Back to Journals » OncoTargets and Therapy » Volume 9
The expression of ERCC1 and BRCA1 predicts prognosis of platinum-based chemotherapy in urothelial cancer
Received 25 November 2015
Accepted for publication 9 March 2016
Published 15 June 2016 Volume 2016:9 Pages 3465—3471
DOI https://doi.org/10.2147/OTT.S101319
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Min Li
Wenhui Song, Hongshun Ma
Department of Urology, Tianjin First Center Hospital, Tianjin, People’s Republic of China
Objective: To investigate the expression and clinical significance of ERCC1 and BRCA1 genes in urothelial cancer patients.
Methods: Forty-two urothelial cancer patients who did not receive platinum-based chemotherapy during January 2009 to May 2013 were enrolled. The expression levels of ERCC1 and BRCA1 were determined by immunohistochemistry and the median survival time (MST) for these patients was calculated.
Results: ERCC1-positive patients who received oxaliplatin-based chemotherapy had a shorter MST than ERCC1-negative patients (P<0.05), whereas there is no difference of MST between BRCA1-positive and -negative patients. Furthermore, MST in ERCC1 and BRCA1 double-positive patients was shorter than ERCC1 and BRCA1 double-negative patients (P<0.05). The positive expression of ERCC1 had a significant positive correlation with BRCA1 (r=0.313, P=0.044).
Conclusion: The expression level of ERCC1 may be used as a prognostic marker for urothelial cancer patients who received postoperative adjuvant chemotherapy.
Keywords: urothelial cancer, ERCC1, BRCA1, chemotherapy, prognosis
Introduction
Urothelial cancer is the most common urinary reproductive system tumor that can occur in the urinary tract epithelium of renal pelvis, calyces, ureter, bladder, and other parts. For the late-stage urothelial cancer, especially muscular invasive and metastatic cancer, adjuvant chemotherapy, especially platinum-based chemotherapy, is still the most widely used treatment. European Organization for Research and Treatment of Cancer conducted a 7-year follow-up of 142 patients with invasive bladder cancer and found that 5-year overall survival rates for patients who did not receive chemotherapy and those who received paclitaxel, gemcitabine, or cisplatin chemotherapy at random after surgery were significantly different (31% and 60%, respectively).1 However, resistance to chemotherapy is increasingly prominent. Thus, studying the mechanisms for drug resistance has become a hot topic. Studies showed that the abnormal expression of the DNA damage repair-related genes, such as excision repair cross-complementation group 1 (ERCC1) and breast cancer 1, early onset (BRCA1), is closely related to cancer multidrug resistance.2 ERCC1 and BRCA1 are key genes in the nucleotide excision repair (NER) pathway, which are closely related to platinum resistance.3 The NER system is the main defense mechanism for repairing DNA damage caused by carcinogens and chemotherapy drugs.4
This study analyzed the protein expression levels of ERCC1 and BRCA1 in 42 cases of urothelial cancer specimens and investigated their relationships with clinical features, including the median survival time (MST) in patients who received platinum-based chemotherapy. We also tried to further explore whether: 1) the expression level of ERCC1 and BRCA1 is related to the sensitivity of platinum drugs and 2) the joint detection of ERCC1 and BRCA1 is more valuable than a single molecule detection in terms of predicting the sensitivity of platinum drugs.
Materials and methods
Patient characteristics
Forty-two patients, including 31 males and eleven females, were enrolled (Table 1). They were aged from 53 to 78 years, and the median age is 67.2 years. All cases were suffering from the locally advanced muscular invasive or metastatic cancer of the urinary tract, including 23 cases of bladder cancer, eleven cases of ureteral cancer, and eight cases of renal pelvis cancer. Among them, there were five cases of liver metastasis, 13 cases of pulmonary metastasis, three cases of bone metastasis, and 36 cases of pelvic lymph node and retroperitoneal metastases. Before chemotherapy, the Karnofsky scores of all patients were above 60 points, which means the patients require occasional assistance, but are able to care for most of their personal needs.5 The routine blood test, liver and kidney function, and appetite in these patients were normal. The life expectancy of patients was over 3 months, and they had not received any general chemotherapy. The normal control group was from urothelial tract tissue in patients, such as benign prostatic hyperplasia, calculus of ureter, and pyelolithiasis. This study was approved by the Ethics Committee of the Tianjin First Center Hospital. All the patients signed the informed consent form before chemotherapy.
  | Table 1 Clinical characteristics in patients |
Chemotherapy
Before chemotherapy, patients were given 10 mg dexamethasone and 0.3 mg ramosetron to prevent side effects and vomiting, respectively. The specific chemotherapy scheme was used as following: gemcitabine 750 mg/m2 was dissolved in 100 mL normal saline and given by an intravenous drip within half an hour on days 1, 8, and 15. Oxaliplatin 100 mg/m2 was dissolved in 500 mL glucose solution and given by an intravenous drip within 2 to 3 hours on day 2. Twenty-eight days were considered as a cycle and generally four cycles in total or continuous treatment for more than two cycles were evaluated.
Immunohistochemical staining
All specimens of urothelial cancer lesions were fixed with 4% formaldehyde, embedded in paraffin, and sectioned into slices at 5 μm. After tissue paraffin sections were dewaxed and hydrated, they were incubated with 3% H2O2 for 10 minutes to block endogenous peroxidase. Following washing with distilled water three times, Mouse Anti-Human ERCC1 Monoclonal Antibody or Mouse Anti-Human BRCA1 Monoclonal Antibody (Shanghai Sun Biological Technology Co., Ltd., Shanghai, People’s Republic of China) were applied onto the sections and incubated for 2 hours at the room temperature. Antimouse secondary antibody was used. Diaminobenzidine was used as a chromogenic agent. Brownish yellow particle precipitation in the nucleus or the cytoplasm was considered as positive. Two pathologists blindly scored the staining results based on the Fromowitz comprehensive scoring method.6 The results were calculated by the percentage of the positive stained cells and the tinting strength of cells. The percentage of the positive cells was calculated in ten visual fields: <5%, 0 point; 5%–25%, 1 point; 26%–50%, 2 points; 51%–75%, 3 points; and >75%, 4 points. The tinting strength of cells was calculated as: no color, 0 point; faint yellow, 1 point; brownish yellow, 2 points; and sepia, 3 points. The two kinds of points were added together: 0–1 point, negative; 2 points and above, positive.
Statistical analysis
The SPSS 16.0 (SPSS Inc., Chicago, IL, USA) statistical package was used for the statistical analysis. Enumeration data were examined with χ2 analysis. Correlation was analyzed with Pearson correlation analysis. The overall survival was expressed as Kaplan–Meier survival curve, and the difference was analyzed by log rank test. P<0.05 was considered as statistically significant.
Results
The expression of ERCC1 in urothelial cancer
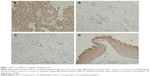
The positive expression of ERCC1 protein was located in the nucleus as brownish yellow particles. Twenty cases of normal bladder mucous tissues were used for comparison, and among them four cases of ERCC1 immunohistochemical staining were positive (20.0%). Among 42 cases of urothelial cancer available for evaluation, 20 cases of ERCC1 immunohistochemical staining were positive (47.6%) (Figure 1). The positive expression rate of ERCC1 in urothelial cancer group was significantly higher than the normal control group (P=0.037).
The effects of clinical features on the expression of ERCC1 were further assessed (Table 2). The positive expression of ERCC1 in urothelial cancer was not affected by sex, age, smoking history, clinical staging, and pathological grading.
  | Table 2 The relationship between ERCC1 and clinical features |
The expression of BRCA1 in urothelial cancer
The positive expression of BRCA1 protein was located in the nucleus or cytoplasm as brownish yellow particles. Twenty cases of normal bladder mucous tissues were used for comparison, and among them seven cases of BRCA1 immunohistochemical staining were positive (35.0%). Among 42 cases of urothelial cancer available for evaluation, 27 cases of BRCA1 immunohistochemical staining were positive (64.3%) (Figure 1). The positive expression rate of ERCC1 in urothelial cancer group was significantly higher than the normal control group (P=0.030).
The effects of clinical features on the expression of BRCA1 were further assessed (Table 3). The positive expression of BRCA1 in urothelial cancer was not affected by sex, age, smoking history, clinical staging, and pathological grading.
  | Table 3 The relationship between BRCA1 and clinical features |
The relationship between the MST and ERCC1 or BRCA1
The MSTs of the ERCC1-negative and -positive groups were 22.2 (95% confidence interval [CI]: 12.29–32.11) and 16.1 months (95% CI: 11.72–11.72), respectively. Thus, the MST of the ERCC1-negative group was significantly longer than that of ERCC1-positive group (P=0.042) (Figure 2).
  | Figure 2 Comparison of the median survival curves between the ERCC1-positive and -negative groups. |
The MSTs of the BRCA1-negative and -positive groups were 22.1 (95% CI: 10.22–34.18) and 17.1 months (95% CI: 14.30–14.30), respectively. However, the MST of the BRCA1-negative group was not statistically different than that of BRCA1-positive group (P=0.551) (Figure 3).
  | Figure 3 Comparison of the median survival curves between the BRCA1-positive and -negative groups. |
The MSTs for those groups with ERCC1 and BRCA1 dual-negative or -positive were 26.6 (95% CI: 19.97–33.24) or 16.6 months (95% CI: 7.98–25.22), respectively.
Statistical analysis showed that the MST of urothelial cancer patients with negative ERCC1 and BRCA1 was significantly longer than those with positive ERCC1 and BRCA1 (P=0.018) (Figure 4).
  | Figure 4 Comparison of the median survival curves between the ERCA1 and BRCA1 double-positive and -negative groups. |
Correlation analysis of the expressions of ERCC1 and BRCA1 in urothelial cancer tissues
Correlation analysis indicated that the expressions of ERCC1 and BRCA1 were significantly correlated to each other (P=0.044).
Discussion
Personalized chemotherapy is a hot topic in tumor biology. However, many tumors will eventually develop resistance to chemotherapy drugs through some not yet fully understood mechanisms. Cytotoxic drugs often directly or indirectly cause different kinds of DNA damage, which will activate DNA repair-related genes in malignant tumors. The abnormal expression of these DNA repair-related genes in tumor cells is considered as one of the mechanisms for the development of chemotherapy drug resistance.2
ERCC1, located on chromosome 19, is one of the important members of the NER family. This protein contains 297 amino acids, forms a heterodimer with xeroderma pigmentosum, complementation group F endonuclease (also known as ERCC4), and plays a role in excising the 5′ end of the damaged DNA single strand. In vivo and in vitro studies have confirmed that the expression level of ERCC1 is related to the effect of platinum-based chemotherapy drugs.7 It is reported that cisplatin treatment can increase the expression level of ERCC1 in tumor cells. The overexpression of ERCC1 can quickly repair the damaged DNA of cells which stagnated at G2/M phase, leading to cisplatin resistance.8 Previously, in a cohort of 57 cases of advanced bladder cancer, the patients with low expression levels of ERCC1 were found to have an obviously longer MST (25.4 months) than those with high expression levels of ERCC1 (15.4 months).9 This is similar to our current study. Interestingly, ERCC1-negative patients were also reported to have better survival rate than ERCC1-positive patients in gemcitabine plus platinum-based chemotherapy.10 In addition, ERCC1 also correlates with ionizing radiation therapy.11 Thus, ERCC1 may be used to predict the prognosis and survival rate of bladder cancer patients receiving platinum-based chemotherapy.
BRCA1 is a tumor suppressor that is located on human chromosome 17q21 containing 24 exons.12 The mutation rate of BRCA1 is ~35%–40% in familial breast and ovarian cancer. BRCA1 is one of top 20 upregulated genes in bladder cancer.13 BRCA1 participates in DNA damage repair by NER and homologous recombination repair.14 BRCA1 also plays an important role in the regulation of cell cycle, mRNA transcription, protein capture, apoptosis, ubiquitination, and so on.15 It was reported that among 57 patients with locally advanced urothelial cancer who received neoadjuvant chemotherapy, the MST for those with low expression of BRCA1 (168 months) was significantly longer than those with high expression of BRCA1 (34 months, P=0.01). Among many factors analyzed for prognosis, only the low expression of BRCA1 and lymph node metastasis were independent prognostic factors.16
Cisplatin is an important drug in adjuvant chemotherapy of urothelial cancer. Its main target of combating cancer is DNA, which creates a cisplatin–DNA compound with cisplatin in covalent bonding.17 This leads to interstrand cross-linking or intrastrand cross-linking, therefore causing DNA damage which will prevent DNA replication and transcription and cause the death of cells.18 The DNA damage repair gene ERCC1 and BRCA1 can repair DNA damage caused by cisplatin and other chemotherapy drugs. ERCC1 mainly participates in the damaged NER of DNA and can recognize and excise the damaged part of DNA.19 Besides this, BRCA1, a tumor suppressor, mainly participates in the double-strand break repair. The overexpression of ERCC1 and BRCA1 can quickly repair the damaged DNA, contributing to the resistance of tumor cells to cisplatin.20
BRCA1 is mainly expressed at the nucleus, with a total positive expression rate of 74.55%. ERCC1 is mainly expressed at the nucleus of urothelial cancer with a little expression in the cytoplasm and the total positive expression rate is 59.09%. The expression intensity of ERCC1 and BRCA1 has nothing to do with sex, age, histological type, degree of tumor cell differentiation, clinical stage, and lymph node metastasis. Jointly detecting the expression level of BRCA1 and ERCC1 can better predict the sensitivity of cisplatin chemotherapy. The mRNA expression of ERCC1 and BRCA1 is highly related to each other and has a positive synergistic effect on influencing the sensitivity and efficacy to cisplatin.21–23 BRCA1 mainly participates in the double-strand break repair, but is also indispensable for the NER. ERCC1 not only participates in the NER of DNA damage but also plays a role in the double-strand break repair, especially those caused by intrastrand cross-linking.22 This may be the reason why their expression is correlated. However, the exact mechanism of their synergistic effect is yet to be further explored.
Conclusion
ERCC1 and BRCA1, key factors in DNA damage repair, are often overexpressed in urothelial cancer and lead to resistance to platinum-based chemotherapy drugs. Tumors with low expressions of ERCC1 and BRCA1 are generally sensitive to platinum-based chemotherapy and patients will have a good prognosis and a higher survival rate. Detecting the levels of both ERCC1 and BRCA1 can better explain the sensitivity of tumor cells to platinum-based chemotherapy. Thus, ERCC1 and BRCA1 are potential prognostic biomarkers for patients who have received cisplatin chemotherapy.
Acknowledgments
This study was supported by Traditional Chinese and Western Medicine Combine Research Subject of Administration of Traditional Chinese Medicine in Tianjin (No 13121). The authors would like to express their gratitude to all the physicians participating in this work.
Disclosure
The authors report no conflicts of interest in this work.
References
Paz-Ares L, Solsona E, Esteban E, et al. Randomized phase III trial comparing adjuvant paclitaxel/gemcitabine/cisplatin (PGC) to observation in patients with resected invasive bladder cancer: Results of the Spanish Oncology Genitourinary Group (SOGUG) 99/01 study. Paper presented at: Chicago, IL: ASCO Annual Meeting Proceedings; 2010. | ||
Raj GV, Karavadia S, Schlomer B, et al. Contemporary use of perioperative cisplatin-based chemotherapy in patients with muscle-invasive bladder cancer. Cancer. 2011;117(2):276–282. | ||
Huang ZL, Cao X, Luo RZ, Chen YF, Zhu LC, Wen Z. Analysis of ERCC1, BRCA1, RRM1 and TUBB3 as predictors of prognosis in patients with non-small cell lung cancer who received cisplatin-based adjuvant chemotherapy: a prospective study. Oncol Lett. 2016;11(1):299–305. | ||
Karran P. Mechanisms of tolerance to DNA damaging therapeutic drugs. Carcinogenesis. 2001;22(12):1931–1937. | ||
Crooks V, Waller S, Smith T, Hahn TJ. The use of the Karnofsky Performance Scale in determining outcomes and risk in geriatric outpatients. J Gerontol. 1991;46(4):M139–M144. | ||
Fromowitz FB, Viola MV, Chao S, et al. ras p21 expression in the progression of breast cancer. Hum Pathol. 1987;18(12):1268–1275. | ||
Fedeli U, Fedewa SA, Ward EM. Treatment of muscle invasive bladder cancer: evidence from the National Cancer Database, 2003 to 2007. J Urol. 2011;185(1):72–78. | ||
Kim KH, Do IG, Kim HS, et al. Excision repair cross-complementation group 1 (ERCC1) expression in advanced urothelial carcinoma patients receiving cisplatin-based chemotherapy. APMIS. 2010;118(12):941–948. | ||
Bellmunt J, Paz-Ares L, Cuello M, et al. Gene expression of ERCC1 as a novel prognostic marker in advanced bladder cancer patients receiving cisplatin-based chemotherapy. Ann Oncol. 2007;18(3):522–528. | ||
Sun JM, Sung JY, Park SH, et al. ERCC1 as a biomarker for bladder cancer patients likely to benefit from adjuvant chemotherapy. BMC Cancer. 2012;12:187. | ||
Kawashima A, Takayama H, Tsujimura A. A review of ERCC1 gene in bladder cancer: implications for carcinogenesis and resistance to chemoradiotherapy. Adv Urol. 2012;2012:812398. | ||
Petrucelli N, Daly MB, Feldman GL. Hereditary breast and ovarian cancer due to mutations in BRCA1 and BRCA2. Genet Med. 2010;12(5):245–259. | ||
Stone R 2nd, Sabichi AL, Gill J, et al. Identification of genes correlated with early-stage bladder cancer progression. Cancer Prev Res. 2010;3(6):776–786. | ||
Taron M, Rosell R, Felip E, et al. BRCA1 mRNA expression levels as an indicator of chemoresistance in lung cancer. Hum Mol Genet. 2004;13(20):2443–2449. | ||
Neveling K, Kalb R, Florl AR, et al. Disruption of the FA/BRCA pathway in bladder cancer. Cytogenet Genome Res. 2007;118(2–4):166–176. | ||
Font A, Taron M, Gago JL, et al. BRCA1 mRNA expression and outcome to neoadjuvant cisplatin-based chemotherapy in bladder cancer. Ann Oncol. 2011;22(1):139–144. | ||
Tassone P, Di Martino MT, Ventura M, et al. Loss of BRCA1 function increases the antitumor activity of cisplatin against human breast cancer xenografts in vivo. Cancer Biol Ther. 2009;8(7):648–653. | ||
Stewart DJ. Mechanisms of resistance to cisplatin and carboplatin. Crit Rev Oncol Hematol. 2007;63(1):12–31. | ||
Simon GR, Ismail-Khan R, Bepler G. Nuclear excision repair-based personalized therapy for non-small cell lung cancer: from hypothesis to reality. Int J Biochem Cell Biol. 2007;39(7–8):1318–1328. | ||
Michalski CW, Erkan M, Sauliunaite D, et al. Ex vivo chemosensitivity testing and gene expression profiling predict response towards adjuvant gemcitabine treatment in pancreatic cancer. Br J Cancer. 2008;99(5):760–767. | ||
Olaussen KA, Dunant A, Fouret P, et al. DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N Engl J Med. 2006;355(10):983–991. | ||
Rosell R, Skrzypski M, Jassem E, et al. BRCA1: a novel prognostic factor in resected non-small-cell lung cancer. PLoS One. 2007;2(11):e1129. | ||
Gregg SQ, Robinson AR, Niedernhofer LJ. Physiological consequences of defects in ERCC1-XPF DNA repair endonuclease. DNA Repair (Amst). 2011;10(7):781–791. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.