Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 18
The Exacerbation of Chronic Obstructive Pulmonary Disease: Which Symptom is Most Important to Monitor?
Authors Jacobson PK , Lind L , Persson HL
Received 18 April 2023
Accepted for publication 14 June 2023
Published 20 July 2023 Volume 2023:18 Pages 1533—1541
DOI https://doi.org/10.2147/COPD.S417735
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jill Ohar
Petra Kristina Jacobson,1,2 Leili Lind,3,4 Hans Lennart Persson1,2
1Department of Health, Medicine and Caring Sciences, Linköping University, Linköping, Sweden; 2Department of Respiratory Medicine in Linköping, Linköping University, Linköping, Sweden; 3Department of Biomedical Engineering/Health Informatics, Linköping University, Linköping, Sweden; 4Digital Systems Division, Unit Digital Health, RISE Research Institutes of Sweden, Linköping, Sweden
Correspondence: Petra Kristina Jacobson, Department of Respiratory Medicine in Linköping, Linköping University, Linköping, SE-581 85, Sweden, Tel +46 0 10 1031162, Email [email protected]
Background: GOLD 2023 defines an exacerbation of COPD (ECOPD) by a deterioration of breathlessness at rest (BaR), mucus and cough. The severity of an ECOPD is determined by the degree of BaR, ranging from 0 to 10. However, it is not known which symptom is the most important one to detect early of an ECOPD, and which symptom that predicts future ECOPDs best. Thus, the purpose of the present study was to find out which symptom is the most important one to monitor.
Methods: We analysed data on COPD symptoms from the telehealth study The eHealth Diary. Frequent exacerbators (n = 27) were asked to daily monitor BaR and breathlessness at physical activity (BaPA), mucus and cough, employing a digital pen and symptom scales (0– 10). Twenty-seven patients with 105 ECOPDs were analysed. The association between symptom development and the occurrence of exacerbations was evaluated using the Andersen–Gill formulation of the Cox proportional hazards model for the analysis of recurrent time-to-event data with time-varying predictors.
Results: According to the criteria proposed by GOLD 2023, 42% ECOPDs were mild, 48% were moderate and 5% were severe, while 6% were undefinable. Mucus and cough improved over study time, while BaR and BaPA deteriorated. Mucus appeared earliest, which was the most prominent feature of the average exacerbation, and worsening of mucus increased the risk for a future ECOPD. There was a 58% increase in the risk of exacerbation per unit increase in mucus score.
Conclusion: This study suggests that mucus worsening is the most important COPD symptom to monitor to detect ECOPDs early and to predict future risk för ECOPDs. In the present study, we also noticed a pronounced difference between GOLD 2022 and 2023. Hence, GOLD 2023 defined the ECOPD severity much lower than GOLD 2022 did.
Keywords: Rome proposal, telemonitoring, telemedicine, COPD management, COPD symptoms, future exacerbations
Introduction
Chronic obstructive pulmonary disease (COPD) is a leading cause of morbidity and the third leading cause of death worldwide.1 COPD is also associated with a significant economic burden. The total direct costs of respiratory disease in the European Union are estimated to be about 6% of the total annual healthcare budget, with COPD accounting for 56% (38.6 billion euros) of the cost of respiratory disease.2 The total cost of COPD in the United States is expected to reach $800.90 billion over the next 20 years, with an annual cost of $40 billion.3 The highest proportion of human and economic cost for COPD is associated with hospital admissions for severe exacerbations (ECOPD).4,5 Beside the economic burden, ECOPDs increase the risk of progression of disease, which leads to premature death, and suffering, and cause diminished quality of life.6–8 Accurate and early detection of ECOPDs will enable early management and will reduce mortality.7–9
The real cause of ECOPDs is not fully understood, and it can be difficult to get the correct diagnosis since symptoms of an ECOPD overlap with symptoms of pneumonia and other diseases such as deterioration of heart failure.1 Severe ECOPDs, that is, those requiring hospitalisation, are associated with significant mortality and morbidity.6,10 Patient recovery is slow and severe ECOPDs are a major burden for health services.10 Remote monitoring of COPD patients by tele-health and care at home with the aim of early detection of ECOPD is therefore of paramount importance and is one of the goals of the respiratory community worldwide.
Normally, having trouble breathing is a hallmark symptom of COPD. Thus, the typical symptoms of an ECOPD include dyspnoea (or breathlessness), cough, and mucus production. In the GOLD 2023 guidelines, the Rome proposal determines the severity of an ECOPD by grading breathlessness at rest (BaR), employing a scale from 0 to 10.11 In the present study, we used the Rome proposal to define the severity of ECOPDs. The aim of the study was to understand which of the COPD symptoms, BaR, breathlessness at physical activity (BaPA), cough or mucus, was the most important one to monitor in order to detect ECOPDs early and to predict future ECOPDs.
Materials and Methods
Study Subjects and Study Procedures
The present study analysed data collected in The eHealth Diary, a 12-month longitudinal telemonitoring study conducted between 2013 and 2017 in Östergötland, Sweden. The Health Diary telemonitoring system, based on digital pen technology, is used by patients when assessing and reporting their daily health state to a specialised hospital-based home care (HBHC) unit.5,7,8 Study subjects were instructed to register their COPD symptoms, BaR, BaPA, mucus and cough, every day, applying a scale from 0 to 10. The underlying assumption behind the telemonitoring is that patients with frequent ECOPDs would undergo fewer ECOPDs due to regular telemonitoring from the patient’s home. Once enrolled in the study, according to the criteria described in Lyth et al, patients were introduced to the telemonitoring system and received continuous supervision from the HBHC unit,5 which was also responsible for providing healthcare during the study period. The study included patients with COPD, aged ≥65 years, who were frequently hospitalised due to ECOPDs – at least two inpatient episodes within the last 12 months. Twenty-seven patients, participating at least 75 days and presenting with totally 105 ECOPDs, were analysed. Previous statistical and qualitative analysis report that the number of hospitalisations was significantly reduced, and quality of life was improved for COPD patients participating in telemonitoring.7,8
Blood Analyses, Dynamic Spirometry, BMI and Comorbidities
At inclusion, we assessed blood oxygenation (SAT) and CRP in venous blood. On ECOPDs, we assessed respiratory rate (RR), heart rate, SAT, CRP and arterial blood gases (ABG), the latter at rest on air breathing or ongoing oxygen supplementation. To measure forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC) and to calculate the ratio of these volumes, dynamic spirometry was performed after bronchodilatation at inclusion using Hedenström as a reference.12,13 COPD was staged by airway obstruction, that is, FEV1% predicted 80–100%=GOLD stage I, 50–79%=stage II, 30–49%=stage III, and <30%=stage IV (GOLD 2023). At inclusion comorbidities were graded using the “Charlson Comorbidity Index” (CCI),14 and body mass index (BMI) was calculated (weight in kg divided by the height in m2). Underweight was defined as BMI <18.5 kg/m2, normal weight as 18.5≤BMI<25 kg/m2, overweight as 25≤BMI<30 kg/m2 and obesity as BMI ≥30 kg/m2.15 Patients’ smoking habits were also registered at inclusion.
Exacerbation Severity According to GOLD 2022 and GOLD 2023
All exacerbations were defined regarding the severity applying the definitions described by GOLD 2022 and GOLD 2023, the latter employing the Rome proposal.11 GOLD 2022 defines the severity of ECOPDs by the action necessary to take due to worsening. Thus, a mild ECOPD requires only increased inhalation therapy, a moderate ECOPD demands additional therapy with steroids and/or antibiotics, while those ECOPDs leading to emergency room visit and/or hospitalisation are defined as severe. In contrast, definition of ECOPD severity according to GOLD 2023 depends on assessment of BaR, RR, heart rate, SAT, CRP and ABG. A mild ECOPD is defined by BaR <5, RR <24 breaths/min, heart rate <95 bpm, resting SAT ≥92% breathing ambient air (or patient’s usual oxygen prescription) and change ≤3% (when known), CRP <10mg/L (if obtained). A moderate one is defined when at least three of five following parameters are stated: BaR ≥5, RR ≥24 breaths/min, heart rate ≥95 bpm, resting SAT <92% breathing ambient air (or patient’s usual oxygen prescription), and/or change >3% (when known), CRP ≥10mg/L. Also, if obtained, ABG may show hypoxemia (PaO2 ≤8 kPa) and/or hypercapnia (PaCO2 >6 kPa) but no acidosis. A severe ECOPD is defined by an ABG showing hypercapnia (PaCO2 >6 kPa) and acidosis (pH <7.35).11
Patient and Public Involvement
Patients and the public were not involved in the design or conduct or reporting of the present research. Results were not disseminated to study participants.
Statistical Analysis
Data are presented as mean ± 1 SD for continuous variables and percentage for categorical variables. Correlations were calculated with Pearson’s correlation coefficient (r), when variables fulfilled the normality criterium; otherwise, Spearman’s Rank Test (ρ) was used. Normality was tested with the Kolmogorov–Smirnov test. The level of significance was 0.05 and all p-values were two-tailed. The evolution of the COPD symptoms was analysed using linear regression. The association between this evolution and the occurrence of exacerbations was evaluated using the Andersen–Gill formulation of the Cox proportional hazards model for the analysis of recurrent time-to-event data with time-varying predictors. All analyses were undertaken using IBM SPSS Statistics, vs 27.0.0.0 (IBM SPSS, Chicago, IL, USA).
Results
Sample COPD: Descriptive Statistics
Table 1 summarises the descriptive statistics of the study subjects (n = 27). Most study subjects were elderly women with a history of smoking, severe COPD stages and a great burden of comorbidities. Eleven patients were on oxygen supplementation. Compliance to symptom registration was high, that is, 90% of all study days demonstrated registrations of the four symptoms recorded. In general, GOLD 2023 definition of COPD severity resulted in a down-staging to lower severity. Thus, compared to GOLD 2022, most ECOPDs were defined mild or moderate according to the Rome proposal for ECOPD severity. Only 6 of 105 ECOPDs remained undefined regarding their severity, when the GOLD 2023 criteria were applied.
 |
Table 1 Descriptive Statistics. Mean ± 1SD |
Symptom Progression Over Study Time
At baseline mean values ± 1 SD for BaR, BaPA, mucus and cough were 1.5±1.6, 5.5±2.2, 2.5±2.5 and 2.0±2.0, respectively. Twenty-two patients (81%) exhibited BaR values from 0 to 3 at inclusion. The progression of symptoms over study time, analysed using linear regression, is shown in Figure 1. Mucus and cough improved significantly over study time (negative slope), while BaR and BaPA deteriorated (positive slope).
Symptom Progression During Exacerbation
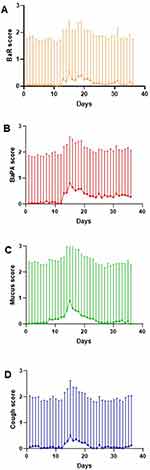
Progression of symptoms for the average ECOPD before, during and after treatment start is shown in Figures 2 and 3. The diagnosis ECOPD was made at day 15, when treatment with steroids and/or antibiotics was started. Figure 2 shows the mean values (adjusted to baseline) of all 105 ECOPDs for all four symptoms in the same Figure. Figure 3 shows the symptoms separately; mean values (adjusted to baseline) of 105 exacerbations are indicated and positive bars represents 1 SD. Of 105 ECOPDs, only 15 exhibited a peak value of BaR ≥5. In comparison, 91 ECOPDs demonstrated BaPa ≥5 at the peak of the ECOPD.
Mucus appeared earliest and was the most prominent feature of the average exacerbation. When the ECOPDs were diagnosed by the HBHC staff and, if applicable, treatments with steroids and/or antibiotics were started the same day (at day 15), the scores of mucus and cough returned at some point to base-line, while the scores for BaR and BaPA did not (Figures 2 and 3).
Prediction of Future Exacerbations
The association between the progression of the four symptoms recorded and the occurrence of exacerbations was evaluated using the Andersen-Gill formulation of the Cox proportional hazards model for the analysis of recurrent time-to-event data with time-varying predictors.16 The hazard ratios (HR) for each symptom associated with the risk for future ECOPDs are shown in Table 2. Worsening of mucus score with one unit increased the risk for future ECOPD with 58% (p ˂0.001).
Discussion
In the present study, we report for the first time that mucus was the most important symptom to telemonitor, preferentially at daily basis, to early detect the start of an ECOPD among patients with frequent ECOPDs (Figure 2). Worsening of the mucus score was also the symptom that best predicted future exacerbations in the present study (Table 2). In line with previous knowledge, this study also demonstrated the negative effects frequent ECOPDs have on symptom progression, that is, a deterioration of BaR and BaPA over study time (Figure 1). Also, in line with present knowledge, as ECOPDs became less frequent, as previously reported by us,7 the symptoms of bronchitis, that is, mucus and cough, became less prominent (Figure 1).
We also investigated how different definitions of ECOPD influenced on the scoring of ECOPD severity applying the definitions of GOLD 2022 and GOLD 2023. We know of only one previous study doing so.17 In contrast to the present study, there was no data on BaR in that study. Because all patients (364 patients displaying 364 ECOPDs) were hospitalised due to an ECOPD the authors assumed that all patients would express BaR ≥5.17 In the present study, we noticed a pronounced difference between GOLD 2022 and 2023. Hence, GOLD 2023 defined the ECOPD severity much lower than GOLD 2022 did. This is in line with the findings by Reumkens et al, who concluded that most of the ECOPDs, classified as severe because of the hospitalization according to GOLD 2022, were classified as moderate ECOPDs.17 However, in contrast to that study, we find in the present study that down-staging to less ECOPD severity mainly depended on the assessment of BaR. Thus, of 105 ECOPDs, only 15 exhibited a peak value of BaR ≥5. Considering that 42 ECOPDs were classified as severe according to GOLD 2022, we conclude that it is not safe to assume that BaR ≥5 is mandatory for ECOPDs regarded as severe from the GOLD 2022 criteria. In comparison, 91 ECOPDs demonstrated BaPa ≥5 at the peak of the ECOPD. The diagnosis of the ECOPDs in the eHealth Diary study was based on the symptom assessments from the study participant, together with an assessment from the experienced physicians at HBHC. When the HBHC staff became aware of a worsening of symptoms, they made a home visit and if the HBHC physician diagnosed an ECOPD, it was treated with steroids and/or antibiotics. According to GOLD 2022, such treatment per se defines a moderate ECOPD. One might argue that the prescriptions of oral antibiotics and/or steroids upon diagnosis of an ECOPD in the eHealth Diary study might have been too excessive, thus, defining too many ECOPDs as moderate. As the diagnosis of an ECOPD was made by a highly qualified physician, knowing the patient well, this explanation seems to us less likely. As the cohort consisted of elderly, multimorbid and very fragile patients, one might assume that treatment with steroids and/or antibiotics was mainly governed by the clinical context, with the aim to prevent further – possibly devastating – deterioration.
One might also wonder whether the severity of the ECOPDs differed so much between GOLD 2022 and GOLD 2023 due to the settings of the criteria. Clearly, few ECOPDs demonstrated BaR score ≥5. Possibly, this reason might explain why the ECOPD severity was so different, when GOLD 2022 and GOLD 2023 criteria were employed. Maybe the limit for BaR is set too high to be classified as a moderate exacerbation. The Rome proposal states that the BaR values range from 0 to 3 in patients with COPD in a stable phase.11,18–20 In the present study, 22 patients (81%) exhibited BaR values from 0 to 3 at inclusion. Moreover, the Rome proposal states that in the emergency ward, or in the hospital, values are higher than 4.11,21–23 It was therefore agreed that BaR score ≥5 (on a scale of 0–10) in the context of a suspected ECOPD would indicate severe dyspnoea.11 It might be that a BaR score ≥5 would be better for a severe ECOPD and a lower one for a moderate ECOPD.
It is also stated in the Rome proposal that worsening of cough and mucus may be the most relevant symptoms or signs.11,24 However, in previous studies, the intensity of these symptoms has not been properly measured, which has made it difficult to include them in an ECOPD severity classification.11 Therefore, it was agreed that worsening of BaR is the most relevant symptom for most patients and most ECOPDs. Notably, the present study suggests that assessment of mucus, employing the same scale as for BaR (0–10), may be of great importance. Zero means no mucus and 10 means the worst mucus ever experienced, in line with what the Rome proposal suggest for assessment of BaR.
As far as we know, there is only one telehealth study previously published reporting on ECOPD prediction in relation to scoring COPD symptoms. Thus, Rassouli et al performed a telehealth study on patients with COPD, assessing COPD symptoms weekly by the COPD assessment test (CAT; score 0–40 points).16 As in the present study, they applied the Andersen–Gill formulation of the Cox proportional hazards model for the analysis of recurrent time-to-event data with time-varying predictors to understand how CAT predicted future ECOPD. They found that a 1-point weekly increase in CAT (scale 0–5) was associated with an 8% increase in the risk for future ECOPD. In comparison, the present study shows that a 1-unit (scale 0–10) daily increase of the mucus score was associated with a 58% increase in the risk for future ECOPD. As Rassouli et al worked with weekly CAT assessment and the present study assessed symptoms at daily basis, we can assume that our way of telemonitoring would detect an ECOPD much earlier than the model applied by Rassouli.16 More recently, Sun et al reported that worsening of morning symptoms of COPD predicted future ECOPDs.25 However, this study was not a telehealth study. Another study by Mokhtar et al predicted future ECOPDs applying telehealth and machine learning, but the prediction was not based on COPD symptoms.26
This study has obvious limitations. It is a single centre study, and the studied COPD cohort consisted of only 27 multimorbid frequent exacerbators. Thus, our observations only apply to this very particular phenotype of the COPD population and cannot be generalised to ECOPDs experienced by the very large population of patients presenting with mild-to-moderate COPD.
Conclusion
This study suggests that worsening of mucus is the most important COPD symptom to monitor to detect ECOPDs early and to predict future risk för ECOPDs. In the present study, we also noticed a pronounced difference between GOLD 2022 and 2023. Hence, GOLD 2023 defined the ECOPD severity much lower than GOLD 2022 did.
Abbreviations
ABG, arterial blood gas; BMI, body mass index; BPM, beats per minute; BaR, breathlessness at rest; BaPa, breathlessness at physical activity; CAT, COPD Assessment Test; CCI, Charlson Comorbidity Index; CI, Confidence Interval; COPD, chronic obstructive pulmonary disease; ECOPD, exacerbation of COPD; FEV1, forced expiratory volume in one second; FEV1(% of predicted), forced expiratory volume in one second expressed as % of predicted; FVC, forced vital capacity; GOLD, global initiative for chronic obstructive lung disease; HBHC, hospital-based home care; HR, hazard ratio; RR, respiratory rate; SAT, blood oxygen saturation; SD, standard deviation.
Data Sharing Statement
The data upon which this analysis was based are available from Professor Hans Lennart Persson in anonymised form, upon receipt of a reasonable request. Contact details for Professor Hans Lennart Persson are Department of Respiratory Medicine in Linköping, Linköping University, SE-581 85 Linköping, Sweden. E-mail: [email protected]
Ethics Statement and Study Registration
The study was approved by the Swedish Ethical Review Authority (dnr: 2020-03308; L. Lind) according to the guidelines of the Declaration of Helsinki. All patients were included following verbal and written information of the study and after informed consent to participate. The study was registered at ISRCTN (ISRCTN34252610).
Acknowledgments
The authors thank all study patients, their next-of-kin and the HBHC staff for making this study possible.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by grants to P.K.J. and H.L.P from the Medical Research Council of Southeast Sweden (FORSS) (Grant No. FORSS-969385, FORSS-980999) and grants to L.L. and H.L.P. from Sweden’s innovation agency Vinnova (Dnr: 2019-05402) in Swelife’s and Medtech 4 Health’s Collaborative projects for better health programme. The study sponsors had no role in study design, data collection, analysis, and interpretation; in the writing of the manuscript; nor in the decision to submit the manuscript for publication.
Disclosure
HLP reports honoraria for advisory boards/lectures from AstraZeneca, Boehringer Ingelheim, GlaxoSmithCline, Intermune, Roche and the Swedish Medical Products Agency. The authors report no other conflicts of interest in this work.
References
1. Global Initiative for Chronic Obstructive Lung Disease GOLD. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease 2023 report; 2023. Available from: https://goldcopd.org/2023-gold-report-2/.
2. Rehman AU, Hassali MAA, Muhammed SA, et al. The economic burden of chronic obstructive disease (COPD) in the USA, Europe and Asia: results from a systematic review of the literature. Expert Rev Pharmacoecon Outcomes Res. 2020;20(6):661–672. doi:10.1080/14737167.2020.1678385
3. Zafari Z, Li S, Eakin MN, Bellanger M, Reed RM. Projecting long-term health and economic burden of COPD in the United States. Chest. 2021;159(4):1400–1410. doi:10.1016/j.chest.2020.09.255
4. Halpin DM, Miravitlles M, Metzdorf N, Celli B. Impact and prevention of severe exacerbations of COPD: a review of the evidence. Int J Chron Obstruct Pulmon Dis. 2017;12:2891–2908. doi:10.2147/COPD.S139470
5. Lyth J, Lind L, Persson HL, Wiréhn AB. Can a telemonitoring system lead to decreased hospitalization in elderly patients? J telemed telecare. 2021;27(1):46–53. doi:10.1177/1357633X19858178
6. Rothnie K, Müllerova H, Smeeth L, Quint J. Natural history of chronic obstructive pulmonary disease exacerbations in a general practice-based population with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;15(198):464–471. doi:10.1164/rccm.201710-2029OC
7. Persson HL, Lyth J, Wiréhn AB, Lind L. Elderly patients with COPD require more health care than elderly heart failure patients do in a hospital-based home care setting. Int J Chron Obstruct Pulmon Dis. 2019;2019(14):1569–1581. doi:10.2147/COPD.S207621
8. Persson HL, Lyth J, Lind L. The health diary telemonitoring and hospital-based home care improve quality of life among elderly multimorbid COPD and chronic heart failure subjects. Int J Chron Obstruct Pulmon Dis. 2020;2020(15):527–541. doi:10.2147/COPD.S236192
9. Wu C, Li G, Huang C, et al. Acute exacerbation of a chronic obstructive pulmonary disease prediction system using wearable device data, machine learning, and deep learning: development and cohort study. JMIR Mhealth Uhealth. 2021;9(5):e22591. doi:10.2196/22591
10. Anzueto A. Impact of exacerbations on COPD. Eur Respir Rev. 2010;19(116):113–118. doi:10.1183/09059180.00002610
11. Celli B, Fabbri LM, Aaron SD, et al. An updated definition and severity classification of chronic obstructive pulmonary disease exacerbations: the Rome proposal. ATS J. 2021;204(11):1251–1258. doi:10.1164/rccm.202108-1819PP
12. Hedenström H, Malmberg P. Agarwal K. Reference values for lung function tests in females. regression equations with smoking variables. Bull Eur Physiopathol Respir. 1985;21(6):551–557.
13. Hedenström H, Malmberg P, Fridriksson H. Reference values for lung function tests in men: regression equations with smoking variables. Ups J Med Sci. 1986;91(3):299–310. doi:10.3109/03009738609178670
14. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi:10.1016/0021-9681(87)90171-8
15. A healthy lifestyle. Available from: https://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi.
16. Rassouli F, Baty F, Stolz D, et al. Longitudinal change of COPD assessment test (CAT) in a telehealthcare cohort is associated with exacerbation risk. Int J Chron Obstruct Pulmon Dis. 2017;12:3103–3109. doi:10.2147/COPD.S141646
17. Reumkens C, Endres A, Simons SO, Savelkoul PHM, Sprooten RTM, Franssen FME. Application of the Rome severity classification of COPD exacerbations in a real-world cohort of hospitalized patients. Eur Respiratory Soc. 2023;9(2):doi: 10.1183/23120541.00569–2022.
18. Scioscia G, Blanco I, Arismendi E, et al. Different dyspnoea perception in COPD patients with frequent and infrequent exacerbations. Thorax. 2017;72(2):117–121. doi:10.1136/thoraxjnl-2016-208332
19. Gift AG, Narsavage G. Validity of the numeric rating scale as a measure of dyspnea. Am J Crit Care. 1998;7(3):200–204. doi:10.4037/ajcc1998.7.3.200
20. Marin JM, Montes de Oca M, Rassulo J, Celli BR. Ventilatory drive at rest and perception of exertional dyspnea in severe COPD. Chest. 1999;115(5):1293–1300. doi:10.1378/chest.115.5.1293
21. Pinto-Plata VM, Livnat G, Girish M, et al. Systemic cytokines, clinical and physiological changes in patients hospitalized for exacerbation of COPD. Chest. 2007;131(1):37–43. doi:10.1378/chest.06-0668
22. O’Donnell DE, Parker CM. COPD exacerbations 3: pathophysiology. Thorax. 2006;61(4):354–361. doi:10.1136/thx.2005.041830
23. Noell G, Cosío BG, Faner R, et al. Multi-level differential network analysis of COPD exacerbations. Eur Respir J. 2017;50(3):1700075. doi:10.1183/13993003.00075-2017
24. Stockley RA, O’Brien C, Pye A, Hill SL. Relationship of sputum color to nature and outpatient management of acute exacerbations of COPD. Chest. 2000;117(6):1638–1645. doi:10.1378/chest.117.6.1638
25. Sun T, Li X, Cheng W, et al. The relationship between morning symptoms and the risk of future exacerbations in COPD. Int J Chron Obstruct Pulmon Dis. 2020;15:1899–1907. doi:10.2147/COPD.S255030
26. Mohktar MS, Redmond SJ, Antoniades NC, et al. Predicting the risk of exacerbation in patients with chronic obstructive pulmonary disease using home telehealth measurement data. Artif Intell Med. 2015;63(1):51–59. doi:10.1016/j.artmed.2014.12.003
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.