International Publishing; 2020. Available from: https://www.ncbi.nlm.nih.gov/books/NBK565742/.
11. World Health Organization. (2015) Global action plan on antimicrobial resistance; 2017. Available from: www.who.int/antimicrobial-resistance/publications/global-action-plan/en/.
12. Klein EY, Van Boeckel TP, Martinez EM, et al. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc Natl Acad Sci U S A. 2018;115(15):E3463–E3470. doi:10.1073/pnas.1717295115
13. Baktygul K, Marat B, Ashirali Z, Harun-Or-rashid M, Sakamoto J. An assessment of antibiotics prescribed at the secondary health-care level in the Kyrgyz Republic. Nagoya J Med Sci. 2011;73(3–4):157–168.
14. Fridkin SK, Hageman J, McDougal LK, et al. Vancomycin-Intermediate Staphylococcus aureus Epidemiology Study Group. Epidemiological and microbiological characterization of infections caused by Staphylococcus aureus with reduced susceptibility to vancomycin, United States, 1997–2001. Clin Infect Dis. 2003;36:429–439. doi:10.1086/346207
15. Long SW, Olsen RJ, Mehta SC, et al. PBP2a mutations causing high-level Ceftaroline resistance in clinical methicillin-resistant Staphylococcus aureus isolates. Antimicrob Agents Chemother. 2014;58:6668–6674. doi:10.1128/AAC.03622-14
16. Sproston NR, Ashworth JJ. Role of C-reactive protein at sites of inflammation and infection. Front Immunol. 2018;9:1–11. doi:10.3389/fimmu.2018.00754
17. Bernstein D, Coster D, Berliner S, et al. C-reactive protein velocity discriminates between acute viral and bacterial infections in patients who present with relatively low CRP concentrations. BMC Infect Dis. 2021;21(1):1–7. doi:10.1186/s12879-021-06878-y
18. Lelubre C, Anselin S, Zouaoui Boudjeltia K, Biston P, Piagnerelli M. Interpretation of c-reactive protein concentrations in critically Ill patients. BioMed Res Int. 2013;2013:1–11. doi:10.1155/2013/124021
19. Smedemark SA, Aabenhus R, Llor C, Fournaise A, Olsen O, Jørgensen KJ. Biomarkers as point-of-care tests to guide prescription of antibiotics in people with acute respiratory infections in primary care. Cochrane Database Syst Rev. 2022;10(10):CD010130. doi:10.1002/14651858.CD010130.pub3
20. Cooke J, Llor C, Hopstaken R, Dryden M, Butler C. Respiratory tract infections (RTIs) in primary care: narrative review of C reactive protein (CRP) point-of-care testing (POCT) and antibacterial use in patients who present with symptoms of RTI. BMJ Open Respir Res. 2020;7:1. doi:10.1136/bmjresp-2020-000624
21. Wakeman M, Cork T, Watwood D. Point-of-care C-reactive protein testing in community pharmacy to deliver appropriate interventions in respiratory tract infections. Clin Pharm CP. 2018;10:5. doi:10.1211/PJ.2018.20204635
22. Martínez-González NA, Keizer E, Plate A, et al. Point-of-care C-reactive protein testing to reduce antibiotic prescribing for respiratory tract infections in primary care: systematic review and meta-analysis of randomised controlled trials. Antibiotics. 2020;9:9. doi:10.3390/antibiotics9090610
23. Althaus T, Greer RC, Swe MMM, et al. Effect of point-of-care C-reactive protein testing on antibiotic prescription in febrile patients attending primary care in Thailand and Myanmar: an open-label, randomised, controlled trial. Lancet Glob Heal. 2019;7(1):e119–e131. doi:10.1016/S2214-109X(18)30444-3
24. Samet JM, Wipfli H, Platz EA, Bhavsar N. A dictionary of epidemiology, fifth edition: edited by Miquel Porta. Am J Epidemiol. 2009;170(11):1449–1451. doi:10.1093/aje/kwp322
25. Isaeva E, Bloch J, Poulsen A, et al. C reactive protein-guided prescription of antibiotics for children under 12 years with respiratory symptoms in Kyrgyzstan: protocol for a randomised controlled clinical trial with 14 days follow-up. BMJ Open. 2023;13(4):e066806. doi:10.1136/bmjopen-2022-066806
26. Sealed Envelope Ltd. Simple randomisation service; 2022. Available from: https://www.sealedenvelope.com/simple-randomiser/v1/.
27. Nabulsi M, Khalil Y, Makhoul J. Parental attitudes towards and perceptions of their children’s participation in clinical research: a developing-country perspective. J Med Ethics. 2011;37(7):420–423. doi:10.1136/jme.2010.035899
28. Hardy V, Thompson M, Keppel GA, et al. Qualitative study of primary care clinicians’ views on point-of-care testing for C-reactive protein for acute respiratory tract infections in family medicine. BMJ Open. 2017;7(1):1–6. doi:10.1136/bmjopen-2016-012503
29. Jenish N. ICT-driven technological and industrial upgrading in Afghanistan, Kyrgyzstan and Tajikistan: current realities and opportunities. SSRN Electron J. 2021. doi:10.2139/ssrn.3807763
30. Huang Y, Chen R, Wu T, Wei X, Guo A. Association between point-of-care CRP testing and antibiotic prescribing in respiratory tract infections: a systematic review and meta-analysis of primary care studies. Br J Gen Pract. 2013;63(616):787–794. doi:10.3399/bjgp13X674477
31. Yebyo H, Medhanyie AA, Spigt M, Hopstaken R. C-reactive protein point-of-care testing and antibiotic prescribing for acute respiratory tract infections in rural primary health centres of North Ethiopia: a cross-sectional study. NPJ Prim Care Respir Med. 2016;26:15076. doi:10.1038/npjpcrm.2015.76
32. Joshi A, Perin DP, Gehle A, Nsiah-Kumi PA. Feasibility of using C-reactive protein for point-of-care testing. Technol Heal Care off J Eur Soc Eng Med. 2013;21(3):233–240. doi:10.3233/THC-130720
33. Jakobsen KA, Melbye H, Kelly MJ, et al. Influence of CRP testing and clinical findings on antibiotic prescribing in adults presenting with acute cough in primary care. Scand J Prim Health Care. 2010;28(4):229–236. doi:10.3109/02813432.2010.506995
34. Melbye H, Stocks N. Point of care testing for C-reactive protein - A new path for Australian GPs? Aust Fam Physician. 2006;35(7):513–517.
35. Marquez LR. Improving health care in low- and middle-income countries. Cham. 2020. doi:10.1007/978-3-030-43112-9
36. Zh.Ysykeeva NA. Medicine Prices, Availability, Affordability in Kyrgyz Republic Report of a survey conducted September to October 2015 MeTA Project in Kyrgyzstan (Medicines Transparency Alliance) Bishkek; 2015.
37. Dyson MP, Shave K, Gates A, et al. Which outcomes are important to patients and families who have experienced paediatric acute respiratory illness? Findings from a mixed methods sequential exploratory study. BMJ open. 2017;7(12):e018199. doi:10.1136/bmjopen-2017-018199
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.