Back to Journals » Cancer Management and Research » Volume 12
The Expression of VEGF and CD31 in Endometrial Lesions and Its Associations with Blood Flow Parameters of Transvaginal 3D Power Doppler Ultrasonography: A Preliminary Study
Authors Liu M, Cai L, Li Q, Chen X, Gao L, Jiang L
Received 24 August 2020
Accepted for publication 9 October 2020
Published 4 November 2020 Volume 2020:12 Pages 11211—11218
DOI https://doi.org/10.2147/CMAR.S277274
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eileen O'Reilly
Meijuan Liu,1,* Li Cai,2,* Qifan Li,3 Xiaoran Chen,1 Lingyun Gao,1 Lei Jiang2
1Department of Ultrasound, The Affiliated Yantai Yuhuangding Hospital of Qingdao University, Yantai, Shandong, People’s Republic of China; 2Department of Pathology, The Affiliated Yantai Yuhuangding Hospital of Qingdao University, Yantai, Shandong, People’s Republic of China; 3Department of Ultrasound, RongCheng Maternal and Child Care Service Centre, Weihai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Lei Jiang
Department of Pathology, The Affiliated Yantai Yuhuangding Hospital of Qingdao University, Yantai, Shandong, People’s Republic of China
Email [email protected]
Aim: To investigate the association of the blood flow parameters measured by transvaginal three-dimensional power Doppler ultrasound and the angiogenesis of endometrial cancer.
Material and Methods: The expressions of vascular endothelial growth factor (VEGF) and CD31 in benign and malignant endometrial lesions, and in malignant lesions with different clinical and pathological features were analyzed. The correlations of the blood flow parameters (vascularization index [VI], blood flow index [FI], and vascularization-blood flow index [VFI]) of transvaginal 3D power Doppler ultrasound, and VEGF expressions, microvessel density (MVD) were also evaluated.
Results: The VEGF-positive rates and the MVD values in benign and malignant endometrial lesions were significantly different (both P< 0.001). The differences of VEGF-positive rates (P < 0.001) and MVD values (P = 0.021) between type I and type II lesions of endometrial cancer were statistically significant. There was no significant difference in the VEGF-positive rate and MVD value between stage IA and IB (P=0.443, P=0.311). The difference of VEGF expression and MVD in stage IA, stage IB and stage II and above was statistically significant (P=0.003, P=0.017). The VEGF-positive rate and MVD value were not significantly different in IAG1 and IAG2 lesions of endometrioid adenocarcinoma (P=0.709, P=0.792). There was no significant correlation between VI, FI, VFI and VEGF expression and MVD in endometrial cancer.
Conclusion: The VEGF-positive rates and MVD values were relatively high in malignant endometrial lesions, type II and stage II and above lesions of type I endometrial cancer, indicating that the angiogenesis of endometrial cancer tissues might play a crucial role in the tumor classification, and pelvic metastasis.
Keywords: three-dimensional power Doppler ultrasound, VEGF, CD31
Introduction
Most patients with benign and malignant endometrial lesions are referred to hospital due to abnormal vaginal bleeding and discharge. It is believed that there are no specific imaging findings of different lesions with similar clinical manifestations. According to the different clinical and pathological features of the tumor, as well as the biological behavior characteristics, endometrial cancer is classified into type I and type II. Type I is estrogen-dependent, and accounts for about 80% to 90%, of which the most pathological types are endometrioid adenocarcinoma, and only a small proportion are mucinous carcinoma. The prognosis of type I is relatively good. The rest 10–20% are type II, a non-estrogen-dependent type, with a poor prognosis, of which the pathological type is endometrial serous carcinoma, clear cell carcinoma, etc.1 Endometrial carcinoma approximately accounts for 20–30% of malignant tumors of female reproductive system in China,2 and an upward trend of the incidence rate can be observed in recent years. Most endometrioid adenocarcinomas are confined to the uterine body at the time of diagnosis, and the 5-year survival is 80%3 but if metastasis occurs, the median survival is only 7–12 months.
The genesis and degeneration of endometrial vessels are the rudimentary pathophysiological events of endometrial microenvironment. Endometrium is an important target organ of ovarian estrogen, and the abnormality of estrogen will lead to endometrial bleeding. Endometrial carcinoma can occur in women of childbearing age to post-menopause. The invasion and metastasis of endometrial carcinoma cause the majority of death of the patients4 and current studies indicate that angiogenesis is responsible for the poor outcome.5,6 Vascular endothelial growth factor (VEGF) is a heparin-binding growth factor, and specific to vascular endothelium. VEGF can provide nutrition for the growth, infiltration, and remote metastasis of tumor cells by stimulating the proliferation of the vascular endothelial cells and increasing the permeability of the vessels. It is regarded as the strongest factor of tumor-angiogenesis. Microvessel density (MVD), or microvessel count (MVC), or intratumoral microvessel density (IMD), is defined as the amount of microvessels per unit of volume and can be quantified through staining of some antibodies of endothelial cells, such as epithelial adhesion CD31. It is regarded as the golden standard to evaluate tumoral angiogenesis currently.
Previous studies pertaining to endometrial lesions were mostly the in-vitro and retrospective studies about the expression of VEGF and measurement of MVD of post-surgical endometrial tissues, unable to evaluate the tumoral vasculature effectively before surgery. Currently, ultrasound is the optical presurgical examination to detect tumoral angiogenesis noninvasively, by measuring resistance index (RI) and pulsatility index (PI) of the blood flow using pulse Doppler ultrasound. Nonetheless, only regional blood flow of the lesions can be assessed through this way, and the overall assessment of the whole endometrial lesions cannot be implemented effectively, which is more meaningful for guiding treatment and predicting prognosis. In this study, we aimed to use in-vivo flow parameters, including vascularization index (VI), blood flow index (FI), and vascularization-blood flow index (VFI) of transvaginal 3D power Doppler ultrasound, to evaluate angiogenesis of endometrial cancers preliminarily.
In this study, blood flow parameters, including VI, FI, and VFI, of the endometrial lesions were measured using transvaginal 3D power Doppler ultrasound before surgery or diagnostic curettage, and VEGF expressions and CD31-labelled MVD values of the lesions were detected using immunohistochemistry (IHC) method post-surgically. The VEGF expressions and MVD values in benign and malignant lesions and in lesions with different clinical and pathological features were evaluated, as well as the associations of VEGF expressions and MVD values with blood flow parameters, aiming to provide an imaging evaluation of tumoral angiogenesis of endometrial cancers before surgery.
Methods and Materials
Patient Recruitment
The study was designed as a retrospective study. From July 2018 to August 2019, a total of 143 patients who were diagnosed with endometrial lesions in Yantai Yuhuangding Hospital, among which 67 were benign, and 76 were malignant. The inclusion criteria: 1) transvaginal 3D power Doppler ultrasound was performed before endoscopy or surgery; 2) hysteroscopy, diagnostic curettage and post-surgical pathological examination were done within one week after ultrasound examination in the hospital; 3) the clinical data, imaging data and pathological data could be acquired entirely. The exclusion criteria: 1) the samples for pathological examinations were too small to obtain the pathological results; 2) patients who were unable to determine the pathological stage due to their older age, complications, or no malignant finding of intraoperative rapid pathology (confirmed malignant by postoperative IHC); 3) the malignant results were shown after hysteroscopy or diagnostic curettage, but the patients rejected further surgical treatment; 4) patients taking hirsutism drugs, digitalis, phenytoin, antidepressants, hormones (HRT-tamoxifen), corticosteroids, anticoagulants, and patients with bleeding and thyroid-related diseases.
Ultrasound Examination
Routine Transvaginal 2D Ultrasound Examination
All patients underwent routine transvaginal 2D ultrasound examination before surgery. Before the examination, the patients were asked to empty the bladder and take the bladder lithotomy position for the US operation. Coupling gel was smeared on the surface of the probe, and it was put into the posterior fornix of the vagina for performing US imaging. An intraluminal volume probe (Voluson E10, system edition: E70297, GE, USA) was used in this study. The long axis of the endometrium was shown on the sagittal section, and the thickness of the bilayer endometrium was measured by 2D transvaginal ultrasound. The standard for endometrial thickening was greater than 0.4cm.7
The Evaluation of Muscular Layer Infiltration Using Routine Ultrasound Examination
The classification of the muscular layer infiltration is defined as follows. No muscular layer infiltration: the boundary between the basal layer and the muscular layer of the endometrium is clear. Muscular layer infiltration: the basal layer is blurred or disappeared, and the endometrium echoes into the muscular layer, showing serrated or finger-like protrusions. Superficial muscle infiltration: the ratio of the thickness of the residual normal myometrium in the section showing the deepest section of the lesion’s muscle infiltration and the normal myometrial thickness was greater than 0.5. Deep muscle infiltration: the above ratio was less than or equal to 0.5.
Transvaginal 3D Power Doppler Ultrasound Examination
The color blood flows of endometrial lesions were obtained using transvaginal ultrasound Rflow stereo blood flow imaging mode, and 3D energy Doppler ultrasound was performed subsequently. The size of the sampling frame was adjusted to completely envelop the uterus, and the scanning angle was kept between 100° and 120°. Power Doppler settings were set to achieve maximum sensitivity to detect low-velocity flow without noise (contrast dynamic,7/gray map 7; frame filter 5/enhance 3; speckle reduction imaging II 2/compound resolution imaging 2; power doppler map 5; pulse repetition frequency, 0.8 kHz). Asking the patient to remain as still as possible, volume acquisition was performed during a 15–20-s time interval.
Quantitative Analysis of 3D Power Doppler Ultrasound
Glass body software and virtual organ computer-aided analysis (VOCAL) were utilized to perform area tracing of the endometrial lesions and measure blood flow parameters, including VI, FI, and VFI. One plane was selected every 15° in the plane A, and a total of 12 sections were selected in each lesion. The lesion area was manually traced, and the boundary of lesion was further modified by playing back the section until satisfactory. VI represents a color voxel/(total element – background voxel), indicating the amount of blood vessels in the targeted tissue, and demonstrated as a percentage; FI was a weighted color voxel/color voxel, indicating the intensity of blood flow during 3D scanning period; VFI was weighted color voxels/(total elements – background voxels), which represented the sum of blood flow and vascularization, also known as tissue perfusion. All cases were measured by an experienced radiologist on the same instrument, and each area was measured 3 times for averaging. For those patients with unclear endometrial contours, multiple measurements were done and the average value was adopted for reducing errors.
Pathological Examination and Immunohistochemistry (IHC)
The post-surgical specimens were fixed with 10% neutral formaldehyde, embedded in paraffin, and serially sectioned at 4um thickness. The CD31 antibodies (ZM44) and VEGF antibodies (2M-0265) were bought from Jinqiao Biotechnology Co.Ltd., Beijing, China. The DAB dye (F0065) was bought from Roche Ltd, Switzerland. The samples were stained with an automatic IHC instrument, BenchMark (Roche, Switzerland). Then, they were sealed with neutral gum, and examined by microscopy.
The Staging of Endometrial Cancer and the Interpretation of VEGF Expressions and MVD Values
The criteria recommended by the International Federation of Obstetricians and Gynecologists (FIGO) in 2009 was adopted for the surgery-pathological staging of the patients.8 Stage I tumors were confined to the uterus. IA stage was defined as the depth of tumor invasion <1/2 muscle layer, and IB as the tumor infiltration depth ≥1/2 muscle layer. Stage II was that the tumor extended to cervical interstitial, but within the corpus. Stage III was the ones that had local and/or regional spread. IIIA was the involvement of the serosa layer and/or the adnexa. IIIB tumor was the involvement of the vagina and/or paracervical regions. IIIC was the metastasis of pelvic lymph nodes, and/or para-aortic lymph nodes. Stage IV represented invasion to the bladder and/or rectal mucosa, and/or distant metastasis. Tumor invasion of the bladder and/or rectal mucosa stood for stage IVA, and distant metastases, including intra-abdominal and/or inguinal lymph node metastasis was stage IVB. According to the FIGO criteria, the endometrial cancer was divided into 3 levels in histology. Grade 1 (G1) was defined as more than 95% of the glandular tumor area; Grade 2 (G2) was defined as 5% to 50% of the tumor’s internal components; Grade 3 (G3) was defined as a solidity of more than 50% of the internal components of the tumor.
The brown particles represented positive expression of VEGF, mainly located in the cytoplasm of tumor cells. The nucleus is not stained, and in some samples, the stained particles could also be seen on the cell membrane. The samples were deemed as VEGF-negative if the cells were consistent with background color. The positive samples were identified when the VEGF-stained cells were clearly marked with brown. The percentage of the positive cells detected by microscopy and the staining intensity was scored separately. The percentage of positive cells: 0% ~ 5% was score 0, 6% ~ 25% was score 1, 26% ~ 50% was 2 points, 51% ~ 75% was score 3, 76% or more was score 4. The positive coloring intensity: the colorless was score 0, the light yellow mark was score 1, the brownish yellow mark was score 2, and the tan mark was score 3. The expression grade was acquired by multiplying the scores of the two groups. The 0 to 4 points were marked as negative, and 5 to 12 points were positive.
The CD31-stained MVD values were calculated with the reference to the Weidner method.9 CD31 was mainly expressed in the vascular endothelial cells, except for hemorrhagic regions, necrotic regions and areas of marginal reactions. Firstly, the entire slide was viewed under a 100-fold light microscope, and three “hot spots” with high blood vessel density were found. Then, the cells within the “hot spots” were counted after switching to a 400-fold microscope, and the average value was taken. Any single cell or a cell cluster that was stained brown by CD31, whether or not it formed a lumen, was considered as a countable microvessel as long as it was clearly separated from surrounding microvessels, tumor cells, and other connected tissues. Even if the “head” and the “tail” of one blood vessel happened to visualize in the same section, they would be thought to be two different microvessels. The interference from large vascular was avoided when counting. The microvessels in the sclerotic regions of the tumor and the soft tissues at the junctions of the tumor were excluded from counting. The smooth muscle wall and the blood vessels with a diameter larger than 8 red blood cells were also excluded.
Statistical Analysis
The statistical analysis was performed using the SPSS22.0 software (IBM, SPSS STATISTICS 22.0, USA). The enumeration and measurement data were described by frequency (rate) and mean (standard deviation), respectively. The χ2 test was used to make the comparison of the enumeration data. The comparison of the measurement data was performed by the Mann–Whitney U rank sum test for the violation of equal variances. The correlation analysis was performed using Pearson correlation coefficients. We took a=0.05 as the statistically significant level.
Results
A total of 143 patients were recruited in this study. There were 67 of benign lesions (46.9%), with a mean age of 52.3±9.5 years old (range from 39 to 78), and 76 of malignant lesions (53.1%), with a mean age of 60.2±9.3 years old (range from 28 to 76).
There were 67 patients with benign endometrial lesions, including 14 of endometrial polypoid hyperplasia (14/67, 20.9%), 11 of endometrial polyps (11/67, 16.4%), and 11 of cystic polyps (11/67, 16.4%), 10 of polypoid-like hyperplasia (10/67, 14.9%), 5 of polyp with hemorrhagic necrosis (5/67, 7.5%), 7 of atrophic endometrium (7/67, 10.4%), 5 of submucosal fibroids (5/67, 7.5%), 2 of simple hyperplasia with mulberry-like squamous (2/67, 3.0%) during the secretory period, hemorrhage and suppurative inflammation in the background, 2 of broken intimal glands were seen (2/67, 3.0%).
A total of 76 patients were diagnosed with endometrial cancer, including 60 patients with type I endometrioid adenocarcinoma (60/76, 78.9%), 6 patients with type II serous carcinoma (6/76, 7.9%), 4 patients with mixed cancer (adenocarcinoma and dedifferentiated carcinoma) (4/76, 5.3%), and 6 of complex hyperplasia of lesions with locally differentiated adenocarcinoma (6/76, 7.9%). Among the 60 patients with endometrioid adenocarcinoma, 40 (40/60, 66.7%) had IA (10 in IAG1, 26 in IAG2, 4 in IAG3), and 14 (14/60, 23.3%) in IB (6 in IBG2 and 8 in IBG3), and 6 (6/60, 10%) of stage III (2 in IIIAG3 and 4 in IIICG2).
The VEGF Expressions and CD31 in Benign and Malignant Endometrial Lesions, and in Lesions with Varied Clinical and Pathological Features
VEGF was mainly stained in the cytoplasm of tumor tissues in endometrial disease tissues and was also expressed in the surrounding stroma and normal endometrial tissues (Figure 1). CD31 was strongly expressed in the microvessels of tumor tissues and was mainly stained in the cytoplasm of vascular endothelial cells in endometrial lesions (Figure 2). The VEGF expressions and the values of CD31-labeled MVD malignant lesions were both significantly higher than the benign groups (P<0.001). The VEGF expression and the values of CD31-labeled MVD in the type I tissues of endometrial cancers were also significantly different that in the type II tissues (P< 0.001, P=0.021). There was no significant difference in VEGF expression and MVD values between stage IA and stage IB (P=0.443, P=0.311). While the difference of VEGF expression and MVD values between stage IA and stage II and above were statistically significant (P=0.003, P=0.047), as well as in stage IB and stage II and above (P= 0.017, P=0.010). The VEGF expression and MVD values in IAG1 had no significant differences with that in stageIAG2 lesions (P= 0.709, P=0.37). (Table 1)
 |
Table 1 VEGF Expressions and MVD Values in Benign and Malignant Endometrial Lesions, in Endometrial Lesions with Different Clinical-Pathological Features |
 |
Figure 1 VEGF expression in the tissues of moderate-differentiated endometrial carcinoma (SPx100). |
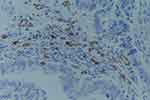 |
Figure 2 CD31 expression in the tissues of moderate-differentiated endometrial carcinoma (SPx400). |
The Associations of the Blood Flow Parameters (VI, FI, and VFI) of Transvaginal 3D Power Doppler Ultrasound, and VEGF Expression and MVD Values
Correlation coefficient of VI with VEGF and MVD (r=0.168, P=0.359; r=−0.121, P=0.510). Correlation coefficient of FI with VEGF and MVD (r=0.159, P=0.384; r=−0.207, P=0.255). Correlation coefficient of VFI with VEGF and MVD (r=0.189, P=0.300; r=−0.098, P=0.595) (Table 2, Figure 3). There was no significant correlation between blood flow parameters (VI, FI, and VFI) and VEGF expression and MVD in endometrial cancers.
 |
Table 2 The Correlations Between Blood Flow Parameters and VEGF Expressions, MVD Values |
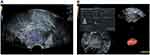 |
Figure 3 The same case as Figure 2 a 78-year-old woman with endometrioid adenocarcinoma (IBG2). (A) The blood flow pattern in Rflow color-Doppler flow imaging (in accordance with the brown areas represent the angiogenesis of tumor tissues as Figure 2). (B) The blood flow parameters (VI, FI, and VFI) of transvaginal 3D power Doppler ultrasound. |
Discussion
The mechanism of occurrence and metastasis of endometrial cancer has not been fully understood. The tumor microenvironment is mainly composed of tumor cells, stromal cells and extracellular matrix (ECM), which works as the local internal environment that promotes tumorigenesis, progression, invasion and metastasis of the neoplasm. VEGF is a highly specific pro-vascular endothelial growth factor that facilitates the proliferation, migration of vascular endothelial cells and microvascular formation. It plays an essential role in the invasion and metastasis of endometrial cancer, which may be a key factor to predict the prognosis of the patients. The angiogenic state is evaluated by assessing the formation of microvessels through counting the number of blood vessels in the “hot spots” selected by microscopy.
The Association of the Expression of VEGF and the Types of Endometrial Lesions, and Their Clinical and Pathological Features
VEGF can induce a large number of new capillaries to provide the oxygen and nutrients necessary for tumor growth, thereby accelerating the growth of tumor cells. The previous studies have found that VEGF is highly expressed in endometrial cancer. However, the results about its correlation with clinicopathological features of endometrial cancer are conflicting in different studies. Saito et al10 found that the expression of VEGF in moderately and highly differentiated endometrioid adenocarcinoma was significantly lower than that of poorly differentiated tumors, and the amount of VEGF expression was associated with the tumor grade. Laxmanan7 found that by binding to the VEGFR-1 receptor, VEGF-A can alter the function of dendritic cells, to reduce the immune ability to tumor cells of the body, which in turn leads to the growth and metastasis of tumor cells. This finding indicated that VEGF played an important role in the development of the biological behavior of endometrial cancers. The expression of VEGF protein in endometrial cancer tissues was significantly higher than that in endometrial atypical hyperplasia and proliferative endometrial tissues, with significant difference.11 According to Sallinen et al,12 the expression of VEGF was significantly higher in patients with malignant tumors than in benign ones, suggesting that the VEGF expression is closely related to endometrial cancer.
Consistent with previous studies, this study showed that VEGF expression in endometrial malignant lesions was significantly higher than that in benign lesions (85.5% vs 22.4%, P < 0.001). Type I endometrial cancer had better tumor differentiation, and the positive rate of estrogen and progesterone receptors was also high. The pathological morphology of type II endometrial cancer was rare, and the tumor was inclined to have high malignancy and poor differentiation. The positive expression of VEGF in type I (85.0%) and type II (100.0%) tissues was also significantly different in this study (P < 0.001).
Odeh et al2 and Merce et al13,14 validated that the angiogenesis of endometrial cancer was more distinct than that of endometrial hyperplasia. There was no difference in the expression of VEGF between stage IA and IB (82.5% vs 85.7%, P =0.443), indicating that VEGF had no significant difference in deep myometrial invasion and superficial myometrial invasion of endometrial cancer. However, there were significant differences between stage IA and II (82.5% vs 100%, P=0.003), and stage IB and Stage II and above (85.7% vs 100%, P=0.017), indicating that the expression of VEGF was possible to have a close relationship with pelvic metastasis of endometrial cancer.
The Association of MVD Value and the Types of Endometrial Lesions, and Their Clinical and Pathological Features
CD31, also known as platelet-endothelial cell adhesion molecule, is widely expressed on the surface of vascular endothelial cells and is a key marker of angiogenesis. MVD is calculated by counting the number of blood vessels by microscopy with the use of IHC methods to stain endothelial cells with some antigens, such as CD31 and CD34. Ozalp15 and Ozuysal16 declared that MVD of endometrial cancer was positively correlated with the FIGO stage, meaning that the surgical pathological stage of endometrial cancer was associated with the tumor angiogenesis, which might help to evaluate the malignancy of the lesions and predict the patients’ prognosis. Wang et al17 included 29 studies in 2517 patients about MVD in the endometrial lesions, and they believed that MVD was associated with deep myometrial invasion, lymphatic infiltration, lymph node metastasis, and lower survival. Angiogenesis can serve as a useful indicator to evaluate the clinical-pathological features and prognosis for patients.
In this study, the CD31 mouse anti-human monoclonal antibody was used to express MVD in 143 patients with benign and malignant endometrial lesions. The results showed that significant differences of MVD were identified between benign and malignant endometrial lesions, and type I and type II, and some of the stages (IA and II and above, IB and II and above). No significant difference was detected between stage IA and IB, as well as grade IAG1 and IAG2. This study suggested that the expression of CD31 in malignant lesions was significantly higher than that of benign lesions; and the lesions of late stages were significantly higher than those of early stages. And the later the stage, the higher was the MVD value.
In this study, the MVD in the poorly differentiated tumor tissues was less than that in the moderately differentiated tumor tissues. This might be due to the reader-dependence in the selection of the hot spots, and it might also be caused by the solid components of poorly differentiated endometrial cancer. The microvessels were seen in the cell nest interstitial, not within the cells, because there were many solid tumor cells with a large area, but a small area of the tumor stroma. This might partially account for the non-difference in the lesions of IAG1 and IAG2.
The reasons why this study was different from previous studies might lie on: (1) the number of samples and differences between groups, intra-groups were relatively small, and the number of cases in stage II and above was also small; (2) the selections of “hot spots” were reader-dependent, and also the differences in the inclusion and exclusion criteria. Future research should increase the sample size, reduce differences between groups, and minimize the subjective differences in physician interpretation of MVD.
The Associations Between the Blood Flow Parameters (VI, FI, and VFI) of Transvaginal 3D Power Doppler Ultrasound and VEGF and MVD
Currently, there is no relevant study on the correlation between blood flow parameters of endometrial cancer of transvaginal 3D power Doppler ultrasonography and VEGF and MVD. These studies showed that the blood flow parameters enabled pre-surgical evaluation of the angiogenesis of endometrial cancer, providing an important reference to help predict the prognosis of patients before treatment. There were no significant correlations between blood flow parameters VI, FI, VFI and VEGF and MVD in our study, which might be due to the variance of the sample size in each subtype of different lesions, because there were less patients with stage II and above. More studies with larger sample size are needed for further research in this field.
Most of the previous studies investigated the expression of VEGF and MVD in endometrial cancer tissues after surgery. The innovation of our study is that in this study, transvaginal 3D power Doppler ultrasound was used to visualize the vascular architecture before surgery and quantify the blood flow parameters VI, FI and VFI by VOCAL software, which might achieve the same efficacy in estimating angiogenesis as the postoperative evaluation of VEGF expression and MVD, helping clinical decision-making and prediction of prognosis. The limitations of the study include the small sample size of endometrial cancer, intra-group differences of sample sizes and the subjectivity of MVD interpretation, which might partially affect the accuracy of the results.
Ethics
This study was conducted in accordance with the Declaration of Helsinki.
Acknowledgments
The authors would like to thank Dr Qian Li (Department of research department, The Affiliated Yantai Yuhuangding Hospital of Qingdao University) for her proofreading the manuscript. Meijuan Liu and Li Cai are co-first authors for this study.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Kuramoto H, Iwami Y, Sugimoto N, Miyagawa J. Cytological characteristics of endometrial phasing using the specimens prepared with the liquid-based procedure: comparison with the conventional procedure. Acta Cytol. 2016;60:429–437. doi:10.1159/000448872
2. Odeh M, Vainerovsky I, Grinin V, Kais M. Three-dimensional endometrial volume and 3-dimensional power Doppler analysis in predicting endometrial carcinoma and hyperplasia. Gynecol Oncol. 2007;106:348–353. doi:10.1016/j.ygyno.2007.04.021
3. Savelli L, Ceccarini M, Ludovisi M, et al. Preoperative local staging of endometrial cancer: transvaginal sonography vs. Magnetic Resonance Imaging Ultrasound Obstet Gynecol. 2008;31:560–566. doi:10.1002/uog.5295
4. Bartosch C, Manuel Lopes J. Endometrial carcinomas: a review emphasizing overlapping and distinctive morphological and immunohistochemical features. Adv Anat Pathol. 2011;18:415–437. doi:10.1097/PAP.0b013e318234ab18
5. Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi:10.1038/35025220
6. Morgan KG, Wilkinson N, Buckley CH. Angiogenesis in normal, hyperplastic, and neoplastic endometrium. J Pathol. 1996;179:317–320. doi:10.1002/(SICI)1096-9896(199607)179:3<317::AID-PATH598>3.0.CO;2-T
7. Laxmanan S, Robertson SW, Wang E, Lau JS, Briscoe DM. Vascular endothelial growth factor impairs the functional ability of dendritic cells through Id pathways. Biochem Biophys Res Commun. 2005;334:193–198. doi:10.1016/j.bbrc.2005.06.065
8. Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009;105:103–104.
9. Weidner N, Carroll PR, Flax J. Tumor angiogenesis correlates with metastasis in invasive prostate carcinoma. Am J Pathol. 1993;143:401–409.
10. Saito M, Sato Y, Watanabe J, Kuramoto H. Angiogenic factors in normal endometrium and endometrial adenocarcinoma. Pathol Int. 2007;57:140–147. doi:10.1111/j.1440-1827.2006.02071.x
11. Xu H, Sun X, Sun WJ. Expression and clinical correlation of NGAL and VEGF in endometrial carcinoma. Eur Rev Med Pharmacol Sci. 2018;22:632–636.
12. Sallinen H, Heikura T, Koponen J, et al. Serum angiopoietin-2 and soluble VEGFR-2 levels predict malignancy of ovarian neoplasm and poor prognosis in epithelial ovarian cancer. BMC Cancer. 2014;14:696. doi:10.1186/1471-2407-14-696
13. Merce LT, Alcazar JL, Engels V, Troyano J. Endometrial volume and vascularity measurements by transvaginal three-dimensional ultrasonography and power Doppler angiography in stimulated and tumoral endometria: intraobserver reproducibility. Gynecol Oncol. 2006;100:544–550. doi:10.1016/j.ygyno.2005.09.024
14. Merce LT, Alcazar JL, Lopez C, et al. Clinical usefulness of 3-dimensional sonography and power Doppler angiography for diagnosis of endometrial carcinoma. J Ultrasound Med. 2007;26:1279–1287. doi:10.7863/jum.2007.26.10.1279
15. Ozalp S, Yalcin OT, Acikalin M, Tanir HM, Oner U, Akkoyunlu A. Microvessel density (MVD) as a prognosticator in endometrial carcinoma. Eur J Gynaecol Oncol. 2003;24:305–308.
16. Ozuysal S, Bilgin T, Ozan H, Kara HF. Angiogenesis in endometrial carcinoma: correlation with survival and clinicopathologic risk factors. Gynecol Obstet Invest. 2003;55:173–177. doi:10.1159/000071533
17. Wang JZ, Xiong YJ, Man GCW, Chen XY. Clinicopathological and prognostic significance of blood microvessel density in endometrial cancer: a meta-analysis and subgroup analysis. Arch Gynecol Obstet. 2018;297:731–740.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
