Back to Journals » Journal of Inflammation Research » Volume 16
The Eradication of Carcinogenic Viruses in Established Solid Cancers
Authors Elkoshi Z
Received 12 July 2023
Accepted for publication 12 December 2023
Published 20 December 2023 Volume 2023:16 Pages 6227—6239
DOI https://doi.org/10.2147/JIR.S430315
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Ning Quan
Zeev Elkoshi
Research and Development Department, Taro Pharmaceutical Industries Ltd, Haifa, Israel
Correspondence: Zeev Elkoshi, Email [email protected]
Abstract: Carcinogenic viruses (oncoviruses) can initiate cancer, but their impact on established cancer varies. Some of these viruses prolong survival while others shorten it. This study classifies oncoviruses into two categories: viruses which induce a strong CD8+T cell reaction in non-cancerous tissues, and viruses which induce a weak CD8+ T cell reaction in non-cancerous tissues. The classification proves useful in predicting the effect of oncoviruses on the prognosis of solid cancers. Therefore, while eliminating carcinogenic viruses in healthy individuals (for example by immunization) may be important for cancer prevention, this study suggests that only viruses which induce a weak CD8+ T cell reaction should be eradicated in established solid tumors. The model correctly predicts the effect of oncoviruses on survival for six out of seven known oncoviruses, indicating that immune modulation by oncoviruses has a prominent effect on prognosis. It seems that CD8+ T cell response to oncoviruses observed in infected benign tissues is retained in infected tumors. Clinical significance: the effect of oncoviruses on solid cancer prognosis can be predicted with confidence based on immunological responses when clinical data are unavailable.
Keywords: carcinogenic viruses, oncoviruses, cancer prognosis, CD8+ T cell, Treg cells
Introduction
Carcinogenic viruses drive the initiation of several types of cancer. Human papillomavirus (HPV) drives cervical cancer, head and neck cancer, and other less common cancer types such as vulva, vagina, penis, anus, and rectum cancers. Hepatitis B virus (HBV) and hepatitis C virus (HCV) drive hepatocellular carcinoma (HCC), human gamma herpesvirus 8 (HHV-8) triggers Kaposi’s sarcoma, human T-cell lymphoma virus 1 (HTLV1) triggers adult T-cell lymphoma/leukemia (ATLL), Epstein–Barr virus (EBV) triggers Burkitt’s lymphoma, nasopharyngeal carcinoma and Hodgkin’s lymphoma (HL), Merkel cell polyomavirus (MCPyV) induces Merkel cell carcinoma (MCC).1 HBV and HPV infections are responsible for 23–59% of HCC cases and for 100% of cervical cancer cases, respectively.1 EBV is detected in the tumor tissue of almost 100% of the cases of endemic Burkitt lymphoma. Evidence of HTLV-1 infection was found in at least 90% of ATLL cases,2 HHV-8 is present in 80–95% of Kaposi sarcoma lesions.3 MCPyV was detected in 77% of all MCCs.4 Although different carcinogenic agents use different mechanisms to induce their tumorigenic effects, they may broadly be divided into two classes: direct carcinogens which express viral oncogenes that directly contribute to cancer cell transformation (HPV, HTLV-1, EBV, MCPyV, and HHV-8), and indirect carcinogens that presumably cause cancer through chronic infection and inflammation, which eventually leads to carcinogenic mutations in host cells (HBV, HCV).1,5 Oncoviruses hijack host cycle regulation machinery to promote their own genomic replication. Presumably, as part of this process, viruses target anti-cancer genes such as p53 and RB1 to delay host cell apoptosis, facilitating efficient virus production and export before cell death.5
Viruses elicit strong innate immune responses by activating diverse pathogen recognition receptors (PRRs), including toll-like receptors (TLR) on plasma or endosomal membranes, and cytoplasmic receptors such as cGAS for viral DNA, and RIG-I and MDA-5 for viral RNA. These receptors initiate separate signaling cascades that converge to activate transcription factors. This activation results in the expression of pro-inflammatory cytokines, including IL-1α, IL-1β, IL-18, IL-6, and TNFα through NF-κB activation. Additionally, type I and type III interferons are expressed through interferon response factors (IRFs), mainly IRF3 and IRF7.6 Upon antigen-driven activation, IFN-I directs naive CD4+ T cells to the Th1 phenotype, facilitates the activation, expansion, and IFNγ secretion of cytotoxic CD8+ T cells, and enhances NK cell activation and IFNγ secretion.7 IFNγ plays a crucial role in coordinating immune responses by promoting IgG production, enhancing macrophage function, and improving dendritic cell antigen presentation.8 Oncoviruses play a role in multistep tumorigenesis alongside chronic inflammation and immunosuppression. They enhance the expression of viral-encoded oncogenes in infected cells, including MCPyV-related T antigen, EBV-related LMP1, LMP2A, LMP2B, EBNA1, and EBNA2, as well as HTLV-1-related Tax and HPV-related E5, E6, and E7. Additionally, oncoviruses regulate the host epigenetic machinery, induce genomic instability through insertional mutagenesis,9 and influence the mitochondrial function of infected cells.10 They also impact tumor suppressor pathways (eg, p53 and pRB) and host signaling pathways (eg, PI3K–AKT–mTOR; MAPK; Notch; WNT/β-catenin; NF-κB).11
Eliminating several carcinogenic pathogens in healthy populations is recommended as a preventive measure to reduce the risk of cancer. It is unclear, however, whether a specific carcinogenic virus should be eradicated in populations of cancer patients, since some of these viruses have a beneficial effect on cancer prognosis while others have an adverse effect. This work proposes the use of CD8+T cell reaction to viral infection in healthy organisms, to determine whether or not an oncogenic virus should be eradicated in established solid tumors. It is demonstrated that viruses with impaired CD8+ T cell reaction, in the context of a chronic infection of an otherwise healthy organism (henceforth: “chronic infection”), have a negative effect on solid cancer prognosis, and should be eradicated in established solid tumors, while an oncovirus that elicit a strong CD 8+T cell reaction in the context of chronic infection, improves prognosis, and therefore should remain untouched in solid cancers.
The Immune Reaction to Carcinogenic Viruses
Carcinogenic viruses are classified as strong or weak inducers of cytotoxic immune response. Various methods have been employed to assess the extent of this response. The evaluation of CD8+ T cell reactivity may involve (a) measuring the capacity of CD8+ T cells to proliferate after exposure to the virus or its epitope, in experimental animal models or infected human populations;12,13 (b) assessing the ability of CD8+ T cells to produce cytotoxic agents such as IL-2, IFNγ, TNFα, perforins, and granzymes;14 (c) examining the down- or up-regulation of CD8+ T cell gene expression associated with anti-viral activity;15 (d) detecting virus-specific CD8+ T cells in a seronegative recipient of a graft infected with the virus.16 While the patient remaining aviremic following graft implantation indicates strong anti-viral cytotoxicity, this method does not allow for the quantification of this anti-viral effect; (e) analyzing the down- or up-regulation of immune checkpoint gene expression (such as CD137, PD-1, TIM-3, CTLA −4, 2B4, ICOS, BTLA, LAG-3, KLRG-1, TIGIT) in CD8+ T cells;17–23 and (f) determining the killing rate of infected splenocytes by virus-specific CD8+ T cells. This involves pulsing a population of mouse splenocytes with the viral epitope and transferring them into syngeneic hosts along with a population of unpulsed splenocytes. Loss of peptide-pulsed targets is measured in the mouse spleen, and the percent killing is estimated from the reduction in the ratio of pulsed target cells to unpulsed cells, corrected for the initial ratio.24 It should be noted that this list of methods used to assess CD8+ T cell response is not exhaustive.
Next, CD8+T cell response to oncoviruses, in the context of non-cancerous tissue infection, is reviewed:
HPV
Human papillomavirus is a strong inducer of CD8+ T cell reaction during both acute and chronic infections. The infiltration of cytotoxic CD8+ T cells into HPV-infected human lesions is considered a crucial immunological characteristic in persistent papillomavirus infection and disease regression.25 In female mice, intravaginal immunization with HPV followed by boosting with different HPV serotypes resulted in a 10-fold increase in cervicovaginal antigen-specific CD8+ T cells compared to priming alone.12 While “exhausted” CD1+ HPV-specific CD8+ T cell clusters have been observed in the context of HPV-positive head and neck squamous cell carcinoma (HNSCC),26,27 this cluster contributes to persistent anti-tumor immunity due to their extended cell survival and specialized cytotoxic capacity.26 Moreover, it has been associated with longer survival in HPV-positive HNSCC.26 Notably, no reports exist of exhausted anti-HPV CD8+ T cells in the context of HPV infection in non-cancerous tissue.
HBV
Hepatitis B virus triggers an exhausted CD8+ T cell reaction in chronic infection. Most acute adult-onset HBV infections usually elicit strong, multifunctional CD8+T cell responses, while chronic hepatitis B is characterized by quantitatively and qualitatively weak HBV-specific CD8+T cell responses with markedly reduced capacity to proliferate or to produce IL-2, IFNγ, TNFα, perforins and granzymes.14,28–31
HCV
Hepatitis C virus triggers an exhausted CD8+ T cell reaction in chronic infection. Similar to HBV, HCV acute infection results in a vigorous CD8+ T cell response. Chronic HCV infection, on the other hand, may lead to a dysfunctional CD8+ T cell response.32 For example, Barili et al33 have shown that during chronic infection, exhaustion of HCV-specific T cell responses is characterized by a broad gene downregulation associated with a wide metabolic and anti-viral function impairment. It was also demonstrated that a TCF1+CD127+PD-1 HCV-specific CD8+ T cell subset with phenotypic features of T-cell exhaustion and memory exists in chronically infected patients, both before and after treatment with direct acting antiviral agents (ie, after HCV eradication). This exhausted population expands after antigen re-challenge. Side- by-side with this expansion, terminally exhausted TCF1-CD127-PD-1hi HCV-specific CD8+ T cells emerge.15 The relationship between memory-like and terminally exhausted HCV-specific CD8+ T cells can be regarded as progenitor–progeny relationship.34
HHV-8
Human herpes virus 8 infection triggers a strong CD8+ T cell response. Cytotoxic T cell reaction to HHV-8, in patients that control the infection are frequent, diverse, and strongly differentiated toward a phenotype which expressed perforin (an effector phenotype). In addition, HHV-8-specific CD8+ T cells were observed in a seronegative recipient of an HHV-8 infected graft who remained persistently aviremic and antibody negative, suggesting that specific CD8+ T lymphocytes may provide protection from persistent HHV-8 infection.16 HHV-8 epitopes activate monofunctional and polyfunctional CD8+ T cells that produce various combinations of IFNγ, IL-2, TNFα, the chemokine MIP- 1β, and cytotoxic degranulation marker CD107a in healthy HHV-8-seropositive individuals.35 However, there are also data describing HHV-8-induced immune evasion through the downregulation of MHC-I molecule expression36 and by enhancing endocytosis of MHC I from the cell surface.37 Another study notes the absence of HHV-8-specific T cells in Kaposi sarcoma.38 This finding is supported by a more recent analysis of biopsies from Kaposi sarcoma patients. In this study, CD8+ T cells were not detected in Kaposi sarcoma tissues with evidence of HHV-8 infected cells, but CD8+ T cells were readily detectable in tumors without HHV-8 infected cells.39 These results may suggest that a lack of specific CD8+ T cells allows HHV-8 infection to progress. Overall, HHV-8 induces a robust specific CD8+ T cell response, which persists despite the virus’s evasion mechanisms.
HTLV1
Human T cell leukemia virus type 1 induces dysfunctional CD8+ T cells during HTLV1 infection. Kozako et al18 examined PD-1/PD-L1 expression on cells from 27 asymptomatic HTLV-1 carriers (ACs) and 27 adult T-cell leukemia/lymphoma (ATLL) patients in comparison with cells from 18 healthy controls. PD-1 expression on HTLV-1-specific CD8+T cells from ACs and ATLL patients was substantially higher compared to healthy controls. Anti-PD-L1 antibody treatment upregulated HTLV1-specific CD8+T-cell response. Clements et al20 compared the expression of negative-checkpoint-immune-receptors (T cell co-receptors that negatively regulate T cell activation), expressed by circulating CD8+T lymphocytes between three groups: a group of patients with myelopathy/tropical spastic paraparesis (HAM/TSP) infected with HTLV1, a group of patients with asymptomatic HTLV-1 infection and a group of seronegative controls. Global CD8+T cells from HAM/TSP patients co-expressed multiple negative checkpoint receptors at much higher frequencies than asymptomatic carriers. In addition, TIM-3 expression in CD8+T cells from asymptomatic carriers was significantly higher compared to seronegative controls. In agreement, a model of immunodeficient NSG mice reconstituted with a functional human immune system demonstrated exhausted CD8+ T cells pattern following HTLV1 infection. These CD8+ T cells expressed reduced IFNγ expression, even though granzyme B and perforin were fully expressed.40 It was also reported that HTLV1 protein expression in naturally infected CD8+T lymphocytes renders them susceptible to killing mediated by autologous HTLVI-specific CD8+T lymphocytes.41 Taken together, HTLV1 infection downregulates cytotoxic T cell activities.
EBV
Epstein–Barr infection triggers a strong CD8+ T cell response that is retained following prolonged exposure to high antigen levels. Infectious mononucleosis is characterized by high viral loads and prolonged CD8+T cell stimulation.42 In most cases, EBV enters latency and is under lifelong immune control. Using tetrameric MHC-peptide complexes to directly visualize antigen-specific CD8+T cells during the primary immune response to EBV infection in humans, Callan et al43 demonstrated a massive expansion of activated, antigen-specific CD8+ T cells during the primary response to this virus. Responses to lytic cycle proteins account for most of the vigorous CD8+T cell expansion seen in acute infectious mononucleosis [Abbott]. Chatterjee et al19 detected an expansion of PD-1 positive CD8+ T cells together with high frequencies of TIM-3, 2B4, and KLRG-1 expression in the plasma of patients with infectious mononucleosis and in the serum of a mouse model. These PD-1 positive CD8+ T cells, however, conserved cytokine production, proliferation, and cytotoxicity. PD-1 blocking resulted in virus-associated lymphomagenesis due to compromised EBV-specific immune control. Conflicting results were reported by Ma et al.21 Using an EBV-infected cord blood-reconstituted huNSG mouse model, these authors demonstrated that blocking PD-1 and CTLA4 activity led to a reduced tumor burden. However, as noted by Chatterjee et al,19 this model was deemed inappropriate as it does not induce efficient EBV-specific immune control of lymphoma. In summary, EBV induces a long-lasting high CD8+ T cell response.
MCPyV
Merkel cell polyomavirus specific CD8+ T cells demonstrate strong anti-viral activity during persistent infection. In immunocompetent individuals, polyomavirus (PyV) infection is asymptomatic and persists lifelong, primarily in the urogenital tract, in the central nervous system (CNS), and in hematopoietic cells.44 Ganusov et al24 have demonstrated a strong CD8+T cell anti-viral response to polyomavirus during persistent infection in a mouse model. Another study in a mouse model, by the same group, confirmed the functionality of virus-specific CD8+T cells recruited during persistent infection. In contrast, CD8+ T cells recruited early in MPyV infection express an exhausted functionality.45 Long-lived antiviral CD8+ T cells are defective in self-renewal; however, newly generated T cells preserve antiviral CD8+ T cell populations during chronic PyV infection.46
The Effect of Carcinogenic Viruses on Survival/Clinical Practice
HPV
An analysis of 2845 patients with cervical cancer revealed 43% lower mortality of patients with high-risk HPV compared with patients without high-risk HPV.47
Among a group of 303 patients with stage IVC head and neck squamous cell carcinoma, HPV- positive patients had better head and neck cancer specific survival (P < 0.001) and overall survival (P < 0.001) compared to those with HPV-negative disease.48
HBV
In a retrospective study with 175 hepatocellular carcinoma (HCC) patients, the overall survival (OS) rate of non-HBV non-HCV HCC patients was significantly better than that of HBV-HCC patients.49 Another retrospective study with 562 HCC patients demonstrated a significantly better OS rate of non-HBV non-HCV patients with a history of HBV infection (self-resolved infection or HBV eradicated) compared to that of HCC patients with a current HBV infection.50 A retrospective analysis of 756 HBV-associated HCC patients at the Beijing Ditan Hospital, China, demonstrated reduced mortality in HCC patients with low HBV-DNA levels after antiviral therapy.51
HCV
A retrospective analysis of overall survival outcomes in 1389 HCV-related HCC patients revealed that HCV eradication after HCC development was associated with improved survival.52 Another retrospective analysis of HCC patients concludes that HCV elimination substantially improved OS in HCC patients with active HCV infection, and patients who did not receive antiviral therapy had a poorer prognosis.53 Studies addressing the relationship between DAA-induced eradication and risk of HCC recurrence have produced contradictory data.54 However, a recent review by Muzica et al55 concludes that most recent and relevant articles suggest a reduced incidence rate of both de novo and recurrent HCC in patients with chronic hepatitis C, HCV-related cirrhosis, and HCV-related HCC, after achieving sustained viral response with direct-acting antiviral therapy.
HHV-8
Antiviral therapy is not included in the very recent S1 guidelines for the Kaposi sarcoma.56
HTLV1
A combination of two antiviral agents: zidovudine (AZT) and interferon-alpha (IFNα) is a standard treatment in adult T-cell lymphoma/leukemia (ATL). It induces a high rate of response and significantly improves survival in the “leukemic-chronic” and “smoldering” subtypes of ATL.57,58 The smoldering and the “favorable” chronic subtypes are regarded as indolent ATL subtypes.57
EBV
In a meta-analysis of clinical studies investigating the effect of EBV status on the clinical outcome in patients with classical Hodgkin’s lymphoma (cHL), almost all studies of older adults or elderly patients with cHL report a deleterious effect of an EBV- positive status on prognosis. In younger adults, there is either a moderate beneficial effect or no effect of EBV status on prognosis. The results of adolescents and children studies conflict. However, the largest study (N = 842) of children and adolescents reports a statistically significant shorter OS of EBV-positive patients compared to EBV- negative patients.59 In this large study, LMP1 positivity was associated with poorer OS (RR = 3.08).60 In a pooled analysis of 26 studies, EBV-positive nasopharyngeal carcinoma patients demonstrated worse OS and progression-free survival, compared to EBV-negative patients.61
MCPyV
A recent meta-analysis of 14 studies reveals a significant correlation between MCPyV positivity and improved OS of Merkel cell carcinoma patients.62
Which Viruses Should Be Eradicated and Which Should Be Left Untouched?
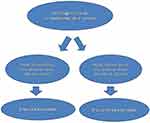
Two mechanisms are utilized by viruses to exert their direct tumorigenic effect: (a) an infliction of DNA damage and carcinogenic mutations in the host cell genome following the induction of chronic inflammation (b) an upregulation of pro-cancer genes or downregulation of anti-cancer genes following integration of the viral genome into the host cell chromosomes or following persistency of a latent form of the viral genome in an infected cell.63 As a counter-reaction, an anti-viral immunity is triggered. Viruses (and intracellular bacteria) induce an MHC-I-mediated CD8+T activation. By releasing perforin, granzyme, granulysin and supramolecular attack particles, these activated CD8 cells destroy the host cells as a means of virus elimination.64,65 Since the host is a cancer cell, the result is an anti-cancer effect. When this anti-cancer response is intensive enough, it may overcome the direct pro-tumor effects of the virus and improve prognosis. However, if this response is weak, the pro-tumor effects of the pathogen will prevail and exacerbate prognosis. Based on these arguments, the present work proposes a division of oncoviruses into two classes: viruses that induce strong anti-viral CD8+ T cell reaction and viruses that induce weak anti-viral CD8+ T cell reaction. In established solid tumors, viruses that induce strong cytotoxic reaction will prolong survival and should therefore be left untouched, while viruses that induce weak cytotoxic reaction will shorten survival and should better be eradicated. Figure 1 depicts a schematic representation of the model. Figure 2 presents a decision tree with respect to the eradication of viruses (and intracellular bacteria) in established solid tumors.
 |
Figure 1 A schematic presentation of the binary model for CD8+ T cell response to oncoviruses (and intracellular carcinogenic bacteria). |
Table 1 presents, side by side, the binary classification of known oncoviruses, the eradication recommendations based on the binary model, and the effect of each virus on patients’ prognosis, as reported in the literature.
 |
Table 1 Binary Classification of CD8+T Cell Reaction to Viral Infection of Benign Tissues, Eradication Recommendations, and Clinical Evidence Regarding the Effect of the Virus on Patients’ Prognosis |
Discussion
Cytotoxic CD8+ T cells employ multiple mechanisms to eliminate viruses and other intracellular pathogens. These mechanisms include the secretion of IFNγ and TNFα, as well as the utilization of pathways mediated by perforin, FasL, and the TNF-related apoptosis-inducing ligand (TRAIL).66 Ultimately, these modalities lead to the elimination of the host cell. If the host cell is a cancer cell, this results in an anti-tumor effect. However, in the case of dysfunctional CD8+ T cells, this anti-tumor effect is impaired.
An earlier publication introduced a binary criterion for selecting drugs for the treatment of severe COVID-19 infection.67 The study demonstrated that drugs suppressing CD8+ T cell activity are ineffective in treating the severe state of the disease. Additionally, Kumagai et al68 proposed a binary classification of CD8+ T cell responses in the tumor microenvironment (TME) of non-small cell lung cancer (NSCLC) and gastric cancer to assess survival outcomes following immunotherapy. These authors illustrated that in patients treated with a monoclonal antibody to PD-1, a high frequency (>57%) of PD-1+ CD8+ T cells in the TME is linked to improved survival compared to a low frequency (<57%). Several studies suggest that elevated expression of inhibitory checkpoint molecules in CD8+ T cells is associated with their exhaustion.15,69–71 The cytotoxic activity in the TME is evidently associated with the frequency of non-exhausted CD8+ T cells. Therefore, Kumagai et al’s study proposes classifying cancer patients into two groups based on CD8+ T cell activity: those with robust activity and those with diminished activity. Patients with diminished activity, characterized by a high frequency of exhausted CD8+ T cells in the TME, exhibit a more favorable prognosis following immunotherapy. This result is likely attributed to the reactivation of these exhausted cells by PD-1 antibodies.
The current study suggests a binary classification of CD8+ T cell response to an oncovirus as either high or low. This classification aims to evaluate whether the virus should be eradicated in established solid tumors. This study assumes that, in most cases, viruses elicit a similar CD8+ T cell reaction regardless of whether the infected cell is benign or malignant. Based on this presumption, it becomes possible to classify viruses as “cytotoxic” or “non-cytotoxic” when infecting healthy tissues, allowing for the prediction of their effect on cancer cells. As shown in Table 1, the prediction is accurate for six out of the seven known oncoviruses. Epstein–Barr virus is an exception. The immune response to EBV may vary, depending on the host’s pathological state.72 While EBV elicits a strong CD8+ T cell reaction in patients with mononucleosis,19,42,43 a reduced frequency and impaired CD8+ T cell response is reported in diffuse large B cell lymphoma (DLBCL).73 In nasopharyngeal cancer, CD8+ T cell activities are suppressed by cancer-derived miR-21 and the generation of suppressive B cells.74 A recent review by Zheng et al75 explores CD8+T cell exhaustion in various EBV-associated malignancies. The binary model fails to predict the effect of EBV on cancer prognosis due to the plasticity of the immune response against EBV. In addition, as will be discussed later in this chapter, Tregs rather than CD8+ T cells display anti-cancer activity in HL. This renders Hodgkin lymphoma an inappropriate candidate for the application of the present binary model.
The probability of correct predictions for all 6 out of 6 pairs by arbitrary chance (assuming events are independent) is very small (P-value = 0.56 = 0.0156), indicating a strong predictive power of 0.984 when the immune response is robust (ie, when the immune response is insensitive to the pathological condition of the patient). The strong correlation observed between the model’s prediction and clinical practice suggests that the impact of immune modulation by oncoviruses on prognosis outweighs the direct (pro-tumor) effects of the virus on host cells (for most viruses). Moreover, it seems that highly activated CD8+ T cells, observed during a persistent infection of non-cancerous tissue, retain their anti-cancer (and anti-viral) activity within the TME of infected cancer cells, although the TME is considered a highly suppressive environment in advanced cancers.
Indeed, the binary classification approach could potentially be extended to encompass any intracellular pathogen, regardless of its oncogenic nature. The current study puts forth the hypothesis that any intracellular pathogen capable of eliciting a robust CD8+ T cell response might induce an anti-cancer effect in solid tumors. For instance, the BCG vaccine, derived from a strain of attenuated live bovine tuberculosis bacillus called Mycobacterium bovis, has been widely utilized as the standard treatment for patients diagnosed with non-muscle-invasive bladder cancer since 1977.76,77 Notably, BCG vaccination stimulates an augmented CD8+ T cell response,76,78 and both M. tuberculosis and M. bovis are intracellular bacteria.79,80
It is not advisable to apply the binary model to extracellular pathogens, since CD8+ T cells are specifically designed to eliminate intracellular pathogens.81 A direct assault of CD8+ T cells on an extracellular pathogen will not necessarily lead to the destruction of host cells, as they do not harbor the pathogen. In fact, the main immune reaction against extracellular pathogens is antibacterial antibodies, which usually do not penetrate into the cytoplasm.82 The immune response to Helicobacter pylori (H. pylori) is an example.
Ninety percent of noncardia gastric cancer and 86% of non-Hodgkin lymphoma (NHL) of gastric location are attributable to H. pylori infection.1 H. pylori, can primarily be considered an extracellular bacterium, although in H. pylori infection, 1–5% of epithelial cells appear to harbor an intracellular population of the bacterium.83 As other extracellular oncopathogens, H. pylori is an indirect carcinogenic pathogen.1 The effector protein CagA, which is part of the cag (cytotoxin-associated gene) pathogenicity island (PAI), the secreted toxin vacuolating cytotoxin A (VacA), the blood-group antigen-binding adhesin (BabA), and H. pylori neutrophil-activating protein (HP-NAP) are four of the most important pathogenicity factors of H. pylori.84 H. pylori DNA vaccines encoding fragments of CagA, VacA, and BabA can induce Th1 shift to Th2 response in immunized BALB/c mice.85 On the other hand, HP-NAP-activated dendritic cells present a Th1 cytokine secretion profile, with high IL-12 and relatively low IL-10 secretion.86 Cytotoxic T cell reaction is attenuated in H. pylori persistent infection: In rodents, CD4 T cells are the main cellular infiltrate in chronic gastritis. Cytotoxic CD8+ T cell response to H. pylori infection is scarcely detected in rodents due to the suppression of CD8+ T cell proliferation by CD4 T cells.87 A recent report suggests that CagA-specific gastric CD8+ tissue-resident T cells restrict H. pylori activity during the early phase of infection in mice and humans, however, this phenotype is lost following prolonged gastritis, and is replaced by CD4 T cells.88 In line with this observation, it was found that persistent H. pylori infection suppresses virus-specific CD8+ T cell response.89 Therefore, the anti-cancer response of the adaptive system observed in H. pylori infected cancer cells, is probably related to Th1 cytokines. As will be explained later, the TME of indolent lymphoma is characterized by low Treg levels, which allow an effective Th1 anti-cancer response by H. pylori. In contrast, the immune response within TME of most solid cancers is suppressed by high Treg levels, which inhibit the Th1 related anti-cancer effect of this bacterium. In agreement, a meta-analysis of 59 published studies concludes that non-cardia gastric cancer patients with H. pylori have a better OS and a better disease-free survival than patients that do not harbor H. pylori.90 On the other hand, H. pylori eradication therapy is recommended as a first-line treatment in all gastric MALT lymphomas regardless of their clinical stage.91 Taken together: (a) the anti-tumor reaction associated with H. pylori is not driven by CD8+ T cell immunity (b) in contrast to (most) oncoviruses, this antitumor activity is sensitive to the tumor microenvironment. It may be speculated that a robust immune response to an extracellular pathogen during a persistent infection of a benign tissue will not necessarily result in an anti-tumor effect in cancerous tissue infected by the same pathogen. On the other hand, as demonstrated above, a strong CD8+ T cell response to an intracellular pathogen during a persistent infection of a benign tissue evidently persists in infected cancerous tissue, and favors prognosis.
The growth of solid tumors is regulated by the cytotoxic activity of CD8+ T cells.92 In contrast, the proliferation of various lymphomas (DLBCL for example) is controlled by a specific subset of regulatory T cells (Tregs).93 Correspondingly, a meta-analysis involving 14 studies on patients with DLBCL demonstrated a correlation between increased expression of FOXP3+Tregs and an improved survival (when assessed by the number or percentage of FOXP3 positive T cells).94
Additionally, it has been proposed that low levels of Tregs promote indolent lymphoma growth, whereas high Tregs levels exert control over it. Certain lymphomas such as follicular lymphoma (FL) and Hodgkin’s lymphoma (HL) are characterized by a prolonged indolent period lasting several years. During this period, the tumor is sensitive to the suppressive effect of TGFβ secreted by Tregs.95 Indeed, higher infiltration of Tregs was associated with more favorable prognosis in FL,96 and in most studies on HL, Tregs’ infiltration into the TME correlates with prolong survival,97 while a greater abundance of activated (GrB+ TIA1+) CD8+ T cells was linked to poorer survival.98 These findings support the notion that Tregs play a suppressive role in the progression of these lymphomas. A word of caution is warranted: a positive impact of tumor-infiltrating Tregs on prognosis does not necessarily indicate a beneficial effect solely attributable of Treg cells. In certain types of cancer, the infiltration of Tregs into the TME coincides with the infiltration of CD8+T cells, which are responsible for inducing the observed anti-cancer effect.99 However, this scenario is unlikely in the case of lymphoma: in several types of lymphoma, CD8+ T cells are dysfunctional. For example, in B-cell non-Hodgkin’s lymphoma, Tregs suppress the activity of CD8+ T cells.100 This suppressive effect is mediated by soluble and membrane-bound TGFβ101 and upregulated TIM-3 expression.69 In follicular lymphoma study, the majority of CD8+ T effector memory cells were found to express TIGIT (T-cell immunoglobulin and ITIM domain), a co-inhibitory receptor, resulting in dysfunctional TCR signaling in these CD8+ T cells.70 Additionally, the expression of LAG-3 by PD-1+ CD8 T cells has been identified as another mechanism leading to CD8 cell exhaustion in follicular lymphoma.71 In contrast, a recent publication showed the absence of many hallmarks of exhaustion in PD-1+, TIM-3+, and CTLA-4+ CD8+ T cells in DLBCL.102 However, it is important to note that PD-1+ and TIM3+ CD8 T cells constitute less than 10% of CD8+ T cell population in the TME of DLBCL.103 Thus in DLBCL, FL and HL, Tregs exert an anti-tumor effect that leads to an improved survival, while CD8+ T cells experience exhaustion within the TME of these lymphomas. Consequently, the binary classification of CD8 cytotoxic response cannot be applied to these lymphomas.
Contrary to DLBCL, FL and HL, adult T cell leukemia/lymphoma (ATLL) is not controlled by Tregs. In fact, this cancer originates from CD4+T cells with Treg phenotypes.104 For this reason, the binary model may apply to HTLV1-associated ATLL.
Clinical implications of these results: relying on the host’s immunological response to an intracellular oncopathogen (evaluated in animals or humans), the binary model can accurately forecast the pathogen’s impact on survival, particularly when data are scarce. This is applicable, for instance, in the case of a rare pathogen linked to cancer or a newly discovered pathogen associated with indolent cancer. In the first scenario, the limited population size may hinder statistical assessment of survival, while in the second scenario, survival studies may extend over many years.
Summary
This study introduces a binary classification of CD8+ T cell cytotoxic reactions to intracellular oncopathogens in non-cancerous tissue as either weak or strong. The objective is to evaluate the impact of these pathogens on the prognosis of solid cancers. The model accurately predicts the effect of six out of seven known oncoviruses on prognosis. However, it is not applicable to extracellular pathogens, certain lymphomas, and pathogens that elicit a host-sensitive immune response. The strong correlation between the model’s predictions and clinical outcomes suggests that immune modulation by oncoviruses has a more significant impact on prognosis than the direct effects of the virus on host cells. Moreover, the observed CD8+ T cell response to oncoviruses in infected benign tissues is retained in the infected TME, indicating that a strong response is not suppressed in the TME. These findings hold clinical significance: based on the host’s immunological response to an intracellular pathogen (evaluated in animals or humans), the binary model can reliably predict the effect of the pathogen on survival when such data are unavailable.
Acknowledgment
The views and opinions expressed, and/or conclusions drawn, in this article are those of the author and do not necessarily reflect those of Taro Pharmaceutical Industries Ltd., its affiliates, directors or employees.
Disclosure
Zeev Elkoshi is employed by Taro Pharmaceutical Industries Ltd.
References
1. Vandeven N, Nghiem P. Pathogen-driven cancers and emerging immune therapeutic strategies. Cancer Immunol Res. 2014;2(1):9–14. doi:10.1158/2326-6066.CIR-13-0179
2. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Biological agents. IARC Monogr Eval Carcinog Risks Hum. 2012;100(Pt B):1–441.
3. Ablashi DV, Chatlynne LG, Whitman JE, Cesarman E. Spectrum of Kaposi’s sarcoma-associated herpesvirus, or human herpesvirus 8, diseases. Clin Microbiol Rev. 2002;15(3):439–464. doi:10.1128/CMR.15.3.439-464.2002
4. Kuwamoto S, Higaki H, Kanai K, et al. Association of Merkel cell polyomavirus infection with morphologic differences in Merkel cell carcinoma. Hum Pathol. 2011;42(5):632–640. doi:10.1016/j.humpath.2010.09.011
5. Moore PS, Chang Y. Why do viruses cause cancer? Highlights of the first century of human tumour virology. Nat Rev Cancer. 2010;10(12):878–889. doi:10.1038/nrc2961
6. Koyama S, Ishii KJ, Coban C, Akira S. Innate immune response to viral infection. Cytokine. 2008;43(3):336–341. doi:10.1016/j.cyto.2008.07.009
7. Hervas-Stubbs S, Perez-Gracia JL, Rouzaut A, Sanmamed MF, Le Bon A, Melero I. Direct effects of type I interferons on cells of the immune system. Clin Cancer Res. 2011;17(9):2619–2627. doi:10.1158/1078-0432.CCR-10-1114
8. Castro F, Cardoso AP, Gonçalves RM, Serre K, Oliveira MJ. Interferon-Gamma at the Crossroads of Tumor Immune Surveillance or Evasion. Front Immunol. 2018;9:847. doi:10.3389/fimmu.2018.00847
9. Soliman SHA, Orlacchio A, Verginelli F. Viral Manipulation of the Host Epigenome as a Driver of Virus-Induced Oncogenesis. Microorganisms. 2021;9(6):1179. doi:10.3390/microorganisms9061179
10. Song S, Gong S, Singh P, Lyu J, Bai Y. The interaction between mitochondria and oncoviruses. Biochim Biophys Acta Mol Basis Dis. 2018;1864(2):481–487. doi:10.1016/j.bbadis.2017.09.023
11. Kori M, Arga KY. Pathways involved in viral oncogenesis: new perspectives from virus-host protein interactomics. Biochim Biophys Acta Mol Basis Dis. 2020;1866(10):165885. doi:10.1016/j.bbadis.2020.165885
12. Çuburu N, Graham BS, Buck CB, et al. Intravaginal immunization with HPV vectors induces tissue-resident CD8+ T cell responses. J Clin Invest. 2012;122(12):4606–4620. doi:10.1172/JCI63287
13. Abbott RJ, Quinn LL, Leese AM, Scholes HM, Pachnio A, Rickinson AB. CD8+ T cell responses to lytic EBV infection: late antigen specificities as subdominant components of the total response. J Immunol. 2013;191(11):5398–5409. doi:10.4049/jimmunol.1301629
14. Boni C, Fisicaro P, Valdatta C, et al. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J Virol. 2007;81(8):4215–4225. doi:10.1128/JVI.02844-06
15. Wieland D, Kemming J, Schuch A, et al. TCF1+ hepatitis C virus-specific CD8+ T cells are maintained after cessation of chronic antigen stimulation. Nat Commun. 2017;8:15050. doi:10.1038/ncomms15050
16. Lambert M, Gannagé M, Karras A, et al. Differences in the frequency and function of HHV8-specific CD8 T cells between asymptomatic HHV8 infection and Kaposi sarcoma. Blood. 2006;108(12):3871–3880. doi:10.1182/blood-2006-03-014225
17. Wölfl M, Kuball J, Eyrich M, Schlegel PG, Greenberg PD. Use of CD137 to study the full repertoire of CD8+ T cells without the need to know epitope specificities. Cytometry A. 2008;73(11):1043–1049. doi:10.1002/cyto.a.20594
18. Kozako T, Yoshimitsu M, Fujiwara H, et al. PD-1/PD-L1 expression in human T-cell leukemia virus type 1 carriers and adult T-cell leukemia/lymphoma patients. Leukemia. 2009;23(2):375–382. doi:10.1038/leu.2008.272
19. Chatterjee B, Deng Y, Holler A, et al. CD8+ T cells retain protective functions despite sustained inhibitory receptor expression during Epstein-Barr virus infection in vivo. PLoS Pathog. 2019;15(5):e1007748. doi:10.1371/journal.ppat.1007748
20. Clements DM, Crumley B, Chew GM, et al. Phenotypic and Functional Analyses Guiding Combination Immune Checkpoint Immunotherapeutic Strategies in HTLV-1 Infection. Front Immunol. 2021;12:608890. doi:10.3389/fimmu.2021.608890
21. Ma SD, Xu X, Jones R, et al. PD-1/CTLA-4 Blockade Inhibits Epstein-Barr Virus-Induced Lymphoma Growth in a Cord Blood Humanized-Mouse Model. PLoS Pathog. 2016;12(5):e1005642. doi:10.1371/journal.ppat.1005642
22. Blair T, Baird J, Bambina S, et al. ICOS is upregulated on T cells following radiation and agonism combined with radiation results in enhanced tumor control. Sci Rep. 2022;12(1):14954. doi:10.1038/s41598-022-19256-8
23. Derré L, Rivals JP, Jandus C, et al. BTLA mediates inhibition of human tumor-specific CD8+ T cells that can be partially reversed by vaccination. J Clin Invest. 2010;120(1):157–167. doi:10.1172/JCI40070
24. Ganusov VV, Lukacher AE, Byers AM. Persistence of viral infection despite similar killing efficacy of antiviral CD8(+) T cells during acute and chronic phases of infection. Virology. 2010;405(1):193–200. doi:10.1016/j.virol.2010.05.029
25. Hibma MH. The immune response to papillomavirus during infection persistence and regression. Open Virol J. 2012;6:241–248. doi:10.2174/1874357901206010241
26. Cheng D, Qiu K, Rao Y, et al. Proliferative exhausted CD8+ T cells exacerbate long-lasting anti-tumor effects in human papillomavirus-positive head and neck squamous cell carcinoma. Elife. 2023;12:e82705. doi:10.7554/eLife.82705
27. Krishna S, Ulrich P, Wilson E, et al. Human Papilloma Virus Specific Immunogenicity and Dysfunction of CD8+ T Cells in Head and Neck Cancer. Cancer Res. 2018;78(21):6159–6170. doi:10.1158/0008-5472.CAN-18-0163
28. Park JJ, Wong DK, Wahed AS, et al. Hepatitis B Research Network. Hepatitis B Virus--Specific and Global T-Cell Dysfunction in Chronic Hepatitis B. Gastroenterology. 2016;150(3):684–695.e5. doi:10.1053/j.gastro.2015.11.050
29. Yang F, Yu X, Zhou C, et al. Hepatitis B e antigen induces the expansion of monocytic myeloid-derived suppressor cells to dampen T-cell function in chronic hepatitis B virus infection. PLoS Pathog. 2019;15(4):e1007690. doi:10.1371/journal.ppat.1007690
30. Hoogeveen RC, Robidoux MP, Schwarz T, et al. Phenotype and function of HBV-specific T cells is determined by the targeted epitope in addition to the stage of infection. Gut. 2019;68(5):893–904. doi:10.1136/gutjnl-2018-316644
31. Baudi I, Kawashima K, Isogawa M. HBV-Specific CD8+ T-Cell Tolerance in the Liver. Front Immunol. 2021;12:721975. doi:10.3389/fimmu.2021.721975
32. Hofmann M, Tauber C, Hensel N, Thimme R. CD8+ T Cell Responses during HCV Infection and HCC. J Clin Med. 2021;10(5):991. doi:10.3390/jcm10050991
33. Barili V, Fisicaro P, Montanini B, et al. Targeting p53 and histone methyltransferases restores exhausted CD8+ T cells in HCV infection. Nat Commun. 2020;11(1):604. doi:10.1038/s41467-019-14137-7
34. Hensel N, Gu Z, Sagar W, et al. Memory-like HCV-specific CD8+ T cells retain a molecular scar after cure of chronic HCV infection. Nat Immunol. 2021;22(2):229–239. doi:10.1038/s41590-020-00817-w
35. Lepone L, Rappocciolo G, Knowlton E, et al. Monofunctional and polyfunctional CD8+ T cell responses to human herpesvirus 8 lytic and latency proteins. Clin Vaccine Immunol. 2010;17(10):1507–1516. doi:10.1128/CVI.00189-10
36. Ishido S, Wang C, Lee BS, Cohen GB, Jung JU. Downregulation of major histocompatibility complex class I molecules by Kaposi’s sarcoma-associated herpesvirus K3 and K5 proteins. J Virol. 2000;74(11):5300–5309. doi:10.1128/jvi.74.11.5300-5309.2000
37. Coscoy L, Ganem D. Kaposi’s sarcoma-associated herpesvirus encodes two proteins that block cell surface display of MHC class I chains by enhancing their endocytosis. Proc Natl Acad Sci U S A. 2000;97(14):8051–8056. doi:10.1073/pnas.140129797
38. Guihot A, Dupin N, Marcelin AG, et al. Low T cell responses to human herpesvirus 8 in patients with AIDS-related and classic Kaposi sarcoma. J Infect Dis. 2006;194(8):1078–1088. doi:10.1086/507648
39. Lidenge SJ, Tso FY, Ngalamika O, et al. Lack of CD8+ T-cell co-localization with Kaposi’s sarcoma-associated herpesvirus infected cells in Kaposi’s sarcoma tumors. Oncotarget. 2020;11(17):1556–1572. doi:10.18632/oncotarget.27569
40. Espíndola OM, Siteur-van Rijnstra E, Frankin E, et al. Early Effects of HTLV-1 Infection on the Activation, Exhaustion, and Differentiation of T-Cells in Humanized NSG Mice. Cells. 2021;10(10):2514. doi:10.3390/cells10102514
41. Hanon E, Stinchcombe JC, Saito M, et al. Fratricide among CD8(+) T lymphocytes naturally infected with human T cell lymphotropic virus type I. Immunity. 2000;13(5):657–664. doi:10.1016/s1074-7613(00)00065-0
42. Maini MK, Gudgeon N, Wedderburn LR, Rickinson AB, Beverley PC. Clonal expansions in acute EBV infection are detectable in the CD8 and not the CD4 subset and persist with a variable CD45 phenotype. J Immunol. 2000;165(10):5729–5737. doi:10.4049/jimmunol.165.10.5729
43. Callan MF, Tan L, Annels N, et al. Direct visualization of antigen-specific CD8+ T cells during the primary immune response to Epstein-Barr virus In vivo. J Exp Med. 1998;187(9):1395–1402. doi:10.1084/jem.187.9.1395
44. Imperiale MJ, Jiang M. Polyomavirus Persistence. Annu Rev Virol. 2016;3(1):517–532. doi:10.1146/annurev-virology-110615-042226
45. Wilson JJ, Pack CD, Lin E, et al. CD8 T cells recruited early in mouse polyomavirus infection undergo exhaustion. J Immunol. 2012;188(9):4340–4348. doi:10.4049/jimmunol.1103727
46. Vezys V, Masopust D, Kemball CC, et al. Continuous recruitment of naive T cells contributes to heterogeneity of antiviral CD8 T cells during persistent infection. J Exp Med. 2006;203(10):2263–2269. doi:10.1084/jem.20060995
47. Lei J, Arroyo-Mühr LS, Lagheden C, et al. Human Papillomavirus Infection Determines Prognosis in Cervical Cancer. J Clin Oncol. 2022;40(14):1522–1528. doi:10.1200/JCO.21.01930
48. Zhou P, Yu YF, Lian CL, Wang J, Zhuo RG, Wu SG. Survival Outcomes and Treatment Decision by Human Papillomavirus Status Among Patients With Stage IVC Head and Neck Squamous Cell Carcinoma. Front Oncol. 2021;11:668066. doi:10.3389/fonc.2021.668066
49. Xue X, Liao W, Xing Y. Comparison of clinical features and outcomes between HBV- related and non-B non-C hepatocellular carcinoma. Infect Agent Cancer. 2020;15:11. doi:10.1186/s13027-020-0273-2
50. Omichi K, Shindoh J, Yamamoto S, et al. Postoperative Outcomes for Patients with Non-B Non-C Hepatocellular Carcinoma: a Subgroup Analysis of Patients with a History of Hepatitis B Infection. Ann Surg Oncol. 2015;22 Suppl 3:S1034. doi:10.1245/s10434-015-4845-0
51. Wang X, Liu X, Wang P, et al. Antiviral Therapy Reduces Mortality in Hepatocellular Carcinoma Patients with Low-Level Hepatitis B Viremia. J Hepatocell Carcinoma. 2021;8:1253–1267. doi:10.2147/JHC.S330301
52. Yeh ML, Liang PC, Tsai PC, et al. Characteristics and Survival Outcomes of Hepatocellular Carcinoma Developed after HCV SVR. Cancers (Basel). 2021;13(14):3455. doi:10.3390/cancers13143455
53. Luo Y, Zhang Y, Wang D, Shen D, Che YQ. Eradication of Hepatitis C Virus (HCV) Improves Survival of Hepatocellular Carcinoma Patients with Active HCV Infection - A Real-World Cohort Study. Cancer Manag Res. 2020;12:5323–5330. doi:10.2147/CMAR.S254580
54. Tagkou NM, Goossens N, Negro F. Impact of direct-acting antivirals on the recurrence of hepatocellular carcinoma in chronic hepatitis C. Hepatoma Res. 2022;8:28. doi:10.20517/2394-5079.2022.08
55. Muzica CM, Stanciu C, Huiban L, et al. Hepatocellular carcinoma after direct-acting antiviral hepatitis C virus therapy: a debate near the end. World J Gastroenterol. 2020;26(43):6770–6781. doi:10.3748/wjg.v26.i43.6770
56. Esser S, Schöfer H, Hoffmann C, et al. S1 Guidelines for the Kaposi Sarcoma. J Dtsch Dermatol Ges. 2022;20(6):892–904. doi:10.1111/ddg.14788
57. El Hajj H, Tsukasaki K, Cheminant M, Bazarbachi A, Watanabe T, Hermine O. Novel Treatments of Adult T Cell Leukemia Lymphoma. Front Microbiol. 2020;11:1062. doi:10.3389/fmicb.2020.01062
58. Bazarbachi A, Plumelle Y, Carlos Ramos J, et al. Meta-analysis on the use of zidovudine and interferon-alfa in adult T-cell leukemia/lymphoma showing improved survival in the leukemic subtypes. J Clin Oncol. 2010;28(27):4177–4183. doi:10.1200/JCO.2010.28.0669
59. Nohtani M, Vrzalikova K, Ibrahim M, et al. Impact of Tumour Epstein--Barr Virus Status on Clinical Outcome in Patients with Classical Hodgkin Lymphoma (cHL): a Review of the Literature and Analysis of a Clinical Trial Cohort of Children with cHL. Cancers (Basel). 2022;14(17):4297. doi:10.3390/cancers14174297
60. Claviez A, Tiemann M, Lüders H, et al. Impact of latent Epstein--Barr virus infection on outcome in children and adolescents with Hodgkin’s lymphoma. J Clin Oncol. 2005;23(18):4048–4056. doi:10.1200/JCO.2005.01.701
61. Alami IE, Gihbid A, Charoute H, et al. Prognostic value of Epstein--Barr virus DNA load in nasopharyngeal carcinoma: a meta-analysis. Pan Afr Med J. 2022;41:6. doi:10.11604/pamj.2022.41.6.28946
62. Yang A, Wijaya WA, Lie Y, et al. The impact of Merkel Cell polyomavirus positivity on prognosis of Merkel cell carcinoma: a systematic review and meta-analysis. Front Oncol. 2022. doi:10.3389/fonc.2022.1020805
63. Hatta MNA, Mohamad Hanif EA, Chin SF, Neoh HM. Pathogens and Carcinogenesis: a Review. Biology (Basel). 2021;10(6):533. doi:10.3390/biology10060533
64. Baral S, Antia R, Dixit NM. A dynamical motif comprising the interactions between antigens and CD8 T cells may underlie the outcomes of viral infections. Proc Natl Acad Sci U S A. 2019;116(35):17393–17398. doi:10.1073/pnas.1902178116
65. Tian L, Zhou W, Wu X, et al. CTLs: killers of intracellular bacteria. Front Cell Infect Microbiol. 2022;12:967679. doi:10.3389/fcimb.2022.967679
66. Schmidt ME, Varga SM. The CD8 T Cell Response to Respiratory Virus Infections. Front Immunol. 2018;9:678. doi:10.3389/fimmu.2018.00678
67. Elkoshi Z. The Binary Model of Chronic Diseases Applied to COVID-19. Front Immunol. 2021;12:716084. doi:10.3389/fimmu.2021.716084
68. Kumagai S, Togashi Y, Kamada T, et al. The PD-1 expression balance between effector and regulatory T cells predicts the clinical efficacy of PD-1 blockade therapies. Nat Immunol. 2020;21(11):1346–1358. doi:10.1038/s41590-020-0769-3
69. Yang -Z-Z, Grote DM, Ziesmer SC, et al. IL-12 upregulates TIM-3 expression and induces T cell exhaustion in patients with follicular B cell non-Hodgkin lymphoma. J Clin Investig. 2012;122:1271–1282. doi:10.1172/JCI59806
70. Josefsson SE, Huse K, Kolstad A, et al. T Cells Expressing Checkpoint Receptor TIGIT Are Enriched in Follicular Lymphoma Tumors and Characterized by Reversible Suppression of T-cell Receptor Signaling. Clin Cancer Res. 2018;24(4):870.
71. Yang -Z-Z, Kim HJ, Villasboas JC, et al. Expression of LAG-3 defines exhaustion of intratumoral PD-1+ T cells and correlates with poor outcome in follicular lymphoma. Oncotarget. 2017;8:61425–61439. doi:10.18632/oncotarget.18251
72. Elkoshi Z. The Binary Classification Of Chronic Diseases. J Inflamm Res. 2019;12:319–333. doi:10.2147/JIR.S227279
73. Cárdenas D, Vélez G, Orfao A, et al. Epstein-Barr virus-specific CD8(+) T lymphocytes from diffuse large B cell lymphoma patients are functionally impaired. Clin Exp Immunol. 2015;182(2):173–183. doi:10.1111/cei.12682
74. Miao BP, Zhang RS, Li M, et al. Nasopharyngeal cancer-derived microRNA-21 promotes immune suppressive B cells. Cell Mol Immunol. 2015;12(6):750–756. doi:10.1038/cmi.2014.129
75. Zheng X, Huang Y, Li K, Luo R, Cai M, Yun J. Immunosuppressive Tumor Microenvironment and Immunotherapy of Epstein-Barr Virus-Associated Malignancies. Viruses. 2022;14(5):1017. doi:10.3390/v14051017
76. Rentsch CA, Birkhäuser FD, Biot C, et al. Bacillus Calmette-Guérin strain differences have an impact on clinical outcome in bladder cancer immunotherapy. Eur Urol. 2014;66(4):677–688. doi:10.1016/j.eururo.2014.02.061
77. Brandau S, Suttmann H. Thirty years of BCG immunotherapy for non-muscle invasive bladder cancer: a success story with room for improvement. Biomed Pharmacother. 2007;61(6):299–305. doi:10.1016/j.biopha.2007.05.004
78. Kumar NP, Padmapriyadarsini C, Rajamanickam A, et al. BCG vaccination induces enhanced frequencies of memory T cells and altered plasma levels of common γc cytokines in elderly individuals. PLoS One. 2021;16(11):e0258743. doi:10.1371/journal.pone.0258743
79. Chai Q, Zhang Y, Liu CH. Mycobacterium tuberculosis: an Adaptable Pathogen Associated With Multiple Human Diseases. Front Cell Infect Microbiol. 2018;8:158. doi:10.3389/fcimb.2018.00158
80. Cross ML, Aldwell FE, Griffin JF, Mackintosh CG. Intracellular survival of virulent Mycobacterium bovis and M. bovis BCG in ferret macrophages. Vet Microbiol. 1999;66(3):235–243. doi:10.1016/s0378-1135(99)00011-5
81. Nolz JC. Molecular mechanisms of CD8(+) T cell trafficking and localization. Cell Mol Life Sci. 2015;72(13):2461–2473. doi:10.1007/s00018-015-1835-0
82. Immunity to Infection. Primer to the Immune Response. 2014:295–332. doi:10.1016/B978-0-12-385245-8.00013-3
83. Oh JD, Karam SM, Gordon JI. Intracellular Helicobacter pylori in gastric epithelial progenitors. Proc Natl Acad Sci U S A. 2005;102(14):5186–5191. doi:10.1073/pnas.0407657102
84. Deng R, Zheng H, Cai H, Li M, Shi Y, Ding S. Effects of helicobacter pylori on tumor microenvironment and immunotherapy responses. Front Immunol. 2022;13:923477. doi:10.3389/fimmu.2022.923477
85. Xue LJ, Mao XB, Liu XB, et al. Activation of CD3+ T cells by Helicobacter pylori DNA vaccines in potential immunotherapy of gastric carcinoma. Cancer Biol Ther. 2019;20(6):866–876. doi:10.1080/15384047.2019.1579957
86. Ramachandran M, Jin C, Yu D, Eriksson F, Essand M. Vector-encoded Helicobacter pylori neutrophil-activating protein promotes maturation of dendritic cells with Th1 polarization and improved migration. J Immunol. 2014;193(5):2287–2296. doi:10.4049/jimmunol.1400339
87. Tan MP, Pedersen J, Zhan Y, et al. CD8+ T cells are associated with severe gastritis in Helicobacter pylori-infected mice in the absence of CD4+ T cells. Infect Immun. 2008;76(3):1289–1297. doi:10.1128/IAI.00779-07
88. Koch MRA, Gong R, Friedrich V, et al. CagA-specific Gastric CD8+ Tissue-Resident T Cells Control Helicobacter pylori During the Early Infection Phase. Gastroenterology. 2023;164(4):550–566. doi:10.1053/j.gastro.2022.12.016
89. Shirai M, Arichi T, Nakazawa T, Berzofsky JA. Persistent infection by Helicobacter pylori down-modulates virus-specific CD8+ cytotoxic T cell response and prolongs viral infection. J Infect Dis. 1998;177(1):72–80. doi:10.1086/513827
90. Jia Z, Zheng M, Jiang J, et al. Positive H. pylori status predicts better prognosis of non-cardiac gastric cancer patients: results from cohort study and meta-analysis. BMC Cancer. 2022;22(1):155. doi:10.1186/s12885-022-09222-y
91. Ruskoné-Fourmestraux A, Fischbach W, Aleman BM, et al.; EGILS group. EGILS consensus report. Gastric extranodal marginal zone B-cell lymphoma of MALT. Gut. 2011;60(6):747–758. doi:10.1136/gut.2010.224949
92. Xie Q, Ding J, Chen Y. Role of CD8+ T lymphocyte cells: interplay with stromal cells in tumor microenvironment. Acta Pharm Sin B. 2021;11(6):1365–1378. doi:10.1016/j.apsb.2021.03.027
93. Wang J, Ke XY. The four types of Tregs in malignant lymphomas. J Hematol Oncol. 2011;4:50. doi:10.1186/1756-8722-4-50
94. Bai Y, He T, Zhang L, et al. Prognostic value of FOXP3+ regulatory T cells in patients with diffuse large B-et al. lymphoma: a systematic review and meta-analysis. BMJ Open. 2022;12(9):e060659. doi:10.1136/bmjopen-2021-060659
95. Elkoshi Z. “High Treg” Inflammations Promote (Most) Non-Hematologic Cancers While “Low Treg” Inflammations Promote Lymphoid Cancers. J Inflamm Res. 2020;13:209–221. doi:10.2147/JIR.S249384
96. Carreras J, Lopez-Guillermo A, Fox BC, et al. High numbers of tumor-infiltrating FOXP3-positive regulatory T cells are associated with improved overall survival in follicular lymphoma. Blood. 2006;108(9):2957–2964. doi:10.1182/blood-2006-04-018218
97. D’Arena G, Vitale C, Coscia M, et al. Regulatory T Cells and Their Prognostic Relevance in Hematologic Malignancies. J Immunol Res. 2017;2017:1832968. doi:10.1155/2017/1832968
98. Menéndez V, Solórzano JL, Fernández S, Montalbán C, García JF. The Hodgkin Lymphoma Immune Microenvironment: turning Bad News into Good. Cancers (Basel). 2022;14(5):1360. doi:10.3390/cancers14051360
99. Elkoshi Z. On the Prognostic Power of Tumor-Infiltrating Lymphocytes - A Critical Commentary. Front Immunol. 2022;13:892543. doi:10.3389/fimmu.2022.892543
100. Yang ZZ, Novak AJ, Ziesmer SC, Witzig TE, Ansell SM. Attenuation of CD8(+) T-cell function by CD4(+)CD25(+) regulatory T cells in B-cell non-Hodgkin’s lymphoma. Cancer Res. 2006;66(20):10145–10152. doi:10.1158/0008-5472.CAN-06-1822
101. Yang ZZ, Grote DM, Ziesmer SC, et al. Soluble and membrane-bound TGF-β-mediated regulation of intratumoral T cell differentiation and function in B-cell non-Hodgkin lymphoma. PLoS One. 2013;8(3):e59456. doi:10.1371/journal.pone.0059456
102. Greenbaum AM, Fromm JR, Gopal AK, Houghton AM. Diffuse large B-cell lymphoma (DLBCL) is infiltrated with activated CD8+ T-cells despite immune checkpoint signaling. Blood Res. 2022;57(2):117–128. doi:10.5045/br.2022.2021145
103. Autio M, Leivonen SK, Brück O, et al. Immune cell constitution in the tumor microenvironment predicts the outcome in diffuse large B-cell lymphoma. Haematologica. 2021;106(3):718–729. doi:10.3324/haematol.2019.243626
104. Takeuchi M, Miyoshi H, Ohshima K. Tumor microenvironment of adult T-cell leukemia/lymphoma. J Clin Exp Hematop. 2021;61(4):202–209. doi:10.3960/jslrt.21007
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.