Back to Journals » International Journal of General Medicine » Volume 16
The Emerging Roles and Mechanisms of PAQR3 in Human Cancer: Pathophysiology and Therapeutic Implications
Authors Guo Q , Liu XL, Zhai K, Chen C, Ke XX, Zhang J, Xu G
Received 23 May 2023
Accepted for publication 13 July 2023
Published 22 September 2023 Volume 2023:16 Pages 4321—4328
DOI https://doi.org/10.2147/IJGM.S422523
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Qiang Guo,1,2,* Xiao-Li Liu,3,* Kui Zhai,4,* Cheng Chen,1 Xi-Xian Ke,1 Jun Zhang,2 Gang Xu1
1Department of Thoracic Surgery, The Second Affiliated Hospital of Zunyi Medical University, Zunyi, Guizhou, People’s Republic of China; 2Department of Cardiothoracic Surgery, Taihe Hospital, Hubei University of Medicine, Shiyan, Hubei, People’s Republic of China; 3Department of Ultrasound, The People’s Hospital of Jianyang City, Jianyang, Sichuan, People’s Republic of China; 4Department of Thoracic Surgery, Xingyi People’s Hospital, Xinyi, Guizhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Gang Xu; Jun Zhang, Email [email protected]; [email protected]
Abstract: Cancer was one of the common causes of death in the world, and it was increasing year by year. At present, Progestin and AdipoQ receptor family member 3 (PAQR3) was widely studied in cancer. It has been found that PAQR3 was down regulated in various cancers, such as the gastric cancer, osteosarcoma, glioma, hepatocellular carcinoma, acute lymphoblastic leukemia, laryngeal squamous cell carcinoma, esophageal cancer, breast cancer, non-small cell lung cancer, and colorectal cancer. The decreased expression of PAQR3 was associated with short overall survival and disease-free survival in patients with gastric cancer, hepatocellular carcinoma, laryngeal squamous cell carcinoma, esophageal cancer, breast cancer, and non-small cell lung cancer. PAQR3 could inhibit cancer progression by using the Ras/Raf/MEK/ERK, PI3/AKT, EMT and other mechanisms, and was negatively regulated by the miR-543, miR-15b-5p and miR-15b. The roles and signaling mechanisms of PAQR3, and the relationship between the expression of PAQR3 and prognosis in cancer progression are reviewed in this article, and provides new tumor marker and idea to guide cancer treatment.
Keywords: PAQR3, overall survival, disease-free survival, tumor suppressor
Introduction
Cancer has a high morbidity and mortality rate worldwide, imposing a significant burden on society.1 Therefore, new cancer treatment methods are required to reduce the negative impact of cancer on society. In recent years, targeted therapy has given cancer patients new hope for a better prognosis.2–6 Progestin and adipoQ receptor (PAQR) family member 3 (PAQR3) gene is located on human chromosome 4 (4q21.21) and belongs to the PAQR family, which consists of 11 members. The PAQR family is divided into three categories, namely I, II, and III, based on their physical characteristics.7–9 PAQR3 encodes a seven-transmembrane protein that is found on the Golgi apparatus of mammalian cells. It is also known as Raf kinase trapping to Golgi and has recently been identified as a tumor suppressor. PAQR3 plays a crucial role in controlling proliferation, ERK phosphorylation, and metastasis, thereby inhibiting cancer development.10–14 For example, PAQR3 suppresses tumor occurrence and development, as well as inhibits the proliferation and transformation of human malignant melanoma A375 cells.10 In addition, PAQR3 expression is significantly decreased in colorectal cancer (CRC) tissues. Overexpression of PAQR3 has demonstrated an impact on the growth and migration abilities of colorectal cancer (CRC). This implies that PAQR3 could serve as a potential target for tumor treatment by promoting ERK phosphorylation and facilitating the accumulation of β-catenin in the nucleus. Conversely, the knockout of PAQR3 enhances the activity of β-catenin,11 indicating that PAQR3 could be a new target for tumor treatment. Therefore, this review aims to offer recommendations for the clinical investigation of novel tumor markers and approaches to cancer treatment.
PAQR3 Structural Features
The PAQR3 gene is located on human chromosome 4 (4q21.21) and is highly expressed in the brain, testis, and small intestine.15 PAQR3 has seven transmembrane domains, which have low sequence similarity to the seven transmembrane structures of G protein-coupled receptors, which are considered paralogs.15 In addition, Luo et al found that PAQR3 is a type III membrane protein with its N-terminus facing the cytoplasm.16 The second, fourth, and sixth rings of PAQR3 were thought to face the same direction as the N-terminus, while the C-terminus faces the Golgi apparatus. The first ring is required for the positioning of PAQR3 on the Golgi apparatus, while the third ring regulates it and interacts with Raf-1 to perform its function.
PAQR3 Affects the Biological Functions
The Roles of PAQR3 in Tumorigenesis and Development
The Roles of PAQR3 in Cancer Cell Growth
Numerous studies have shown that cell proliferation and apoptosis play important roles in tumor cell growth and were closely related to cell cycle regulation.17 Tumors develop as a result of abnormal cell cycle regulation.17,18 Therefore, understanding tumor growth mechanisms and correcting abnormal cycle regulation may aid in the treatment of tumorigenesis. Recent studies have found that PAQR3 plays an important role by inhibiting cancer cell proliferation, colony formation and inducing cell apoptosis.10,12,14,19–27 For example, Li et al found that overexpression of PAQR3 inhibits the proliferation of non-small cell lung cancer (NSCLC) cells, induces cell apoptosis, and promotes cell cycle arrest in the G0/G1 phase. In contrast, interference with PAQR3 gene expression promotes NSCLC cell proliferation while inhibiting apoptosis. PAQR3 may act as a tumor suppressor in NSCLC cells.19–21 In addition, Tang et al found that increasing PAQR3 expression inhibits the proliferation of glioma cells in vitro and attenuates the growth of nude mouse tumor xenogeneic models in vivo.14 These results demonstrate that PAQR3 functions as a tumor suppressor gene in various types of cancer cells. (Table 1).
 |
Table 1 The Role of PAQR3 in Tumor Growth |
The Roles of PAQR3 in the Metastasis of Malignant Tumors
Metastasis is the most important step in tumor progression. Metastasis of early-stage cancer via lymph nodes or blood pathways may result in a poor long-term prognosis for cancer patients, thereby increasing the economic burden on people all over the world.35–39 The rate of early metastasis in cancer patients has a significant impact on their treatment and prognosis. Numerous studies have confirmed that PAQR3 inhibits tumor metastasis and may improve the long-term prognosis of cancer patients (Table 2). This indicates that PAQR3 overexpression is closely related to the invasion and metastasis of various cancers, including GC, glioma, and prostate cancer.
 |
Table 2 The Role of PAQR3 in Tumor Metastasis |
PAQR3 is Involved in Cancer-Related Mechanisms
Ras/Raf/MEK/ERK Signaling Pathway
The Ras/Raf/MEK/ERK signaling pathway is related to the cell proliferation, apoptosis, differentiation, and the cell cycle,44 and its dysregulation is associated with the progression of various cancers, including CRC, GC, and skin cancer.11,12,27 PAQR3 binds to Raf-1 and anchors it to the Golgi apparatus, thereby inhibiting downstream signaling.10,45 In vitro studies confirmed that deletion of the PAQR3 gene induces ERK phosphorylation and proliferation of epidermal keratinocytes, indicating that PAQR3 acts as a tumor suppressor in skin cancer cells.27 PAQR3 overexpression inhibits ERK phosphorylation in melanoma A375 cells, colon cancer SW-480 cells, osteosarcoma MG-63 cells, laryngeal cancer Hep2 cells, prostate cancer PC3 and DU145 cells, as well as esophageal cancer EC9706, ECA-109 and TE-1 cells.10,11,13,24,25,29–31 Among them, PAQR3 gene silencing in prostate cancer cells increases serum-induced ERK phosphorylation.25 U0126, a specific MEK inhibitor, has been shown in osteosarcoma, laryngeal cancer, colon cancer, and EC cells to reverse the cell proliferation effects of PAQR3 silencing.
PI3K/AKT Signaling Pathway
The PI3K/AKT signaling pathway is one of the most common pathways involved in tumor growth and metastasis. PAQR3 inhibits AKT signaling activation by inhibiting the signal transduction of the G protein Gβγ subunit and the spatial regulation of the PI3K p110a subunit.12,46,47 Knocking out the PAQR3 gene in mouse embryonic fibroblasts (MEFs) under physiological conditions has been found to increase lysophosphatidic acid (LPA)-induced AKT phosphorylation and substrate glycogen synthase kinase 3 beta (GSK3β) phosphorylation, resulting in the increased expression of tumor suppressor p53 and its downstream targets p21 and p16.48 In addition, the upregulation of PAQR3 gene expression in prostate cancer cells inhibits AKT phosphorylation and epithelial-mesenchymal transition (EMT) characteristics. On the contrary, knocking out the PAQR3 gene has been found to increase serum-induced AKT phosphorylation and EMT characteristics.25 Overexpression of PAQR3 in glioma cells inhibits the phosphorylation of PI3K and AKT. Wortmannin, an AKT inhibitor, potentiates the inhibitory effect of PAQR3 on glioma cell proliferation and invasion. The AKT activator SC79 mitigates the inhibitory effect of PAQR3 on glioma cell proliferation and invasion.14
EMT Process
EMT process is an important biological process that allows cells derived from epithelial cells to acquire migration and invasion capabilities. It is critical to understand the molecular mechanism that regulates the EMT process of malignant tumor cells.39 Zhao et al found that miR-15b-5p promotes the migration, invasion, and EMT process in gastric cancer (GC) cells, whereas increasing PAQR3 expression reverses this effect.40,41 Furthermore, Ling et al found that PAQR3 overexpression negatively regulates GC cell migration and EMT.12 Twist1 is also an important transcription factor involved in EMT and tumor metastasis.49,50 Guo et al confirmed that PAQR3 promotes Twist1 degradation and inhibits GC cell metastasis by regulating the EMT process.40 Tang et al found that PAQR3 overexpression inhibits glioma cell migration and invasion and prevents the EMT process.14 Huang et al found that PAQR3 decreases the expression of EMT in prostate cancer PC3 cells and regulates prostate cancer cell invasion and metastasis.25
Autophagy
PAQR3 induces autophagy during starvation via the AMPK and MTORC1 signaling pathways.20 During the growth and proliferation of NSCLC cells, PAQR3 promotes autophagy in cancer cells by EGFR inhibitor (erlotinib), blocking the interaction between BECN1 and the activated form of EGFR, and inhibiting BECN1 tyrosine phosphorylation. This indicates that PAQR3 regulates autophagy via the EGFR and inhibits NSCLC progression.20
Other Signaling Pathways
Abnormal hypermethylation of the CPG island in the promoter region of the tumor suppressor gene could cause its own transcription inactivation, thereby silencing it, which was involved in the occurrence and development of cancer. Lounglaithong et al found that the methylation of the PAQR3 promoter region is increased in prostate cancer. The rate of PAQR3 hypermethylation is associated with nerve infiltration.51 Chen et al found that PAQR3 overexpression or silencing had no effect on HER2 mRNA expression in breast cancer (BC) cells, whereas inhibiting HER2 gene expression increases PAQR3 gene expression in BC cells and inhibits BC cell growth and migration.32,42 The demethylating agent 5-Aza-dC increases PAQR3 gene expression in BC cells while inhibiting clonal formation and invasion.42 Therefore, reducing abnormal methylation of the PAQR3 promoter region may play a direct or indirect role in the development of BC, CRC, and prostate cancer.
In addition, miRNA mainly regulates the expression of target mRNAs by binding to the 3’ untranslated region (UTR), thus playing an important role in human development and various pathological conditions.52,53 Figure 1 shows that miR-543, miR-203a-3p, miR-15b-5p, and miR-15b can inhibit PAQR3 mRNA and protein expression, thereby promoting the proliferation, migration, and invasion of liver cancer, GC, and BC.23,24,33,40,43
 |
Figure 1 The miRNAs-PAQR3 signaling pathway in cancer progression. |
Decreased Expression of PAQR3 is Associated with the Poor Prognosis of Cancer Patients
In general, the expression of PAQR3 is low in pan-cancer tissues, and the levels of PAQR3 expression are associated with the prognosis of EC, HCC, GC, and others (Table 3). Ling et al reported that PAQR3 mRNA and protein expression decreased in GC tissues while the methylation rate increased. PAQR3 expression has been linked to Helicobacter pylori infection, tumor size, tumor stage, vein and lymphatic invasion, distant metastasis, and lymph node metastasis. In GC patients, the rate of PAQR3 methylation has been linked to the depth of tumor invasion, lymph node metastasis, and TNM staging. GC patients with elevated PAQR3 expression were found to have better overall survival (OS) and disease-free survival (DFS).12,24 Wu et al found that the expression of PAQR3 in HCC tissues is significantly decreased and is associated with tumor size, grade, and recurrence. The Kaplan–Meier survival curve revealed a relationship between low PAQR3 expression and poor prognosis in HCC patients. PAQR3 expression is an independent prognostic marker for OS and DFS in HCC patients.22 Liang et al found that the expression of PAQR3 in lung cancer is decreased and is associated with TNM stage, lymph node metastasis, pathological type, and degree of differentiation in lung cancer patients. PAQR3 expression is associated with a poor prognosis.19,21 Bai et al found that the expression of PAQR3 is significantly decreased in esophageal squamous cell carcinoma (ESCC) tissue than in adjacent tissues and is associated with race, tumor size, lymph node metastasis, and local recurrence. Decreased PAQR3 expression has been linked to a poor prognosis and is an independent prognostic indicator in ESCC patients.29 An increase in the methylation rate of the gene promoter region decreases the expression of tumor suppressor genes, resulting in tumor development. Chen et al found that the degree of methylation in the PAQR3 promoter region is increased in BC tissues, and the probability of a poor prognosis in BC patients increases with the rate of methylation.42 These findings indicate that the PAQR3 expression level is closely linked to the prognosis of cancer patients and that increasing PAQR3 expression may improve the prognosis of patients. Therefore, PAQR3 is a potential biomarker for cancer prognosis as well as a target for anti-tumor therapy.
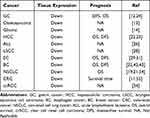 |
Table 3 The Prognostic Value of PAQR3 in Clinical Patients |
Conclusions
PAQR3 has been extensively studied in the past decades, particularly its roles in cancer initiation, progression, metastasis, and clinical outcomes. PAQR3 may act as a tumor suppressor and is related to the poor prognosis for various cancers, such as NSCLC, EC, and HCC, which are associated with hypermethylation of the PAQR3 promoter region and negative regulation of overexpression of miR-543, miR-15b-5p, and miR-15b (Figure 1). PAQR3 can further suppress cancer progression by inhibiting Raf/MEK/ERK, PI3K/AKT, TGF-β/Smad, and autophagy pathways (Figure 2). Although the mechanisms mentioned above have been identified, some questions remain unanswered. Are there, for example, other factors that decrease PAQR3 expression levels? Does PAQR3 play the same tumor suppressor role in cancer resistance? In general, it is essential to conduct comprehensive research on the upstream and downstream mechanisms of PAQR3, as well as its interactions with other molecules. This research holds paramount importance in understanding the role of PAQR3 in cancer, evaluating its correlation with prognosis in cancer patients, and advancing the potential application of PAQR3 in cancer treatment.
 |
Figure 2 Downstream signaling of PAQR3 in cancer progression. |
Abbreviations
PAQR3, progestin and adipoQ receptor family member 3; EMT, epithelial-mesenchymal transition; GC, gastric cancer; HCC, hepatocellular carcinoma; ALL, acute lymphoblastic leukemia; LSCC, laryngeal squamous cell carcinoma; EC, esophageal cancer; BC, breast cancer; NSCLC, non-small cell lung cancer; CRC, colorectal cancer; OS, overall survival; DFS, disease-free survival.
Funding
Not funded.
Disclosure
Qiang Guo, Xiao-Li Liu and Kui Zhai are co-first authors for this study. The authors declare that they have no competing interests in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi:10.3322/caac.21590
2. Ghafoor Q, Baijal S, Taniere P, O’Sullivan B, Evans M, Middleton G. Epidermal Growth Factor Receptor (EGFR) kinase inhibitors and non-small cell lung cancer (NSCLC) - advances in molecular diagnostic techniques to facilitate targeted therapy. Pathol Oncol Res. 2018;24(4):723–731. doi:10.1007/s12253-017-0377-1
3. Hsu PC, Jablons DM, Yang CT, You L. Epidermal Growth Factor Receptor (EGFR) Pathway, Yes-Associated Protein (YAP) and the Regulation of Programmed Death-Ligand 1 (PD-L1) in Non-Small Cell Lung Cancer (NSCLC). Int J Mol Sci. 2019;20(15):3821. doi:10.3390/ijms20153821
4. Zhang Y, Tang M, Guo Q, Xu H, Yang Z, Li D. The value of erlotinib related target molecules in kidney renal cell carcinoma via bioinformatics analysis. Gene. 2022;816:146173. doi:10.1016/j.gene.2021.146173
5. Lee YT, Tan YJ, Oon CE. Molecular targeted therapy: treating cancer with specificity. Eur J Pharmacol. 2018;834:188–196. doi:10.1016/j.ejphar.2018.07.034
6. Bhattacharjee S, Nandi S. Synthetic lethality in DNA repair network: a novel avenue in targeted cancer therapy and combination therapeutics. IUBMB Life. 2017;69(12):929–937. doi:10.1002/iub.1696
7. Villa NY, Moussatche P, Chamberlin SG, Kumar A, Lyons TJ. Phylogenetic and preliminary phenotypic analysis of yeast PAQR receptors: potential antifungal targets. J Mol Evol. 2011;73(3–4):134–152. doi:10.1007/s00239-011-9462-3
8. Huang M, Zhao Z, Cao Q, et al. PAQR3 modulates blood cholesterol level by facilitating interaction between LDLR and PCSK9. Metabolism. 2019;94:88–95. doi:10.1016/j.metabol.2019.02.005
9. Lei L, Ling ZN, Chen XL, Hong LL, Ling ZQ. Characterization of the Golgi scaffold protein PAQR3, and its role in tumor suppression and metabolic pathway compartmentalization. Cancer Manag Res. 2020;12:353–362. doi:10.2147/CMAR.S210919
10. Fan F, Feng L, He J, et al. RKTG sequesters B-Raf to the Golgi apparatus and inhibits the proliferation and tumorigenicity of human malignant melanoma cells. Carcinogenesis. 2008;29(6):1157–1163. doi:10.1093/carcin/bgn119
11. Wang X, Li X, Fan F, et al. PAQR3 plays a suppressive role in the tumorigenesis of colorectal cancers. Carcinogenesis. 2012;33(11):2228–2235. doi:10.1093/carcin/bgs245
12. Ling ZQ, Guo W, Lu XX, et al. A Golgi-specific protein PAQR3 is closely associated with the progression, metastasis and prognosis of human gastric cancers. Ann Oncol. 2014;25(7):1363–1372. doi:10.1093/annonc/mdu168
13. Ma Z, Wang Y, Piao T, et al. The tumor suppressor role of PAQR3 in osteosarcoma. Tumour Biol. 2015;36(5):3319–3324. doi:10.1007/s13277-014-2964-z
14. Tang SL, Gao YL, Hu WZ. PAQR3 inhibits the proliferation, migration and invasion in human glioma cells. Biomed Pharmacother. 2017;92:24–32. doi:10.1016/j.biopha.2017.05.046
15. Tang YT, Hu T, Arterburn M, et al. PAQR proteins: a novel membrane receptor family defined by an ancient 7-transmembrane pass motif. J Mol Evol. 2005;61(3):372–380. doi:10.1007/s00239-004-0375-2
16. Luo X, Feng L, Jiang X, et al. Characterization of the topology and functional domains of RKTG. Biochem J. 2008;414(3):399–406. doi:10.1042/BJ20080948
17. Fu Y, Jia X, Yuan J, et al. Fam72a functions as a cell-cycle-controlled gene during proliferation and antagonizes apoptosis through reprogramming PP2A substrates. Dev Cell. 2023;58:398–415.e7. doi:10.1016/j.devcel.2023.02.006
18. Zhang Z, Shao L, Wang Y, Luo X. MicroRNA-501-3p restricts prostate cancer growth through regulating cell cycle-related and expression-elevated protein in tumor/cyclin D1 signaling. Biochem Biophys Res Commun. 2019;509(3):746–752. doi:10.1016/j.bbrc.2018.12.176
19. Li X, Li M, Chen D, Shi G, Zhao H. PAQR3 inhibits proliferation via suppressing PI3K/AKT signaling pathway in non-small cell lung cancer. Arch Med Sci. 2018;14(6):1289–1297. doi:10.5114/aoms.2017.72220
20. Cao Q, You X, Xu L, Wang L, Chen Y. PAQR3 suppresses the growth of non-small cell lung cancer cells via modulation of EGFR-mediated autophagy. Autophagy. 2020;16(7):1236–1247. doi:10.1080/15548627.2019.1659654
21. Guo Q, Ke XX, Fang SX, et al. PAQR3 inhibits non-small cell lung cancer growth by regulating the NF-κB/p53/Bax Axis. Front Cell Dev Biol. 2020;8:581919. doi:10.3389/fcell.2020.581919
22. Wu HG, Zhang WJ, Ding Q, et al. Identification of PAQR3 as a new candidate tumor suppressor in hepatocellular carcinoma. Oncol Rep. 2014;32(6):2687–2695. doi:10.3892/or.2014.3532
23. Yu L, Zhou L, Cheng Y, et al. MicroRNA-543 acts as an oncogene by targeting PAQR3 in hepatocellular carcinoma. Am J Cancer Res. 2014;4(6):897–906.
24. Wu YL, Hong LL, Ling ZN, et al. Golgi scaffold protein PAQR3 as a candidate suppressor of gastric cardia adenocarcinoma via regulating TGF-β/Smad pathway. J Clin Lab Anal. 2022;36(9):e24617. doi:10.1002/jcla.24617
25. Huang W, Guo W, You X, et al. PAQR3 suppresses the proliferation, migration and tumorigenicity of human prostate cancer cells. Oncotarget. 2016;8(33):53948–53958. doi:10.18632/oncotarget.9807
26. Jin L, Tong L. PAQR3 inhibits proliferation and aggravates ferroptosis in acute lymphoblastic leukemia through modulation Nrf2 stability. Immun Inflamm Dis. 2021;9(3):827–839. doi:10.1002/iid3.437
27. Xie X, Zhang Y, Jiang Y, et al. Suppressive function of RKTG on chemical carcinogen-induced skin carcinogenesis in mouse. Carcinogenesis. 2008;29(8):1632–1638. doi:10.1093/carcin/bgn139
28. Wu Q, Zhuang K, Li H. PAQR3 plays a suppressive role in laryngeal squamous cell carcinoma. Tumour Biol. 2016;37(1):561–565. doi:10.1007/s13277-015-3770-y
29. Bai G, Yang M, Zheng C, Zhang L, Eli M. Suppressor PAQR3 associated with the clinical significance and prognosis in esophageal squamous cell carcinoma. Oncol Lett. 2018;15(4):5703–5711. doi:10.3892/ol.2018.8004
30. Zhou F, Wang S, Wang J. PAQR3 inhibits the proliferation and tumorigenesis in esophageal cancer cells. Oncol Res. 2017;25(5):663–671. doi:10.3727/096504016X14761384026719
31. Bai G, Chu J, Eli M, Bao Y, Wen H. PAQR3 overexpression suppresses the aggressive phenotype of esophageal squamous cell carcinoma cells via inhibition of ERK signaling. Biomed Pharmacother. 2017;94:813–819. doi:10.1016/j.biopha.2017.07.154
32. Li Z, Ling ZQ, Guo W, et al. PAQR3 expression is downregulated in human breast cancers and correlated with HER2 expression. Oncotarget. 2015;6(14):12357–12368. doi:10.18632/oncotarget.3657
33. Zhang C, Liu P, Huang J, et al. Circular RNA hsa_circ_0043280 inhibits cervical cancer tumor growth and metastasis via miR-203a-3p/PAQR3 axis. Cell Death Dis. 2021;12(10):888. doi:10.1038/s41419-021-04193-7
34. Zhang Y, Jiang X, Qin X, et al. RKTG inhibits angiogenesis by suppressing MAPK-mediated autocrine VEGF signaling and is downregulated in clear-cell renal cell carcinoma. Oncogene. 2010;29(39):5404–5415. doi:10.1038/onc.2010.270
35. Takai M, Terai Y, Kawaguchi H, et al. The EMT (epithelial-mesenchymal-transition)-related protein expression indicates the metastatic status and prognosis in patients with ovarian cancer. J Ovarian Res. 2014;7:76. doi:10.1186/1757-2215-7-76
36. Yan H, Li H, Silva MA, et al. LncRNA FLVCR1-AS1 mediates miR-513/YAP1 signaling to promote cell progression, migration, invasion and EMT process in ovarian cancer. J Exp Clin Cancer Res. 2019;38(1):356. doi:10.1186/s13046-019-1356-z
37. Cao R, Yuan L, Ma B, Wang G, Qiu W, Tian Y. An EMT-related gene signature for the prognosis of human bladder cancer. J Cell Mol Med. 2020;24(1):605–617. doi:10.1111/jcmm.14767
38. Yi C, Wan X, Zhang Y, et al. SNORA42 enhances prostate cancer cell viability, migration and EMT and is correlated with prostate cancer poor prognosis. Int J Biochem Cell Biol. 2018;102:138–150. doi:10.1016/j.biocel.2018.07.009
39. Cho ES, Kang HE, Kim NH, Yook JI. Therapeutic implications of cancer epithelial-mesenchymal transition (EMT). Arch Pharm Res. 2019;42(1):14–24. doi:10.1007/s12272-018-01108-7
40. Zhao C, Li Y, Chen G, Wang F, Shen Z, Zhou R. Overexpression of miR-15b-5p promotes gastric cancer metastasis by regulating PAQR3. Oncol Rep. 2017;38(1):352–358. doi:10.3892/or.2017.5673
41. Guo W, You X, Xu D, et al. PAQR3 enhances Twist1 degradation to suppress epithelial-mesenchymal transition and metastasis of gastric cancer cells. Carcinogenesis. 2016;37(4):397–407. doi:10.1093/carcin/bgw013
42. Chen J, Wang F, Xu J, He Z, Lu Y, Wang Z. The role of PAQR3 gene promoter hypermethylation in breast cancer and prognosis. Oncol Rep. 2016;36(3):1612–1618. doi:10.3892/or.2016.4951
43. Qi LQ, Sun B, Yang BB, Lu S. MiR-15b facilitates breast cancer progression via repressing tumor suppressor PAQR3. Eur Rev Med Pharmacol Sci. 2020;24(2):740–748. doi:10.26355/eurrev_202001_20054
44. Shi Z, Hodges VM, Dunlop EA, et al. Erythropoietin-induced activation of the JAK2/STAT5, PI3K/Akt, and Ras/ERK pathways promotes malignant cell behavior in a modified breast cancer cell line. Mol Cancer Res. 2010;8(4):615–626. doi:10.1158/1541-7786.MCR-09-0264
45. Yu X, Li Z, Chan MT, Wu WK. PAQR3: a novel tumor suppressor gene. Am J Cancer Res. 2015;5(9):2562–2568.
46. Jiang Y, Xie X, Zhang Y, et al. Regulation of G-protein signaling by RKTG via Sequestration of the Gβγ subunit to the golgi apparatus. Mol Cell Biol. 2010;30(1):78–90. doi:10.1128/MCB.01038-09
47. Wang X, Wang L, Zhu L, et al. PAQR3 modulates insulin signaling by shunting phosphoinositide 3-kinase p110α to the Golgi apparatus. Diabetes. 2013;62(2):444–456. doi:10.2337/db12-0244
48. Jiang Y, Xie X, Li Z, et al. Functional cooperation of RKTG with p53 in tumorigenesis and epithelial-mesenchymal transition. Cancer Res. 2011;71(8):2959–2968. doi:10.1158/0008-5472.CAN-10-4077
49. Yochum ZA, Cades J, Wang H, et al. Targeting the EMT transcription factor TWIST1 overcomes resistance to EGFR inhibitors in EGFR-mutant non-small-cell lung cancer. Oncogene. 2019;38(5):656–670. doi:10.1038/s41388-018-0482-y
50. Tao Y, Han T, Zhang T, Ma C, Sun C. LncRNA CHRF-induced miR-489 loss promotes metastasis of colorectal cancer via TWIST1/EMT signaling pathway. Oncotarget. 2017;8(22):36410–36422. doi:10.18632/oncotarget.16850
51. Lounglaithong K, Bychkov A, Sampatanukul P. Aberrant promoter methylation of the PAQR3 gene is associated with prostate cancer. Pathol Res Pract. 2018;214(1):126–129. doi:10.1016/j.prp.2017.10.010
52. Shukla GC, Singh J, Barik S. MicroRNAs: processing, maturation, target recognition and regulatory functions. Mol Cell Pharmacol. 2011;3(3):83–92.
53. Brenner B, Hoshen MB, Purim O, et al. MicroRNAs as a potential prognostic factor in gastric cancer. World J Gastroenterol. 2011;17(35):3976–3985. doi:10.3748/wjg.v17.i35.3976
54. Shen XC, Zhan SH, Xu ST, Gu GJ. Prognostic and clinicopathologic significance of PAQR3 and VEGF-A expression in pulmonary adenocarcinoma. Int J Clin Exp Pathol. 2020;13(7):1676–1681.
55. Li RH, Zhang AM, Li S, et al. PAQR3 gene expression and its methylation level in colorectal cancer tissues. Oncol Lett. 2016;12(3):1773–1778. doi:10.3892/ol.2016.4843
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
