Back to Journals » Infection and Drug Resistance » Volume 15
The Emergence of Novel Spoligotypes of Highly Drug-Resistant Mycobacterium tuberculosis Isolates in Fujian, China
Authors Lin S, Wei S , Zhao Y, Dai Z, Lin J, Pang Y
Received 2 July 2022
Accepted for publication 11 September 2022
Published 1 October 2022 Volume 2022:15 Pages 5781—5793
DOI https://doi.org/10.2147/IDR.S380950
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Shufang Lin,1 Shuzhen Wei,1 Yong Zhao,1 Zhisong Dai,1 Jian Lin,1 Yu Pang2
1Fujian Provincial Key Laboratory of Zoonosis Research, Fujian Center for Disease Control and Prevention, Fuzhou, People’s Republic of China; 2Department of Bacteriology and Immunology, Beijing Key Laboratory for Drug-Resistant Tuberculosis Research, Beijing Chest Hospital, Capital Medical University, Beijing Tuberculosis and Thoracic Tumor Institute, Beijing, People’s Republic of China
Correspondence: Shuzhen Wei, Fujian Center for Disease Control and Prevention, No. 386, Chong’an Road, Jin’an District, Fuzhou, Fujian, 350012, People’s Republic of China, Tel/Fax +86 591 8343 1464, Email [email protected] Yu Pang, Beijing Chest Hospital, Capital Medical University, No. 97, Machang, Tongzhou District, Beijing, 101149, People’s Republic of China, Tel/Fax +86 10 8950 9359, Email [email protected]
Background: Here, we aimed to determine the population structure of Mycobacterium tuberculosis (MTB) genotypes in Fujian and explore risk factors associated with infection with the Beijing genotype. We also explored the association between Beijing genotype and drug resistance.
Methods: Representative MTB isolates obtained during provincial-level routine anti-tuberculosis drug resistance surveillance conducted since 2013 in 11 Fujian counties were analyzed using McSpoligotyping. In vitro drug susceptibilities to anti-tuberculosis drugs were determined using the standard Löwenstein–Jensen proportion method.
Results: Overall, 477 MTB isolates were included in the study, of which 245 isolates belonged to the Beijing genotype family and 232 possessed non-Beijing genotypes. Ultimately, a total of 204 spoligotypes were identified that included 58 spoligotype international types (SITs) from the SITVITWEB database and 146 novel spoligotypes. As compared to patients < 25 years of age (control group), elderly patients (≥ 65 years of age) were more likely to be infected with non-Beijing genotypes [aOR 18.69, 95% CI (5.80– 60.26)], with risk of infection with non-Beijing genotypes increasing with age [aOR 3.73, 95% CI (1.67– 8.30) for patients 45– 64 years of age]. Additionally, as compared to isolates with Beijing and other non-Beijing genotypes, significantly greater proportions of isolates with novel spoligotypes exhibited PTO- and PAS-resistance. Moreover, a markedly higher proportion of isolates with novel spoligotypes exhibited OFX-resistance as compared to isolates with other non-Beijing genotypes.
Conclusion: Our data demonstrated that the Beijing genotype is the predominant MTB sublineage in Fujian and that the prevalence rate of infection with this MTB genotype decreases with advancing patient age. Notably, the prevalence rate of this genotype in Fujian TB patients is relatively lower than in other regions of China. In addition, the emergence of novel spoligotypes of highly drug-resistant MTB isolates highlights the urgent need for ongoing molecular genotyping surveillance in Fujian.
Keywords: Mycobacterium tuberculosis, genotype, spoligotype, drug resistant
Introduction
Tuberculosis (TB), an infection caused by Mycobacterium tuberculosis (MTB) complex, remains a major public health concern worldwide.1,2 An estimated 9.9 million new cases of TB and 1.51 million deaths from the disease were reported globally in 2020.1 Despite great progress made in TB control over the past few decades, China still shoulders 8.5% of the global TB burden and is host to the second largest annual number of TB cases globally, with nearly 30,000 deaths reported annually.1 A series of challenges have severely hindered efforts to reduce the TB burden in this country, including a high disease burden in rural areas and high community TB transmission in regions where migrations of large populations occur.3–5 Therefore, understanding the factors associated with TB spread within different populations is of great importance for controlling MTB transmission in China.
Genotyping of MTB isolates has markedly enhanced our understanding of TB epidemiology, with recently developed powerful molecular tools making it possible to determine predominant genotypes circulating in local geographic areas.1–3 Currently, spacer oligonucleotide typing (spoligotyping), a fast and cost-effective genotyping technique, is used to analyze genetic structures of MTB populations.4,5 Despite its limited discriminative power, this technique has been used in numerous studies across China to successfully spoligotype MTB isolates, especially those with the Beijing genotype.6–8 The notorious Beijing genotype, one of the most successful MTB variants, causes more than a quarter of all TB epidemics occurring worldwide and an even greater proportion of epidemics occurring in East and Central Asia. Importantly, this genotype has been shown to exhibit hypervirulence, as supported by experiments using animal models that have shown that infections of animals with Beijing genotype MTB isolates are associated with especially high mortality rates.6–9 Indeed, the successful expansion of the Beijing genotype may stem from its evolutionary acquisition of unique properties that may have favored greater community transmission of this genotype relative to other genotypes, thereby leading to its recent emergence as the predominant MTB population in China.10,11 In fact, results of a recent nationwide study revealed that 62.2% of MTB isolates circulating in China possessed the Beijing genotype, with prevalence rates of this genotype in northern China reported to be significantly higher than corresponding rates in southern China as evidence supporting geographic diversity of prevalence rates of this genotype across China.10 This disparity partly reflects more frequent and larger population migrations occurring in southern versus northern China, especially those related to migrations involving cross-border population movements that have remodeled MTB population dynamics in China.
Fujian, a province of 41.9 million residents that is located in southeastern China, has an extremely high TB prevalence rate of 427.4 cases/100,000 people that may be due, in part, to the historical importance of Fujian as a starting point of the Maritime Silk Road. This major international trade route was established by the end of the second century BCE to connect trade routes across China with trade routes in East Asia, Southeast Asia, and Africa.12 The continued use of this international trade within southeastern China has expectedly supported an ongoingsizeable population flow through this region that has likely hampered TB control efforts. Thus, the question has been raised as to whether ongoing population flow has altered the population structure of circulating MTB in Fujian, prompting this molecular epidemiological investigation. Moreover, we also aimed to explore risk factors associated with infection with the Beijing genotype while also investigating the association between the Beijing genotype and drug resistance.
Materials and Methods
Patients and Bacterial Isolates
Provincial-level anti-tuberculosis drug resistance surveillance has been routinely conducted since 2013 in 11 counties that were randomly selected from the total of 89 counties within Fujian Province. All patients with smear-positive MTB test results who provided written informed consent forms were enrolled in the study. Using well-designed questionnaires, we collected patient information based on multiple demographic and clinical variables for use in conducting comparative analyses of patients infected with Beijing versus non-Beijing MTB genotypes, including demographic characteristics (gender, age, ethnicity, occupation, marital status, education level, and residence area) and clinical characteristics (concurrent conditions such as diabetes and previous TB episodes). Additionally, each patient was required to provide three sputum specimens (spot, night, and morning) for use in bacterial species identification and spoligotyping. Sputum smears were examined microscopically, and mycobacterial cultures of two good quality sputum specimens per patient were generated by county-level laboratories according to national guidelines. Briefly, each sputum sample was decontaminated via addition of an equal volume of 4% NaOH followed by a 15-min incubation at room temperature; then, 0.1 mL of the treated specimen was inoculated into acidic Löwenstein–Jensen (L-J) solid medium. Growth of mycobacteria on the L-J medium surface was recorded weekly for a total of 8 weeks. Thereafter, positive cultures were transported to the Fujian Provincial TB Reference Laboratory for bacterial species identification and drug susceptibility testing (DST). Patient treatment outcomes were obtained from records deposited in the clinical electronic database of TB cases in China. Favorable outcomes included treatment cure and treatment completion, and unfavorable outcomes included treatment failure, death during treatment, and loss to follow-up.13
Drug Susceptibility Testing and Species Identification
The standard L-J proportion method for susceptibility testing of MTB isolates was performed by following previously described procedures based on anti-TB drug concentrations as follows: isoniazid (INH) at 0.2 μg/mL, rifampicin (RIF) at 40 μg/mL, ethambutol (EMB) at 2 μg/mL, streptomycin (SM) at 4 μg/mL, ofloxacin (OFX) at 4 μg/mL, kanamycin (KM) at 30 μg/mL, capreomycin (CPM) at 40 μg/mL, protionamide (PTO) at 40 μg/mL, and para-aminosalicylic acid (PAS) at 1 μg/mL.10 L-J medium was purchased commercially from BASO Biotech (Zhuhai, China). Briefly, 10-fold dilutions of a 1.0 McFarland standard isolate suspension were prepared (10−1 to 10−4 dilutions) in sterile normal saline; then, 10−2 and 10−4 dilutions were inoculated into medium within L-J slants with and without anti-TB drugs, respectively. Thereafter, slants were incubated at 37°C for 8 weeks until MTB colonies were visible on drug-free medium. An isolate was defined as resistant to a specific drug when 1% greater growth on drug-containing medium was observed as compared to growth of the isolate on drug-free medium (the control). Additionally, species identification was performed via growth tests conducted on L-J media containing 500μg/mL para-nitrobenzoic acid (PNB) or 5μg/mL thiophene-2-carboxylic acid hydrazide (TCH). The reference strain H37Rv (ATCC 27294) was used for quality control for each experiment.
Genotyping
Crude genomic DNA extracts generated from fresh bacterial colonies of the 477 isolates were prepared using previously reported methods, and then the extracts were used as DNA templates for molecular analysis.14 McSpoligotyping (Zeesan Biotech, Xiamen, China), a novel multicolor melting curve-based typing assay, was conducted to determine spoligotypes of MTB isolates.15 Briefly, a pair of primers, DRa and DRb, was designed to amplify the 43 unique genomic spacer regions between direct repeat-containing regions within the MTB genome. Meanwhile, probes with unique melting temperatures and fluorophore labels were designed to detect the 43 unique spacers. The probes were divided into three groups; then, each group of probes was added to three separate reactions to detect the presence or absence of each of the 43 unique spacer sequences. Each 25-μL reaction mixture contained 5 μL of sample DNA, 19.75 μL of PCR mixture, and 0.25 μL of DNA polymerase. PCR was initiated using a denaturation step of 95°C for 10 min followed by 50 cycles of 95°C for 10s, 57°C for 15s, and 76°C for 10s. After amplification, melting curve analysis was performed using the following steps: an initial denaturation step at 95°C for 3 min, a hybridization step of 1 min at 35°C, and a continuous fluorescence signal collection step for FAM, HEX, ROX, and CY5 channels as the temperature was increased from 35°C to 90°C at a rate of 0.04°C/s. The results were automatically interpreted using MeltPro Manager (Zeesan Biotech) then the results were automatically exported as 43-digit binary codes for each sample based on the presence or absence of each of the 43 spacer sequences. Binary typing results were compared with SITVIT database spoligotypes to assign spoligotype international types (SITs) to isolates.16
Statistical Analysis
Demographic and clinical characteristics of patients infected with Beijing and non-Beijing strains were compared via Chi-square test. Multivariate logistic regression was used to determine whether statistically significant covariates identified via univariate analysis were independently associated with Beijing genotype status. The extent of each association was described as an odds ratio (OR) and 95% confidence interval (95% CI). Chi-square or Fisher’s exact tests were used (as appropriate) to compare drug resistance rates stratified by spoligotype. A P value of <0.05 was considered statistically significant. All calculations were performed using SPSS 20.0 (IBM Corp., Armonk, NY).
Results
Distribution of Spoligotypes Across Geographic Regions
A collection of 477 representative MTB isolates was analyzed using spoligotyping in this study. Among these clinical isolates, 245 isolates possessed genotypes belonging to the Beijing genotype family and 232 isolates possessed non-Beijing genotypes. Isolates with non-Beijing family genotypes included 28 isolates from the T1 family, 10 from the EAI2-Manila family, 13 from the H family, 21 from the H3 family, and 160 with other non-Beijing genotypes (Table 1). We further analyzed the distribution of Beijing genotypes in different districts of Fujian Province. As shown in Figure 1, the highest Beijing genotype prevalence rate was noted in Quanzhou city (32.65%, 80/245), while the lowest rate was noted in Sanming city (2.45%, 6/245); however, due to small sample sizes, statistical analysis revealed no significant differences in Beijing genotype prevalence rates across different regions of Fujian (P=0.09).
 |  |  |  |
Table 1 Spoligotyping Analyses of MTB Isolates from Fujian, China |
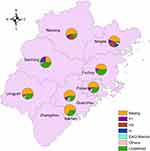 |
Figure 1 Distribution of Beijing genotype strains in ten prefectures of Fujian Province. |
Alignments of spoligotypes of MTB isolates to earlier published spoligo patterns revealed that a total of 204 unique spoligotypes were detected in this study. Among these spoligotypes, 58 of the matched spoligotype international types (SITs) within the SITVITWEB database while the other 146 isolates possessed novel spoligotypes. In addition, spoligotypes of 338 isolates were classified into 9 clusters containing 2 or more isolates per cluster, while 139 of these isolates possessed unique spoligotypes. The most prevalent spoligotype, SIT1, an SIT associated with the Beijing genotype family, accounted for 44.86% (214/477) of all isolates tested, followed by SIT53 (12/477, 2.52%), SIT742 (9/477, 1.89%), SIT190 (9/477, 1.89%), and SIT19 (7/477, 1.47%) (Table 1).
Risk Factors Associated with Non-Beijing Genotype Isolates
A detailed list of characteristics of patients infected with Beijing and non-Beijing genotypes stratified by patient demographic and clinical characteristics is presented in Table 2. Overall, the proportion of male patients infected with Beijing genotype isolates was comparable to that of female patients [adjusted odds ratio (aOR) 1.34, 95% confidence interval (CI) (0.85–2.10)]. Similarly, Beijing genotype distributions did not significantly differ according to patient ethnicity, occupation, or education level. However, based on an age of ˂25 years as the age range used to describe young patients, it was noted that elderly patients (≥65 years of age) were more likely to be infected with non-Beijing genotype isolates than young patients [OR 18.69, 95% CI (5.80–60.26)]. Furthermore, patient risk of infection with non-Beijing genotype MTB increased with advancing age [OR 3.73, 95% CI (1.67–8.30) for patients 45–64 years of age]. Interestingly, results of multivariate regression analysis demonstrated that risk of infection with non-Beijing genotype MTB was lower for diabetic patients than for patients without diabetes [aOR 2.85, 95% CI (1.38–5.86)]. Meanwhile, patients with farm-based occupations were at greater risk of being infected with Beijing genotype MTB than were patients with non-farm-based occupations [aOR 1.99, 95% CI (1.25–3.17)]. By contrast, no significant differences in education level, residence, treatment history, or clinical outcomes were observed between patients infected with Beijing versus non-Beijing genotype isolates (P>0.05).
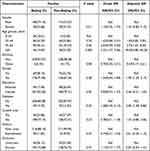 |
Table 2 Characteristics of MTB Strains of Beijing Family Compared with Non-Beijing Family |
Correlations Between Spoligotypes and Drug Resistance Profiles
We further analyzed differences in drug susceptibility profiles between Beijing and non-Beijing genotype isolates. As listed in Table 3, overall rates of drug resistance to INH, RIF, EMB, and SM were 9.64% (46/477), 5.66% (27/477), 2.94% (14/477), and 7.13% (34/477), respectively. Comparisons of drug susceptibility profiles between Beijing versus non-Beijing groups revealed respective rates of drug resistance to OFX (1.22% vs 6.03%), CPM (0% vs 3.02%), PTO (0.82% vs.3.45%), and PAS (0.41% vs 3.02%), thus indicating drug resistance rates of Beijing family genotype isolates were dramatically lower than corresponding resistance rates of non-Beijing genotype isolates (P<0.05).
 |
Table 3 Comparison of Drug Resistant Rates Between Beijing and Non-Beijing Family |
In view of the high diversity of MTB isolates circulating in Fujian, we next investigated whether the predominance of non-Beijing genotypes within the group of drug-resistant MTB isolates was associated with the emergence of novel spoligotypes. As illustrated in Figure 2, the group of isolates with novel spoligotypes exhibited significantly greater PTO and PAS resistance rates as compared to corresponding rates associated with the Beijing genotype family group of isolates and the group of isolates with other non-Beijing family genotypes. In addition, a markedly higher OFX resistance rate was observed for the group of isolates with other non-Beijing genotypes. Meanwhile, CPM resistance rates for the group of isolates with novel spoligotypes and the group of isolates with other non-Beijing genotypes were comparable, with both rates significantly greater than the CPM resistance rate of the group of isolates with the Beijing genotype.
 |
Figure 2 Drug resistance of MTB isolates stratified by different lineages. *P<0.05. |
Discussion
The Beijing genotype is one of the most prevalent MTB sublineages associated with clinical TB cases worldwide, especially in East Asia and Russia.6 The successful emergence of this genotype is associated with hypervirulence, escape from Bacillus Calmette–Guérin vaccination protection and multidrug resistance.6 Indeed, results of a previously reported study conducted in China demonstrated that Beijing genotype isolates comprised the most successful clade detected in that country. In fact, isolates belonging to this clade accounted for 62.2% of the clinical MTB isolates,10 although highly diverse Beijing genotype infection rates across regions were observed. In the present study, the Beijing genotype remained the predominant MTB sublineage in Fujian, although the prevalence rate of this genotype in that province was lower than the corresponding rate reported in almost all other regions of China, with the exception of Guangdong (50%).17 These results may partly reflect different MTB genotype-related adaptations to unique host genotypes and prior infection histories associated with populations residing in this geographic area of China.18,19 Indeed, the correspondingly high spoligotype diversity associated with clinical MTB isolates in Fujian may be linked to extremely large non-resident and cross-border migrant populations living there that tend to engage in frequent migrations that may drive MTB evolution. Mechanistically, such population movements support ongoing frequent clonal MTB expansions that may help these pathogens overcome unique immunological barriers related to specific host populations found in Fujian.20
Intriguingly, a previous study by Anh et al demonstrated that the Beijing genotype was strongly associated with younger age in Vietnam.21 Similar results were obtained in a large study of populations in western European countries that revealed a significant correlation between infection with Beijing genotype MTB and young age in patients with pulmonary TB.22 However, contradictory results were reported in Thailand, where no association between Beijing genotype and young age was found.23 In the present study, we observed that the prevalence rate of Beijing genotype MTB infection decreased with advancing age. We suggest two possible reasons for the predominance of Beijing genotype MTB infections in the young population of Fujian. The first explanation is based on the results of a molecular epidemiological study suggesting that the Beijing MTB genotype diverged from a recent common ancestor.6,24 This newly emerging genotype may more readily infect young individuals without prior MTB exposure than older individuals with prior exposures to previously circulating non-Beijing genotypes. The second explanation is based on accumulated evidence that suggests that the Beijing genotype has achieved successful global dominance by eluding BCG vaccination-based protection.25 Although China has implemented BCG vaccination of newborns since the 1980s, the predominance of the Beijing genotype in young individuals may be partly explained by ineffective BCG vaccine protection against this virulent genotype that has allowed this genotype to outcompete other genotypes in younger hosts.
Another important feature of the Beijing genotype, as revealed in previous studies, is its strong association with drug resistance and multidrug resistance,1 although this correlation was not found in Fujian isolates studied here. As compared to Beijing genotype isolates in Fujian, non-Beijing genotype isolates were associated with significantly higher drug resistance rates, with additional detailed analysis revealing markedly increased drug resistance rates that were mainly associated with isolates with novel spoligotypes. In view of the fact that the Beijing genotype has effectively adapted to the immunological characteristics of host populations in East Asia, emerging MTB genotypes with novel spoligotypes may outcompete aboriginal genotypes by acquiring drug resistance. Although the exact molecular mechanisms underlying the emergence in Fujian of highly drug-resistant MTB isolates with novel spoligotypes remain unclear, our results highlight the urgent need for ongoing molecular genotyping surveillance in that region.
We acknowledge several obvious limitations of the present study. First, spoligotyping rather than whole genome sequencing was used herein to differentiate MTB isolates, even though the lower resolving power of spoligotyping may have hampered identification of novel clades circulating in Fujian. Second, although in Fujian we found that MTB isolates with novel spoligotypes as a group exhibited significantly higher drug resistance rates than groups of Beijing genotype isolates and other non-Beijing genotype isolates, mutations related to observed drug resistance results were not analyzed, given that spoligotyping cannot effectively identify such mutations. Despite these limitations, we consider the automatic spoligotyping assay to be an efficient and practical method for use in monitoring MTB population dynamics of circulating strains in resource-limited settings.
In summary, although our data demonstrate that the Beijing genotype is the predominant MTB sublineage in Fujian, the proportion of clinical isolates with the Beijing genotype is relatively lower than that observed in other regions of China. Moreover, the Beijing MTB genotype infection rate decreases with advancing host age. In addition, the emergence of highly drug-resistant MTB isolates with novel spoligotypes highlights the urgent need for ongoing molecular genotyping-based surveillance in Fujian.
Ethical Approval Statement
This project was approved by the Ethical Committee of the Fujian Province Center for Disease Control and Prevention (CDC). The written informed consent forms were obtained from the enrolled patients prior to study enrolment. Ethics have been respected in the whole period. The study was conducted in accordance with the Declaration of Helsinki.
Funding
This study was sponsored by the Construction of Fujian Provincial Scientific and Technological Innovation Platform (2019Y2001), Fujian provincial health technology project (2019-ZQN-28), the Beijing Hospitals Authority Ascent Plan (DFL20191601), and the Beijing Hospitals Authority Clinical Medicine Development of Special Funding (ZYLX202122). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. World Health Organization. Global Tuberculosis Report 2021. Geneva: World Health Organization; 2021. Licence: CC BY-NC-SA 3.0 IGO.
2. Garrido-Cardenas JA, de Lamo-Sevilla C, Cabezas-Fernandez MT, Manzano-Agugliaro F, and Martinez-Lirola M . Global tuberculosis research and its future prospects. Tuberculosis. 2020;121:101917. doi:10.1016/j.tube.2020.101917
3. Wang L, Zhang H, Ruan Y, et al. Tuberculosis prevalence in China, 1990–2010; a longitudinal analysis of national survey data. Lancet. 2014;383(9934):2057–2064. doi:10.1016/S0140-6736(13)62639-2
4. Yang C, Lu L, Warren JL, et al. Internal migration and transmission dynamics of tuberculosis in Shanghai, China: an epidemiological, spatial, genomic analysis. Lancet Infect Dis. 2018;18(7):788–795. doi:10.1016/S1473-3099(18)30218-4
5. Liang QF, Pang Y, Chen QY, et al. Genetic profile of tuberculosis among the migrant population in Fujian Province, China. Int J Tuberc Lung Dis. 2013;17(5):655–661. doi:10.5588/ijtld.12.0615
6. Parwati I, van Crevel R, van Soolingen D. Possible underlying mechanisms for successful emergence of the Mycobacterium tuberculosis Beijing genotype strains. Lancet Infect Dis. 2010;10(2):103–111. doi:10.1016/S1473-3099(09)70330-5
7. Mokrousov I, Ly HM, Otten T, et al. Origin and primary dispersal of the Mycobacterium tuberculosis Beijing genotype: clues from human phylogeography. Genome Res. 2005;15(10):1357–1364. doi:10.1101/gr.3840605
8. Hanekom M, Gey van Pittius NC, McEvoy C, Victor TC, Van Helden PD, and Warren RM . Mycobacterium tuberculosis Beijing genotype: a template for success. Tuberculosis. 2011;91(6):510–523. doi:10.1016/j.tube.2011.07.005
9. Dormans J, Burger M, Aguilar D, et al. Correlation of virulence, lung pathology, bacterial load and delayed type hypersensitivity responses after infection with different Mycobacterium tuberculosis genotypes in a BALB/c mouse model. Clin Exp Immunol. 2004;137(3):460–468. doi:10.1111/j.1365-2249.2004.02551.x
10. Pang Y, Zhou Y, Zhao B, et al. Spoligotyping and drug resistance analysis of Mycobacterium tuberculosis strains from national survey in China. PLoS One. 2012;7(3):e32976. doi:10.1371/journal.pone.0032976
11. Dong H, Liu Z, Lv B, et al. Spoligotypes of Mycobacterium tuberculosis from different Provinces of China. J Clin Microbiol. 2010;48(11):4102–4106. doi:10.1128/JCM.00549-10
12. Yu X, Chen H, Li C. Evaluate typhoon disasters in 21st century maritime silk road by super-efficiency DEA. Int J Environ Res Public Health. 2019;16(9):1614. doi:10.3390/ijerph16091614
13. Linh NN, Viney K, Gegia M, et al. World Health Organization treatment outcome definitions for tuberculosis: 2021 update. Eur Respir J. 2021;58(2):2100804. doi:10.1183/13993003.00804-2021
14. Zhang Z, Lu J, Liu M, et al. Genotyping and molecular characteristics of multidrug-resistant Mycobacterium tuberculosis isolates from China. J Infect. 2015;70(4):335–345. doi:10.1016/j.jinf.2014.11.008
15. Zeng X, Xu Y, Zhou Y, et al. McSpoligotyping, a one-step melting curve analysis-based protocol for spoligotyping of Mycobacterium tuberculosis. J Clin Microbiol. 2018;56(8). doi:10.1128/JCM.00539-18
16. Brudey K, Driscoll JR, Rigouts L, et al. Mycobacterium tuberculosis complex genetic diversity: mining the fourth international spoligotyping database (SpolDB4) for classification, population genetics and epidemiology. BMC Microbiol. 2006;6(1):23. doi:10.1186/1471-2180-6-23
17. Guo YL, Liu Y, Wang SM, et al. Genotyping and drug resistance patterns of Mycobacterium tuberculosis strains in five provinces of China. Int J Tuberc Lung Dis. 2011;15(6):789–794. doi:10.5588/ijtld.10.0403
18. Dye C, Williams BG. The population dynamics and control of tuberculosis. Science. 2010;328(5980):856–861. doi:10.1126/science.1185449
19. Caws M, Thwaites G, Dunstan S, et al. The influence of host and bacterial genotype on the development of disseminated disease with Mycobacterium tuberculosis. PLoS Pathog. 2008;4(3):e1000034. doi:10.1371/journal.ppat.1000034
20. Gagneux S. Ecology and evolution of Mycobacterium tuberculosis. Nat Rev Microbiol. 2018;16(4):202–213. doi:10.1038/nrmicro.2018.8
21. Anh DD, Borgdorff MW, Van LN, et al. Mycobacterium tuberculosis Beijing genotype emerging in Vietnam. Emerg Infect Dis. 2000;6(3):302–305. doi:10.3201/eid0603.000312
22. European Concerted Action on New Generation Genetic M, Techniques for the E, Control of T. Beijing/W genotype Mycobacterium tuberculosis and drug resistance. Emerg Infect Dis. 2006;12(5):736–743. doi:10.3201/eid1205.050400
23. Prodinger WM, Bunyaratvej P, Prachaktam R, Pavlic M. Mycobacterium tuberculosis isolates of Beijing genotype in Thailand. Emerg Infect Dis. 2001;7(3):483–484. doi:10.3201/eid0703.010330
24. Pang Y, Song Y, Xia H, Zhou Y, Zhao B, Zhao Y. Risk factors and clinical phenotypes of Beijing genotype strains in tuberculosis patients in China. BMC Infect Dis. 2012;12(1):354. doi:10.1186/1471-2334-12-354
25. Kousha A, Farajnia S, Ansarin K, Khalili M, Shariat M, Sahebi L . Does the BCG vaccine have different effects on strains of tuberculosis? Clin Exp Immunol. 2021;203(2):281–285. doi:10.1111/cei.13549
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
