Back to Journals » Cancer Management and Research » Volume 14
The Failure Patterns of Nasopharygeal Carcinoma After Intensity-Modulated Radiotherapy and Implications for Surveillance
Authors Tian Y, Huang WZ, Zeng L, Bai L, Han F, Lan Y
Received 10 November 2021
Accepted for publication 15 March 2022
Published 5 October 2022 Volume 2022:14 Pages 2813—2823
DOI https://doi.org/10.2147/CMAR.S347864
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Harikrishna Nakshatri
Yunming Tian,1 Wei-Zeng Huang,2 Lei Zeng,3 Li Bai,1 Fei Han,4,* Yuhong Lan1,*
1Department of Radiation Oncology, Huizhou Central People’s Hospital, Huizhou, Guangdong Province, People’s Republic of China; 2Department of Medical Oncology, Huizhou First Hospital, Huizhou, Guangdong Province, People’s Republic of China; 3Department of Medical Oncology, The Second Affiliated Hospital of Nanchang University, Nanchang, Jixiang Province, People’s Republic of China; 4Department of Radiation Oncology, Sun Yat-Sen University Cancer Centre, State Key Laboratory of Oncology in South China, Guangzhou, Guangdong Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Fei Han, Department of Radiation Oncology, Sun Yat-Sen University Cancer Center, Guangzhou, Guangdong Province, People’s Republic of China, Email [email protected] Yuhong Lan, Department of Radiation Oncology, Hui Zhou Municipal Central Hostpital, Huizhou, Guangdong Province, People’s Republic of China, Email [email protected]
Objective: To investigate the treatment outcomes, failure patterns and surveillance strategy in patients with nasopharyngeal carcinoma (NPC) after intensity-modulated radiotherapy (IMRT).
Methods: A cohort of patients with NPC who had received the full course of IMRT between 2008 and 2012 were retrospectively analyzed. The failure patterns, time to recurrence, and detection methods were recorded. The survival was calculated using the Kaplan–Meier method. Multivariate proportional hazard regression models were used to test the prognostic factors.
Results: In total, 2607 patients with NPC treated with IMRT were recruited. After the median follow-up of 112 months, 402 (15.4%) patients experienced distant metastasis, 225 (8.6%) patients had local recurrence, and 77 (3.0%) patients had regional recurrences. The 10-year overall survival (OS), local recurrence-free survival (LRFS), and distant metastasis-free survival (DMFS) rates were 74.5%, 90.1%, and 79.3%, respectively. The factors of male sex, age > 50 years, lactate dehydrogenase > 245 IU/L, advanced T classification, and advanced N classification were associated with poor OS. The N disease classification was the most important factor in predicting distant metastasis, and advanced T disease classification for high risk of local recurrence. For patients with T1 disease, the incidence of local recurrence was less than 2%, and the incidence of distant metastasis was less than 5% for patients with N0 disease. About 83% of the recurrence occurred in the first 5 years, and 20% of the recurrences showed no symptoms.
Conclusion: High rate of local-regional control can be achieved for patients with NPC after IMRT, while distant metastasis remains as the major cause of failures. Patients with advanced N classification has high risk to develop distant metastasis, and most occurred within 5 years. Developing rational and individualized surveillance strategies based on the high risk factors of recurrence is helpful to balance the survival benefit and medical cost.
Keywords: failure patterns, intensity-modulated radiotherapy, nasopharyngeal carcinoma, surveillance
Introduction
Nasopharyngeal carcinoma (NPC) is a unique head and neck malignancy with an extremely unbalanced global distribution, which is commonly seen in Southeastern Asia and Northern Africa.1,2 According to the global cancer statistics, in 2020, about 133,354 new cases of nasopharyngeal carcinoma are diagnosed, and more than 70% of new cases are in Southeastern Asia.3 Because of the high sensitivity to radiation and the anatomic constraints, radiotherapy is the main curative treatment modality for patients with non-metastatic NPC.4,5
With the development of radiotherapy techniques, especially for the common application of intensity-modulated radiotherapy (IMRT) in NPC, the incidence of local recurrence has been reduced to less than 10%. However the incidence of distant metastasis is still up to 20–25%, which becomes the most common part of treatment failure.6–8 Regular surveillance after definitive treatment plays an important role in patient management, with earlier detection and better treatment of recurrences. However, the evidence on the optimal surveillance frequency and regimen for patients with NPC is limited. Furthermore the recommendations from the NCCN, ESMO, or other cancer centers on the method and frequency of surveillance are also various and represent the major dilemma in clinical practice.4,5,9 Furthermore, the significantly various schedules in the surveillance always causes unreasonable waste of medical resource because of the misapplied utilization or the delayed diagnosis of recurrence due to the underutilization in certain patients.10 The individual surveillance strategies based on the risk of treatment failure and frequency of recurrence may better balance survival benefit and the cost.
This study was to evaluate: (1) the failure patterns and time to occurrence of failure after definitive chemoradiotherapy; (2) the methods used to detect the recurrence; and (3) the salvage treatment and outcomes for patients with recurrence. The main goal was to investigate the failure patterns and a rational follow-up regimen for surveillance of patients with NPC after IMRT.
Methods and Materials
Materials
The retrospective cohort study included 2607 NPC patients treated with IMRT from 2008 to 2012 from Sun Yat-Sen University Cancer Centre and Huizhou Central People’s Hospital. The inclusion criteria were: (1) pathologically proven NPC (WHO I–III types); (2) no evidence of distant metastasis (classified as M0); (3) completion of the full course of radiotherapy. To make comprehensive evaluation of tumor stage, patients had fiberoptic endoscope examination of the nasopharynx, contrast-enhanced magnetic resonance imaging (MRI) of nasopharynx and neck, X-ray or computed tomography scan (CT) of chest, ultrasound or CT of abdomen, and single photon emission computed tomography (SPECT). Positron emission tomography (PET-CT) was recommended in patients with advanced disease. All the patients were re-staged according to the new 8th edition AJCC staging system.11
The protocol was in accordance with the Helsinki declaration and approved by the institutional ethics committee of Huizhou Central People’s Hospital. Written informed consent was obtained from all participants. All methods were performed in accordance with the relevant guidelines and regulations.
Treatment
All patients underwent intensity-modulated radiotherapy (IMRT). Patients were immobilized in the supine position by a thermoplastic head and shoulder mask. Planning computed tomography (CT) images were obtained from the CT simulator with serial 3-mm slices, and scanning from the vertex to the clavicles. The gross tumor volume (GTV) included the primary tumor in nasopharynx (GTV-nx) and the metastatic lymph nodes in the neck (GTV-nd) as shown by clinical, endoscopic, and radiologic examinations. The clinical target volume (CTV) included the high-risk CTV (CTV1) and the low-risk CTV (CTV2). The CTV1 was defined as the GTV-nx plus a margin of 0.5–1.0 cm encompassing the whole nasopharyngeal mucosa. The CTV2 was defined by adding a margin of 0.5–1.0 cm to CTV1 and the lymph node regions including the posterior nasal cavity, the parapharyngeal space, the skull base, the pterygoid fossae, the inferior sphenoid sinus, the anterior clivus, and upper+lower neck regions. An additional 0.2–0.3 cm margin was added to the CTV2 to create the planning target volume (PTV). The prescribed radiation doses were 66–70 Gy to GTV-nx, 64–66 Gy to GTV-nd, 60 Gy to CTV1, and 50–54 Gy to CTV2 in 30–33 fractions. The details of target contouring and organs at risk (OARs) were described in the previous study.8
According to the treatment recommendations in the center, concurrent chemoradiotherapy (CCRT) was the standard choice for patients with stage II–IVa disease if no major medical comorbidity precluded its use; furthermore, patients with more advanced T stage (i.e T4) or N stage (i.e N2-3) would also receive neoadjuvant (NACT) or adjuvant chemotherapy (AC). Of the 2607 patients, 885 patients were treated with radiotherapy alone and 1722 patients were treated with chemoradiotherapy including 891 patients with CCRT alone, 705 patients with NACT+CCRT, 101 patients with CCRT+AC, and 25 patients with NACT+CCRT+AC.
The platinum-based regimens most commonly used in NACT or AC included TP (docetaxel and cisplatin), PF (cisplatin and 5-fluorouracil), and a triplet of them (docetaxel, cisplatin, and 5-fluorouracil). For patients receiving concurrent chemotherapy, 2–3 cycles of cisplatin (80–100 mg/m2) every 3 weeks were administered.
Definition of Recurrence
When there was local recurrence disease, biopsy was made for the pathology diagnosis; however, when the recurrence disease was deeply seated in skull base, intracranial space, and orbit, the radiological manifestations (i.e contrast-enhancement mass) and clinical symptoms would also be helpful to the diagnosis of local recurrence.
When the regional recurrence was suspected from the neck mass or radiological finding, the fine-needle was administrated to make the histology diagnosis; however, the recurrence of retrospective parapharynx node would also be diagnosed by the radiological evidence because of the difficulty in biopsy.
For the recurrence in the distant organs, most diagnoses were based on the radiological examination including the bone metastasis by the SPECT or MR, the lung metastasis by CT of chest, and the liver metastasis by the CT, MR, or ultra-sound, and the biopsy was also recommended when the metastatic lesions detected by the imaging were atypical.
Follow-Up and Statistical Methods
After the completion of treatment, patients were subsequently followed-up every 1–3 months during the first 2 years, every 6 months in years 2 to 5, and annually thereafter. During the follow-up, a medical history, physical examination, endoscopy, and repeated imaging methods including MRI of nasopharynx and neck, X-ray or CT of chest, ultrasound or CT of abdomen, and SPECT were recommended.
The local recurrence-free survival (LRFS), regional recurrence-free survival (RRFS), local-regional recurrence-free survival (LRRFS), distant metastasis-free survival (DMFS), progression-free survival (PFS), and overall survival (OS) rates were calculated using the Kaplan–Meier method. Endpoints of different groups of patients were compared by using the log rank test. Multivariate analysis was performed using the Cox proportional hazards model. LRFS, RRFS, LRRFS, and DMFS were calculated from the date of histologic diagnosis to the first local, regional, or distant failure. OS was defined to the date of death from any cause.12 All statistical tests were two-sided, and P<0.05 was considered statistically significant.
Results
Patient Characteristics
Table 1 summarizes the patients’ characteristics. The median age for the all patients was 43 years (range, 13–68 years) with a male (n = 1957) to female (n = 650) ratio of 3.0:1. The overall stage distribution was: stage I, 3.2%; stage II, 17.5%; stage III, 46.6%; stage IVA, 32.7%. The T stage distribution was: T1, 9.8%; T2, 24.5%; T3, 45.5%; T4, 20.2%; and the N stage was: N0, 7.4%; N1, 47.2%; N2, 30.8%; N3, 14.3%.
 |
Table 1 Characteristics of 2607 Patients and Survival Outcome |
Survival and Pattern of Failure
After the median follow-up of 112 months (range 1–148 months), 142 (5.4%) patients were lost after 6–83 months. The 5- and 10-year OS rates for all the patients were 82.7% and 74.5%. The 10-year OS rates for patients with stage I, II, III, and IVa disease were 94.2%, 87.3%, 77.5%, and 57.4%, respectively. A total of 675 patients died; distant metastasis was the most common cause in 360 patients, followed by local/regional failure in 201 patients, both in 20 patients, radiation-related complications in 37 patients, other cancer in 29 patients including lung cancer and other head and neck cancer, internal medication in 13 patients, and unknown in 15 patients. The survival curves are shown in Figure 1.
 |
Figure 1 Kaplan–Meier curves showing OS for all the patients (A), stratified by gender (B), age (C), LDH (D), T stage (E), and N stage (F). |
The 5- and 10-year LRFS, RRFS, and LRRFS rates were 92.5%, 97.0%, and 91.5%, and 90.1%, 95.0%, and 88.4%. A total of 289 (11.0%) patients experienced local or regional failure, including 212 patients with local recurrence, 64 patients with regional recurrence, and 13 patients with local-regional recurrence. The median time to the occurrence of local/regional recurrence was 33 months (range, 3–132 months). Furthermore, about 75% (212/289) of the local/regional recurrence occurred in the 5 years after the treatment, and only 25% (77/289) of them occurred after 5 years.
The 10-year actuarial LRFS rates for patients with T1, T2, T3, and T4 disease were 98.0%, 92.5%, 92.1%, and 78.4%, respectively. The patients with T4 disease had significantly poorer local control than those with T1-3 disease (p<0.001). The 10-year actuarial LRFS rates for patients with stage I, II, III, and IVa disease were 97.6%, 94.0%, 91.9%, and 81.1%, respectively. The 10-year actuarial RRFS rates for patients with N0, N1, N2, and N3 disease were 100.0%, 97.0%, 94.5%, and 90.5%, respectively. The patients with N3 disease had significantly poorer regional control than those with N0-2 disease (p<0.001). Furthermore, no patients with N0 disease developed regional recurrence during the follow-up.
The 5- and 10-year DMFS rates for all the patients were 85.4% and 79.3%, respectively, and 83.5% and 74.3% for PFS. The 10-year DMFS rates for patients with stage I, II, III, and IVa/b were 98.8%, 93.2%, 86.3%, and 72.6%, respectively. Patients with advanced disease of primary tumor and nodal metastasis showed higher risk to develop distant metastasis. The 10-year DMFS rates were 89.2% and 75.4% for patients with N0-1 and N2-3, and 90.3% and 79.5% for patients with T1-2 and T3-4.
Distant metastasis occurred in 402 patients (15.3%), including 245 patients with single organ metastasis and 157 patients with multiple organ metastasis. The median time to the occurrence of distant metastasis was 22 months (range, 1–148 months). Similar to the local/regional recurrence, most of distant metastasis occurred in the first 5 years with a rate of 87.7% (352/401), and only 13.3% (49/401) of them occurred after 5 years. The cumulative incidences of LR, RR, and DM are shown in Figure 2.
 |
Figure 2 The cumulative incidence of local recurrence (LR) by T stage (A), regional recurrence (RR) by N stage (B), and distant metastasis (DM) by N stage (C). |
Detection of Recurrence
In the 225 patients with local recurrence, about 77.3% of them had more than one of the suspicious symptoms, including headache, nasal obstruction, ear symptoms, and cranial nerves palsy, which were helpful to the diagnosis of recurrence; however, 22.7% of them were asymptomatic, and their recurrences were only detected by the physical examination, endoscope examination, and/or imaging. Surveillance nasopharyngoscopy could only detect 78% of recurrence (179/225), including all recurrence in rT1 disease (5/5), 84.6% in rT2 disease (33/39), 79.2% in rT3 disease (65/80), and 75.2% in rT4 disease (76/101). Compared to nasopharyngoscopy, MR imaging had a higher probability in detecting the abnormal signs of recurrence with a rate of 90%, including 80% of the recurrence in rT1 disease (4/5), 89.7% in rT2 disease (35/39), 88.7% in rT3 disease (71/80), and 89.2% in rT4 disease (90/101). Finally, about 20% of the recurrences were diagnosed based on the evidence of clinical symptoms and radiology findings when no biopsy of tumor can be acquired.
In the 77 patients with regional recurrence, 60 patients (77.9%) presented with palpable neck mass and 16 patients (22.1%) could be only detected by MR imaging. Finally, 68 patients (88.4%) were pathology-diagnosed by fine-needle biopsy, and 9 patients (11.6%) with recurrence of retropharyngeal lymph node were diagnosed by MR imaging and PET-CT.
Among the 402 patients with distant metastasis, 269 patients (66.9%) presented with suspicious symptoms, and 133 patients (33.1%) were asymptomatic which were detected by routine surveillance including CT of chest and abdomen and SPECT. Biopsy/surgery was administered in 51 patients (12.6%). The details are shown in Table 2.
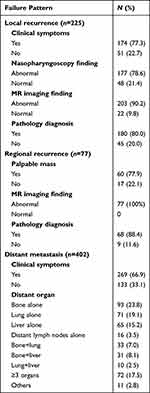 |
Table 2 The Characteristics of Recurrence in NPC Patients |
Salvage Strategies for Patients with Recurrence
The median OS time of the 225 patients with local recurrence (from diagnosis of recurrence) was 23.0 months (95% CI, 2.0 to 108.0 months). As of this analysis, 181 (80.4% of 225) patients have died. The salvage treatments for the 225 patients included 66 patients receiving re-irradiation, 21 patients with surgery, 5 patients with both re-irradiation and surgery, 95 patients with only palliative chemotherapy with or without target drugs, 25 patients with only palliative support, and 13 patients with no record of subsequent therapy. For 76 patients with local recurrence who survived more than 36 months, almost 76% patients (58/76) received local therapy for recurrence of disease including re-irradiation or surgery.
For the 77 patients with regional recurrence, the median OS time was 37.0 months (95% CI, 3.0 to 131.0 months) after the diagnosis, and 40 (51.9% of 77) patients have died. A total of 51 patients received neck dissection with or without chemotherapy, and 16 patients received only chemotherapy±radiotherapy for the extensive disease. After salvage treatment, 28 patients (36.3% of 77) developed distant metastasis.
For the 402 patients with distant metastasis, the median OS time was 18.0 months (95% CI, 2.0 to 125.0 months), and 380 (94.5% of 402) patients have died. A total of 352 (87.5% of 402) patients received at least one cycle of chemotherapy, and 50 (12.5% of 402) declined any treatments. Local therapy including the radiotherapy or surgery to the metastatic disease was administered in 189 (47.0% of 402) patients.
Univariate and Multivariate Analysis
In the univariate analysis, the factors of male sex, lactate dehydrogenase (LDH) >245 IU/L, advanced T classification, N classification, and overall stage were associated with poor OS, PFS, and DMFS. Furthermore the factor of age ≥45 years was also associated with poor OS. The factors of male sex, lactate dehydrogenase (LDH) >245 IU/L, advanced T classification, and overall stage were associated with poor LRFS. The factor of advanced N classification was associated with poor RRFS. However, in the multivariate analysis, the factor of advanced overall stage shows no association with poor OS, PFS, DMFS, and LRFS. The details of the univariate and multivariate analysis are shown in Tables 3 and 4.
 |
Table 3 Univariate Analysis of Prognostic Factors |
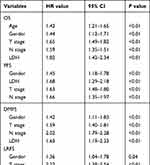 |
Table 4 Multivariate Analysis of Prognostic Factors |
Discussion
With the advantage of dosimetric distribution between target coverage and the adjacent organs, the IMRT has become the main treatment in patients with NPC. Series studies have demonstrated that IMRT provides satisfactory long-term outcomes and reduced complications.13,14 Patients with NPC achieved a high rate of local-regional control of 85–90% after IMRT with or without the additional chemotherapy.6–8,13 Consistent with these results, the present study indicated that the 10-year LRFS rates for patients with T1-3 disease after IMRT were 92–98%, which was significantly higher than those reported in the era of conventional RT with the rate of only 60–70%.15 However, the local control rate for T4 disease was only about 78%, and local failure was still a main cause of treatment failures for these patients. Dosimetric inadequacy of the primary tumor volume constricted by the critical organs may contribute to the recurrence.16 In the study reported by Ng et al, an under-dose GTVp of 3.4 cm3 was associated with high rate of local failure.17 Our previous studies indicated that patients with the early local recurrence still had the chance to achieve long-term survival after salvage treatment.18 However, more than 70% of the local recurrence were advanced, and salvage treatments were difficult. The present study indicated that about 22% of the local recurrences presented with no symptom and were only detected by the regular follow-up. Besides the physical examination, the nasopharyngoscopy and MR imaging are important methods to detect the local recurrence during the follow-up.19–21 Bagri et al found that nasopharyngoscopy showed 77.8% sensitivity and 93.0% specificity in diagnosing the residual/recurrence of tumor, significantly better than the CT scan.19 The main disadvantage of the nasopharyngoscopy is that it may easily neglect the deeply seated recurrences including the skull base and intracranial lesions. A meta-analysis also indicated that the MR showed 78% sensitivity and 76% specificity in diagnosing local recurrence; however, distinction between post-irradiation changes and recurrent tumors was always difficult.21 The present study indicated the MR has higher possibility in detecting the abnormal signs of local recurrence than nasopharyngoscopy since about 20% of the local recurrence was deeply seated. Therefore, the combination of nasopharyngoscopy and MR can be more accurate in detecting the local recurrence during the follow-up.
Although a high rate of local-regional control was achieved after the IMRT, about 15% of the patients developed distant metastasis and this became the most common cause of treatment failure. Our study showed that the factors of male sex, elevated LDH, and advanced T classification and N classification were independent adverse prognostic factors for DMFS. Similar findings were also reported by others.6,7,22 In the study relating to 3328 patients reported by Au et al, the 8-year DMFS rates were 92.1%, 86.6%, 78.0%, and 62–64% for patients with N0, N1, N2, and N3a/3b disease.7 In the study by Lan et al, the 5-year DMFS rate for patients with N3 disease was only 74.9%, significantly poorer than for those with N1-2 disease with the rate of 83–91%.22 In the present study, N classification was also the most important determinant of distant metastasis with hazard risk of 2.0. The 10-year DMFS rates for patients with N0-N1 disease was more than 88%, while the rate decreased to 82.0% and 67% for patients with N2 and N3 disease. Furthermore, the patients with T4 also showed high risk of distant metastasis. The 10-year DMFS rate for patients with T4 disease was only 74.1%, significantly poorer than those with T1-3 disease. The intracranial extension and/or involvement of cranial nerves may lead to the high risk of metastasis, which had been demonstrated in other head and neck cancers after surgery. Batsakis’s study indicated that carcinoma proliferated along the nerves within the lymphatics of the epineurium and the perineural sheaths.23 Liu et al reported that the patients with clinical and/or MRI-detected cranial nerve involvement have much higher risk to develop distant metastasis than those without cranial nerve involvement, with the 3-year DMFS rates of 74.6% vs 84.6%.24 The addition of chemotherapy to radiotherapy is a crucial development to reduce the distant metastasis for those with N2-3 disease and T4 disease. A number of trials have demonstrated the mainstay treatment of concurrent chemoradiotherapy (CCRT) in advanced disease with significant survival benefit.25,26 Furthermore, induction chemotherapy followed by concurrent chemoradiotherapy showed the advantage in patient tolerance and eradicating micrometastases. In a large-scale multicentre phase 3 trial reported by Sun et al, the 3-year DMFS for patients with induction chemotherapy plus CCRT was significantly higher than for those with CCRT alone (90% vs 83%).27 Furthermore, the study by Ou et al indicated that the induction chemotherapy reduced the grade 3–4 late radiation toxicities when compared with the CCRT alone because of the high responsiveness of induction chemotherapy and smaller volume of tumor was irradiated.28 Although the adjuvant chemotherapy of conventional regimens after CCRT shows no survival benefit because of the poor tolerance, series studies indicated that metronomic chemotherapy with the advantages of good compliance, low toxicities, and convenience substantially reduced distant failure and improved survival in high-risk patients.29,30
Patients with various factors showed different patterns of treatment failure; however, the current NCCN guidelines only highlight the use of a pretreatment imaging modality to detect the recurrence, and there are no detailed recommendations from the ESMO guideline.5 Zhou et al analyzed the expenditure to detect the 95% of recurrences for patients with various T disease and found that the cost per recurrence in patients with T1/2 disease was almost three times that in patients with T3/4 disease since there was excellent local control and fewer recurrences in T1/2 patients.10 In the present study, the intensive follow-up strategies applied in the T3-4 disease to detect the local recurrence for patients with T1-2 disease seem not reasonable for the high rate of local control. Patients with T1-3 N0 disease had seldom developed distant metastasis, with a10-year DMFS rate of 98.2%, which indicated that the conventional imaging to detect distant metastasis including CT of chest or abdomen shows little survival benefit during the follow-up. Patients with nodal metastasis have high risk to develop distant metastasis, and most of them occurred within 5 years with the rate of 87.7%. Furthermore, about 33% of patients with distant metastasis were asymptomatic and can only be detected by the routine imaging. Therefore, more intensive follow-up with the routine imaging methods were recommended during the first 5 years for the patients with high risk factors of distant metastasis. In addition, follow-up should also continue because about 12% of the recurrences occur after the first 5 years. Therefore developing individual and accurate follow-up strategies based on the patient’s risk factors of recurrence and the failure pattern will better balance the survival benefit and medical cost.
Our study has some limitations. First, this is a retrospective study from only two institutions; therefore, selection bias related to the treatment might have influenced the results. Second, the serum EBV-DNA has been demonstrated as a valuable factor in detecting the recurrence during the follow-up.31 However, only some patients’ EBV-DNA data were collected in our study, therefore we had excluded the factor to avoid the potential bias.
In conclusion, high rate of local-regional control can be achieved for patients with NPC after IMRT, while distant metastasis remains as the major cause of failure. Patients with advanced N classification have high risk to develop distant metastasis, and most occurred in the first 5 years. Developing rational and individualized follow-up strategies based on the high risk factors of recurrence is helpful to balance the survival benefit and medical cost.
Data Sharing Statement
The data supporting the conclusion of this article are available from Yunming Tian, Fei Han, and Yuhong Lan.
Disclosure
Yunming Tian and Wei-Zeng Huang shared the first authorship. Fei Han and Yuhong Lan share senior authorship.
The authors report no conflicts of interest in this work.
References
1. Lee AW, Ng WT, Chan YH, Sze H, Chan C, Lam TH. The battle against nasopharyngeal cancer. Radiother Oncol. 2012;104(3):272–278. doi:10.1016/j.radonc.2012.08.001
2. Chen YP, Chan A, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394(10192):64–80. doi:10.1016/S0140-6736(19)30956-0
3. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
4. Pfister D. NCCN clinical practice guidelines in oncology: head and neck cancers. Version1; 2020: 88–89.
5. Chan A, Felip E. Nasopharyngeal cancer: eSMOclinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20:123–125. doi:10.1093/annonc/mdp150
6. Sun X, Su S, Chen C, et al. Long-term outcomes of intensity-modulated radiotherapy for 868 patients with nasopharyngeal carcinoma: an analysis of survival and treatment toxicities. Radiother Oncol. 2014;110(3):398–403. doi:10.1016/j.radonc.2013.10.020
7. Au KH, Ngan RK, Ng AW, et al. Treatment outcomes of nasopharyngeal carcinoma in modern era after intensity modulated radiotherapy (IMRT) in Hong Kong: a report of 3328 patients (HKNPCSG 1301 study). Oral Oncol. 2018;77:16–21. doi:10.1016/j.oraloncology.2017.12.004
8. Tian YM, Liu MZ, Zeng L, et al. Long-term outcome and pattern of failure for patients with nasopharyngeal carcinoma treated with intensity-modulated radiotherapy. Head Neck. 2019;41(5):1246–1252. doi:10.1002/hed.25545
9. Felice D, Vincentiis D, Valentini V, et al. Follow-up program in head and neck cancer. Crit Rev Oncol Hematol. 2017;113:151–155. doi:10.1016/j.critrevonc.2017.03.012
10. Zhou GQ, Lv JW, Tang LL, et al. Evaluation of the National Comprehensive Cancer Network and European Society for Medical Oncology nasopharyngeal carcinoma surveillance guidelines. Front Oncol. 2020;10:119. doi:10.3389/fonc.2020.00119
11. Amin MB, Edge SB, Greene FL, et al. AJCC Cancer Staging Manual.
12. Ollier E, Blanchard P, Le Teuff G, et al. Penalized Poisson model for network meta-analysis of individual patient time-to-event data. Stat Med. 2022;41(2):340–355. doi:10.1002/sim.9240
13. Lee AW, Sze WM, Au JSK, et al. Treatment results for nasopharyngeal carcinoma in the modern era: the Hong Kong experience. Int J Radiat Oncol Biol Phys. 2005;61(4):1107–1116. doi:10.1016/j.ijrobp.2004.07.702
14. Lin S, Pan J, Han L, et al. Update report of nasopharyngeal carcinoma treated with reduced-volume intensity-modulated radiation therapy and hypothesis of the optimal margin. Radiother Oncol. 2014;110(3):385–389. doi:10.1016/j.radonc.2014.01.011
15. Lee AW, Poon YF, Foo W, et al. Retrospective analysis of 5037 patients with nasopharyngeal carcinoma treated during 1976–1985: overall survival and patterns of failure. Int J Radiat Oncol Biol Phys. 1992;23(2):261–270. doi:10.1016/0360-3016(92)90740-9
16. Gou X, Duan B, Shi H, et al. The relations of dosimetric parameters with long-term outcomes and late toxicities in advanced T-stage nasopharyngeal carcinoma with IMRT. Head Neck. 2020;42(1):85–92. doi:10.1002/hed.25986
17. Ng W, Lee MCH, Chang ATY, et al. The impact of dosimetric inadequacy on treatment outcome of nasopharyngeal carcinoma with IMRT. Oral Oncol. 2014;50(5):506–512. doi:10.1016/j.oraloncology.2014.01.017
18. Tian YM, Xiao WW, Bai L, et al. Impact of primary tumor volume and location on the prognosis of patients with locally recurrent nasopharyngeal carcinoma. Chin J Cancer. 2015;34(6):247–253. doi:10.1186/s40880-015-0019-5
19. Bagri PK, Singhal MK, Singh D, et al. Diagnosis of post-radiotherapy local failures in nasopharyngeal carcinoma: a prospective institutional study. Iran J Cancer Prev. 2014;7(1):35–39.
20. Chong VF, Fan YF. Detection of recurrent nasopharyngeal carcinoma: MR imaging versus CT. Radiology. 1997;202(2):463–470. doi:10.1148/radiology.202.2.9015075
21. Liu T, Xu W, Yan WL, et al. FDG-PET, CT, MRI for diagnosis of local residual or recurrent nasopharyngeal carcinoma, which one is the best? A systematic review. Radiother Oncol. 2007;85(3):463–470. doi:10.1016/j.radonc.2007.11.002
22. Lan M, Huang Y, Chen C-Y, et al. Prognostic value of cervical nodal necrosis in nasopharyngeal carcinoma: analysis if 1800 patients with positive cervical nodal metastasis at MR imaging. Radiology. 2015;276(2):536–544. doi:10.1148/radiol.15141251
23. Batsakis JG. Nerves and neurotropic carcinomas. Ann Otol Rhinol Laryngol. 1985;94(4 Pt 1):426–427.
24. Liu L, Liang S, Li L, et al. Prognostic impact of magnetic resonance imaging-detected cranial nerve involvement in nasopharyngeal carcinoma. Cancer. 2009;115(9):1995–2003. doi:10.1002/cncr.24201
25. Al-Sarraf M, LeBlanc M, Giri PG, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: Phase III randomized Intergroup study 0099. J Clin Oncol. 1998;16(4):1310–1317. doi:10.1200/JCO.1998.16.4.1310
26. Blanchard P, Lee A, Marguet S, et al. Chemotherapy and radiotherapy in nasopharyngeal carcinoma: an update of the MAC-NPC meta-analysis. Lancet Oncol. 2015;16(6):645–655. doi:10.1016/S1470-2045(15)70126-9
27. Sun Y, Li WF, Chen NY, et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol. 2016;17(11):1509–1520. doi:10.1016/S1470-2045(16)30410-7
28. Ou D, Blanchard P, Khoury C, et al. Induction chemotherapy with docetaxel, cisplatin and fluorouracil followed by concurrent chemoradiotherapy or chemoradiotherapy alone in locally advanced non-endemic nasopharyngeal carcinoma. Oral Oncol. 2016;62:114–121. doi:10.1016/j.oraloncology.2016.10.011
29. Twu CW, Wang W-Y, Chen -C-C, et al. Metronomic adjuvant chemotherapy improves treatment outcome in nasopharyngeal carcinoma patients with postradiation persistently detectable plasma Epstein-Barr virus deoxyribonucleic acid. Int J Radiat Oncol Biol Phys. 2014;89(1):21–29. doi:10.1016/j.ijrobp.2014.01.052
30. Chen YP, Liu X, Zhou Q, et al. Metronomic capecitabine as adjuvant therapy in locoregionally advanced nasopharyngeal carcinoma: a multicentre, open-label, parallel-group, randomised, controlled, phase 3 trial. Lancet. 2021;398(10297):303–313. doi:10.1016/S0140-6736(21)01123-5
31. Leung SF, Chan KC, Ma BB, et al. Plasma Epstein-Barr viral DNA load at midpoint of radiotherapy course predicts outcome in advanced-stage nasopharyngeal carcinoma. Ann Oncol. 2014;25(6):1204–1208. doi:10.1093/annonc/mdu117
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
