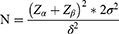Back to Journals » International Journal of General Medicine » Volume 15
The Efficacy of Integrated Rehabilitation for Post-Stroke Anxiety: Study Protocol for a Prospective, Multicenter, Randomized Controlled Trial
Authors Zhou J, Fan L , Hu H , Shen K, Wu L, Lin X, Gao H
Received 13 July 2022
Accepted for publication 24 August 2022
Published 6 September 2022 Volume 2022:15 Pages 7101—7111
DOI https://doi.org/10.2147/IJGM.S381434
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Jie Zhou,1,* Lijuan Fan,2,* Hantong Hu,1 Ke Shen,2 Liya Wu,2 Xiaoqi Lin,2 Hong Gao1
1Department of Acupuncture and Moxibustion, The Third Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou, People’s Republic of China; 2The Third Clinical College of Zhejiang Chinese Medical University, Hangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hong Gao, Department of Acupuncture and Moxibustion, The Third Affiliated Hospital of Zhejiang Chinese Medical University, No. 219 Moganshan Road, Hangzhou, People’s Republic of China, Email [email protected]
Background: Post-stroke anxiety (PSA) remains a challenging medical problem. Integrated rehabilitation involves a combination of traditional Chinese medicine (TCM) and Western conventional rehabilitation techniques. Theoretically, integrated rehabilitation is likely to have significant advantages in treating PSA. Nevertheless, the therapeutic effect of integrated rehabilitation needs to be verified based on large-scale trials with sound methodology. Thus, the aim of this trial is to assess the efficacy and safety of integrated rehabilitation on PSA.
Methods: The study is a prospective, multicenter, randomized, controlled trial involving 188 PSA patients from four clinical centers in China. Eligible participants will be randomly divided into the integrated rehabilitation group or the standard care group. Participants in the integrated rehabilitation group will receive a combination of TCM and Western conventional rehabilitation methods, including acupuncture, repeated transcranial magnetic stimulation, traditional Chinese herbal medicine, and standard care. The primary outcome will be the Hamilton Anxiety Rating Scale (HAM-A). The secondary outcomes will include the Self-Rating Anxiety Scale (SAS), the Activities of Daily Living (ADL) scale, the Montreal Cognitive Assessment (MoCA) scale, the simplified Fugl–Meyer Assessment of motor function (FMA) scale, and the Pittsburgh Sleep Quality Index (PSQI). Outcome measurements will be performed at baseline, at the end of the 4-week treatment and the 8-week follow-up.
Conclusion: Results of this trial will ascertain the efficacy and safety of integrated rehabilitation on PSA, thereby providing evidence regarding integrated rehabilitation strategies for treating PSA. It will also promote up-to-date evidence for patients, clinicians, and policy-makers.
Trial Registration: ClinicalTrials.gov NCT05147077.
Keywords: post-stroke anxiety, traditional Chinese medicine, Western medicine, repeated transcranial magnetic stimulation, rehabilitation, randomized controlled trial
Introduction
Stroke is the second leading cause of death globally.1 The estimated global lifetime risk data show that the lifetime risk of stroke for people aged above 25 years was 24.9%.2 China has the highest estimated lifetime risk of stroke (39.3%) in the world according to global, regional, and country-specific lifetime risk of stroke and the 2017 Global Burden of Disease Study.3 And stroke has been the leading cause of death and disability in China in recent years.4
Post-stroke anxiety (PSA) is a common psychological complication of stroke, impacting the quality of life and functional decline. The estimated global prevalence of PSA ranges from 18% to 25%,5 while in a Jordanian survey, the figure is up to 52.9%.6 PSA aggravates cognitive dysfunction, delays the recovery process, and increases the disability, mortality, and recurrence rates of stroke. Therefore, it is vital to perform early clinical interventions for PSA to improve the prognosis and restore the social function of stroke patients.
To date, there have been no specific and uniform guidelines for the treatment of PSA. For the treatment of PSA, current treatments mainly include pharmacological therapies, psychotherapy, and rehabilitation. In addition, some patients also seek complementary and alternative therapies.7,8
As it is widely believed that anxiety is associated with increased disability and decreased quality of life,9 improving movement function is fundamental for PSA. Thus, rehabilitation therapy is an important treatment approach for PAS. However, it is worth noting that the majority of previous studies focus on the efficacy of specific rehabilitation therapy, but ignore the important concept of “integrated rehabilitation”, thereby failing to achieve optimal efficacy. Thus, it is of significance to develop an integrated rehabilitation scheme for PSA.
The concept of “integrated rehabilitation” in our study is derived from “integrative medicine”, which is defined as “medicine that reaffirms the importance of the relationship between practitioners and patients, focuses on the whole person, and makes use of all appropriate therapeutic approaches, healthcare professionals and disciplines (including conventional and complementary therapies) to achieve optimal health and healing”.10 Notably, in most Asian countries, especially China, with the aim to optimize the therapeutic effect through closer collaborations between traditional Chinese medicine (TCM) and Western medicine, clinicians often adopt an integrative medicine approach to treat a wide range of diseases in hospitals,11,12 rather than just adopt a sole TCM or Western medicine approach. Therefore, the concept of “integrated rehabilitation” in this study can promote the development of a rehabilitation scheme that integrates both TCM and Western medicine, which has promising guiding value in real-world practice. Specifically, our integrated rehabilitation scheme incorporates two TCM therapies (ie, acupuncture and Chinese herbal medicine), repeated transcranial magnetic stimulation (rTMS) and standard care. The rationales for selecting these components are interpreted in detail as follows.
First, acupuncture and Chinese herbal medicine are two important components of TCM, which is an essential part of China’s healthcare system. Acupuncture is commonly used for mental diseases such as anxiety disorders, and previous systematic reviews and meta-analyses reveal promising results that favor the efficacy and safety of acupuncture on anxiety disorders.13,14 Similarly, findings of previous meta-analyses suggest that Chinese herbal medicine has certain beneficial effects on reducing anxiety.15,16 In addition, with increasing numbers of clinical studies, there is growing evidence that anxiety can be treated with rTMS.17,18 Lastly, standard care (eg, pharmacological drugs, conventional rehabilitation) is a fundamental treatment for PSA. Taken together, the integrated rehabilitation scheme incorporating the aforementioned therapies in our study is commonly used and has promising application value for PSA in real-world clinical settings, especially in Asia, so we intend to investigate the therapeutic effect of this integrated rehabilitation protocol.
Materials and Methods
Study Objectives and Hypothesis
The main objective of this trial is to compare the efficacy and safety of integrated rehabilitation with standard care. To further enrich the treatment of PSA in real-world practice, we choose an integrated rehabilitation scheme that combines TCM and Western conventional rehabilitation treatments. We hypothesize that integrated rehabilitation will lead to a more significant improvement in PSA when compared with standard care.
Study Design and Setting
The study is a prospective, multicenter, randomized controlled, clinical trial that includes 188 participants from four clinical centers in China. All patients who meet the enrollment criteria will be randomly assigned to either the integrated rehabilitation group or the standard care group. The study duration will be 12 weeks, which includes a 4-week treatment period, and an 8-week follow-up period.
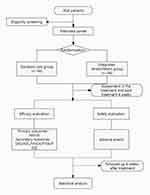
The research flow chart is shown in Figure 1. The trial schedule of enrolment, treatments, and assessments is displayed in Table 1. The reporting of this protocol is strictly based on the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT),19 which is uploaded in Supplementary File 1.
 |
Table 1 Schedule of Enrolment, Treatments, and Assessments |
Randomization and Allocation Concealment
After signing the informed consent, eligible patients will be randomly divided into the integrated rehabilitation group or the standard care group in a 1:1 ratio through a central randomization system. Personnel permission levels in the randomization system are rigorous, and only the highest-level system administrator has access to the randomization scheme. Once a participant is enrolled, an independent staff, who is not involved in participant screening, treatment or outcome assessment, will operate the randomization system to generate a random number to determine the corresponding group that the participant should be assigned to. The researcher who applies for the random number will call the randomization center and enter the subject’s information in the voice form or via a text message. When the central randomization system has received the application for a random number, the patient’s random number and group information will be sent to the researcher. Allocation concealment will be maintained by means of central randomization.
Blinding Implementation
Owing to the characteristic of the complex integrated rehabilitation procedures and distinct differences in interventions between the two groups, it is hard to blind both the manipulators of integrated rehabilitation and patients in this study. However, to ensure the blinding principles and avoid potential bias as much as possible during our trial, the operators, outcome assessors, and statistical analysts will be separated and performed by respective specially assigned persons. The outcome measurement and statistical analyses will be carried out by specially designated researchers who are unaware of the grouping information.
Sample Size Calculation
Participants will be divided into two groups in a 1:1 ratio. Due to the lack of previously published relevant RCTs, the sample size calculation is based on the results of our pilot study. It is assumed that after 4 weeks of intervention, the mean of the HAM-A scores is 12.7 in the integrated rehabilitation and 14.4 in the standard care group, with a pooled standard deviation of 3.27. According to the following sample size formula, to achieve 80% statistical power and keep the type I error rate to <0.05, a total of 156 participants (with 78 in each group) will be required to detect a two-sided significant difference between the two groups. To compensate for an estimated dropout rate of 20%, 188 participants (with 94 in each group) will be ultimately required.
Eligibility Criteria
The inclusion criteria for this trial are as follows:
- Patients meet the diagnostic criteria of cerebral infarction or cerebral hemorrhage with anxiety disorder, and they meanwhile meet the TCM syndrome differentiation of “Liver stagnation transforming into fire” type;
- Consciousness, stable vital signs, ability to understand and follow the instructions, Barthel Index (BI)>20, FMA (0–95);
- 25≤age≥85 years, male or female;
- The first episode of stroke, no personal or family history of mental disability before the stroke;
- Anxiety level as mild or moderate (ie, HAM-A scores ≥7 and≤21);
- Anxiety symptoms occur after the stroke in a clear temporal sequence;
- The duration of the disease range between 2 weeks and 36 months after the stroke;
- Participants can understand the study protocol and written informed consent is signed.
The exclusion criteria are as follows:
- Patients with acute brain trauma, brain infection, effusion, or tumor occupation;
- There are intracranial metals and other foreign bodies (such as orthopedic materials, arterial clips, etc), cardiac pacemakers, deep brain stimulators, and other electronic devices;
- Previous seizures, including primary and secondary seizures;
- Patients have severe complications in cardiovascular, liver, and kidney; or patients have psychiatric history;
- There is a significant cognitive impairment (MMSE: illiteracy ≤17, primary school level ≤20, secondary school level (including technical secondary school) ≤22, and college-level (including junior college) ≤23 points), or hearing impairment, aphasia;
- Patients who are unconscious;
- Patients have taken psychotropic drugs or been treated for anxiety in the last month;
- People with unstable vital signs or patients with other mental disorders.
Participant Recruitment
All PSA patients will be recruited from four clinical centers, which include the Third Affiliated Hospital of Zhejiang Chinese Medical University, Zhejiang General Hospital of the Armed Police, Hangzhou TCM Hospital Affiliated to Zhejiang Chinese Medical University, and The Second Hospital of Jinhua. Recruitment strategies will include posters, public social networks, and advertisements.
Interventions
Standard Care Group
The standard care includes pharmacological drugs, general care, and exercise therapy.
Pharmacological drugs mainly consist of anti-anxiety drugs and cerebrovascular drugs. For anti-anxiety, the selective 5-HT reuptake inhibitor (escitalopram oxalate 10mg qd, 4 weeks) will be used for all participants, which has anxiolytic effects as an antidepressant. Other medications are referred to the Chinese Stroke Association guidelines for clinical management of cerebrovascular disorders.20 When appropriate, corresponding drugs that have the effect of lipid regulation, blood sugar control, antihypertension, and anticoagulation will be used depending on patients’ individual conditions.
In addition, general care and exercise therapy will be provided. General care is the assessment of the patient’s general and psychological condition and family situation by the nursing staff. And the exercise therapy is based on the Chinese Stroke Association guidelines for clinical management of cerebrovascular disorders and the 2019 update of clinical management of stroke rehabilitation.21 The patient’s Brunnstrom stage will be assessed by a professional therapist, and the prescriptions for motor function rehabilitation will be tailored to the changes in muscle strength and tone, and motor function. They include, but not limited to, good limb positioning, joint release and muscle massage on the affected side, muscle strength training, balance training in sitting, and standing positions, gait training, etc. Every individual exercise therapy lasts 30–45 minutes once a day, 5 times per week and the treatment duration lasts 4 weeks.
Integrated Rehabilitation Group
In addition to standard care, participants in the integrated rehabilitation group will receive a combination of TCM and Western conventional rehabilitation methods, including acupuncture, repeated transcranial magnetic stimulation (rTMS), and traditional Chinese herbal medicine.
Acupuncture
Locations of acupoints applied in this trial are summarized in Table 2. The disposable sterile acupuncture needles (Suzhou Hua Tuo Medical Instruments, China) with a diameter of 0.25 mm and length of 40 mm will be used.
 |
Table 2 Location of Acupoints for Treating PSA |
The location standard of scalp acupoints is based on the Standardized manipulations of acupuncture and moxibustion-Part 2: Scalp acupuncture 2021 (GB/T 21709.2–2021). The scalp acupoints include the middle line of forehead (MS1), a front line by the forehead (MS2), and the middle line of vertex (MS5). And the needle inserts the scalp at an angle of 15–30 degrees for 1 cun. The twirling method of acupuncture manipulation should be performed at a speed of 200 times per minute for 10 seconds and the amplitude depends on the patient’s tolerance, generally in the range of 90°–360°. The scalp needles will be kept for 1 hour and twisted twice during needle retention.
The location standard of body acupoints is based on the National Standard Nomenclature and location of meridian points 2021 (GB/T 12346–2021). Following the end of scalp needle insertion, body acupuncture is performed in a supine position. Acupoints include Yintang (GV24+), bilateral Taichong (LR3), bilateral Shenmen (HT7), bilateral Neiguan (PC6), Danzhong (CV17), bilateral Tongli (HT5), and bilateral Xingjian (LR2). The Yintang (GV24+) acupoint is needled for 0.3–0.5 cun using the horizontal insertion. The bilateral Taichong (LR3) and Xingjian (LR2) acupoints are needled for 0.5–0.8 cun using the vertical insertion. The Danzhong (CV17), bilateral Shenmen (HT7), and Tongli (HT5) acupoints are needled for 0.3–0.5 cun using the vertical insertion. The bilateral Neiguan (PC6) acupoints are needled for 0.5–1 cun using the vertical insertion. The needles inserting the body acupoints will be kept for 30 minutes.
The treatment frequency of acupuncture will be 5 times per week. And the treatment duration will last 4 weeks.
rTMS
We will administer rTMS using a CCY-I-type magnetic field stimulator (Wuhan Yiruide Medical Equipment New Technology Company Limited). Active treatment will consist of 900 pulses per session, delivered over the right prefrontal cortex at 90% of motor threshold (MT). We will determine MT at screening and at the beginning of every treatment block (every 5 sessions) using electromyographic measurement of the resting left thumb (left abductor pollicus brevis). Treatment will be delivered at a pulse frequency of 1 Hz with continuous stimulation and each rTMS treatment session last for 15 minutes. Patients will receive 20 sessions of treatment, with a frequency of 5 times per week and the treatment duration will last 4 weeks.
Traditional Chinese Herbal Medicine
Based on syndrome differentiation, traditional Chinese herbal medicine called “danzhixiaoyao power” will be selected and its specific prescriptions are as follows: Danpi 10g, fried Zhizi 10g, Danggui 12g, Baishao 12g, fried Chaihu 6g, Fuling 10g, fried Baishu 10, and roasted Gancao 3g. The dosage of Chinese herbal medicine is 2 times a day for 4 weeks.
Outcomes Measures
Primary Outcome
The primary outcome is the Hamilton Anxiety Rating Scale (HAM-A). This scale will be assessed before treatment, at the end of the 4-week treatment, and at the end of the 8-week follow-up after treatment.
The HAM-A scale is utilized to assess the subjects’ anxiety symptoms. The CCMD-3 Chinese Diagnostic Criteria for Mental Disorders22 lists the HAM-A scale as an important diagnostic tool for anxiety disorders,23 and it is often used clinically as a recognized tool for the diagnosis and classification of anxiety disorders.24 It is widely accepted that the HAM-A scale is a valid and reliable tool for stroke patients25,26 and it is also the most extensive semi-structured assessment scale for evaluating the severity of anxiety,26 so we chose it as the primary outcome.
Secondary Outcomes
Secondary outcomes include Zung’s Self-Rating Anxiety Scale (SAS), Activities of Daily Living (ADL) scale, the Montreal Cognitive Assessment (MoCA) scale, the simplified Fugl–Meyer Assessment of motor function (FMA) scale, and Pittsburgh Sleep Quality Index (PSQI). These scales will be assessed before treatment and at the end of the 4-week treatment. The ADL and PSQI need to be assessed again at the end of the 8-week follow-up. The justifications for selecting them as secondary outcomes are elaborated as follows.
The SAS is used to assess the subjective feelings of patients with anxiety. SAS has good psychometric credentials, and it is suitable for all types of psychiatric disorders in which anxiety symptoms are predominant. There are 20 items on a four-point scale, and higher scores indicate greater anxiety.27,28 SAS has good psychometric credentials28 and can assist in the assessment of patients’ anxiety status.
The ADL scale consists of the following two components: 1) the Physical Self-maintenance Scale (PSMS), which consists of six items (ie, toileting, eating, dressing, grooming, walking, and bathing); 2) the Instrumental Activities of Daily Living Scale (IADL), which consists of eight items (ie, telephone, shopping, meal preparation, housework, laundry, transportation, medication, and economic self-management).29,30 Another aim of this trial is to improve the patient’s ability to perform activities of daily living, so ADL is used as one of the secondary indicators.
The MoCA is based on clinical experience and is referenced to the Mini-mental State Examination, which has been widely used to measure the consciousness of test-takers and is more sensitive to screening for mild cognitive impairment. The scale covers eight cognitive domains, including attention and concentration, executive function, memory, language skills, visual structure, abstract thinking, numeracy, and orientation, among other 11 items. The total score is 30 and a score of more than 26 is considered to be normal. If the subject has less than 12 years of education, one point is added to the total score. The questionnaire is ideal for experimental use because of its sensitivity, coverage of important cognitive domains, and short testing time.31,32 It is worth noting that cognitive decline and anxiety symptoms commonly interact and co-occur33; this is why we set MoCA as one of the secondary outcomes.
It is widely accepted that the FMA is the gold standard for evaluating the motor function in patients with post-stroke hemiparesis,34 with a score of 100 for motor function (66 for upper limb and 34 for lower limb).35 Therefore, we utilize it as one of the main components of secondary outcomes.
The PSQI is a standardized clinical tool that measures a wide variety of sleep quality markers.36 It combines the qualitative and quantitative aspects of sleep to assess the quality of the subject’s sleep over the last month. The scale can be used to evaluate the sleep behavior and habits of the general population, as well as the sleep quality of clinical patients. Notably, sleep disorders can predict depression and anxiety, and short sleep duration and poor sleep quality are identified as risk factors for PSA.37,38 Reduced sleep quality is a common symptom in patients with depressive disorders. Thus, PSQI is adopted as one of the secondary outcomes.
Statistical Analysis
Statistical analysis will be performed using SPSS 25.0 for Windows. Continuous variables in normal distribution will be expressed as means ± standard deviations, while continuous variables in non-normal distribution will be reported as median with quartiles. In addition, categorical variables will be expressed as frequency rates and percentages in each category. For continuous variables in a normal distribution, repeated-measures analysis of variance (repeated measures ANOVA) will be used to perform within-group and between-group comparisons by assessing changes in continuous variables before and after treatment at different time points, while a non-parametric test will be adopted to conduct within-group and between-group comparison for continuous variables in non-normal distribution. For categorical variables, between-group differences will be assessed by the chi-square test or Fisher’s exact test.
All hypothesis tests will be performed as two-sided tests, and differences will be considered statistically significant at P<0.05. The statistical analysis plan will be completed by professional statisticians who are not involved in other procedures of the trial. After all data entry and review, the statistician will complete the analysis in time and produce a written statistical analysis report.
Ethical Approval and Study Registration
The trial will be conducted by the principles of the Declaration of Helsinki. It has been approved by the Medical Ethics Committee of the Third Affiliated Hospital of Zhejiang Chinese Medical University (Approval ZSLL-KY-2021-009-01). Written informed consent will be signed by each participant who is included in the trial.
The study protocol has been prospectively registered in the Clinical Trials Registry with the identification code NCT05147077.
Quality Control
A standard operating procedure (SOP) will be developed by the research group. A special training session will be held 1 month before the official launch of the clinical trial, which provides standard training to all research personnel involved in the trial. The training session will focus on the procedure of the trial and ensure each researcher is familiar with the research process and masters the SOP, thereby guaranteeing the quality of the trial.
Adverse Events
Adverse events (AEs) throughout the study will be assessed and recorded by the investigators in the case report form. After each patient visit, participants will be inquired about using questions related to AEs. In addition, throughout the trial, subjects will also be instructed to report any adverse events to investigators spontaneously. Investigators will record the occurrence date, degree, duration, and treatment measures of AEs.
Discussions
Previous clinical studies mainly focus on post-stroke depression. There are very few studies on PSA. Thus, more studies are urgently needed to be conducted on the pathogenesis and treatment methods of PAA. Currently, although the pathogenesis of PSA remains unclear, it may be related to the following mechanisms: 1) Cerebrovascular circulation disorder leads to brain tissue damage, and there is a correlation between areas of impaired hypoperfusion and the morbidity of anxiety,39 especially in areas of dominant psycho-behavioral functions such as the frontotemporal lobe and cortex40; 2) Activated NF-kB cells in the neuroglia may mediate anxiety behavior by enhancing nNOS-CAPON-Dexras expression and association41; and 3) Impaired neuronal function leads to dysregulation of neurotransmitters such as adrenaline, 5-hydroxytryptamine, and dopamine to trigger depression and anxiety.42
At present, there is a paucity of clinical research on PSA. Previous available studies mainly adopt a solo therapy for PSA, and the integrated rehabilitation incorporating both TCM and Western medicine is rarely used in previously published studies in this field. Based on the available literature, current treatment options include new techniques such as Armeo Spring robot-assisted trainer and the Kinect-based system,43 acupuncture,44 aquatic exercise programs,45 medications (eg, escitalopram,24 Sertraline46) and rehabilitation treatments such as problem-solving therapy,24 self-help relaxation,9,47 etc. To further enrich the treatment of PSA, we choose an integrated rehabilitation scheme in this trial, which integrates both TCM and Western medicine. Such an integrated rehabilitation scheme is widely used and it has significant guiding value in real-world clinical settings, especially in Asian countries. Among the specified components of the integrated rehabilitation protocol, scalp acupuncture can improve anxiety symptoms after stroke and reduces the HAM-A scores by promoting cranial nerve recovery48 and body acupuncture can also decrease the level of anxiety.49 Compared to conventional pharmacotherapy, to a certain extent, Chinese herbal medicine or Chinese herbal compound can significantly relieve anxiety symptoms in PSA patients with fewer adverse events.15 One retrospective study50 indicated that acupuncture combined with Chinese herbal medicine has superior effects in alleviating post-stroke anxiety. In addition, the French guidelines51 for rTMS include anxiety as one of its indications. Another study favored the feasibility and safety of rTMS for improving anxiety symptoms.52 Therefore, we design this present study to evaluate the safety and efficacy of integrated rehabilitation in PSA patients.
Notably, the investigation of the efficacy and safety of our integrated rehabilitation protocol will have significant guiding value in clinical practice. With increasingly closer cooperation between TCM and Western medicine, as well as the popularity of the concept of “integrative medicine”, such integrated rehabilitation strategies that combine TCM and Western medicine in our study are expected to be widely used in real-world practice, especially in Asian countries. Integrative medicine makes use of all appropriate therapeutic approaches, healthcare professionals and disciplines (including conventional and complementary therapies) to achieve optimal health and healing. In our study, the treatment strategy focuses on the improvement of the patient’s overall symptoms through integrated rehabilitation. We believe that the findings of this study will contribute to promoting the application of integrative rehabilitation strategies for PSA.
However, this study has certain limitations. First, limited by research funds, patients could not be followed up for a longer period, making it impossible to observe the long-term effect of the integrated rehabilitation. Second, due to the characteristic of the complex integrated rehabilitation procedures and distinct differences in interventions between the two groups, double-blind and placebo controls are not utilized, which will arise a certain bias and also fails to exclude a placebo effect of integrated rehabilitation. Nonetheless, with the aim to minimize bias, we have applied blindly to the outcome assessors and statistical analysts. Last, since there is a lack of objective evaluation indicators for PSA, our outcome measures mainly rely on scales that are relatively subjective. Nonetheless, to reduce the bias induced by subjective scales, each center of this multicenter trial will assign a professional physician as a fixed outcome assessor to perform outcome measurements of various subjective scales. Moreover, prior to the trial, the outcome assessor from each center will gather together to receive intensive training to practice outcome assessments in a consistent manner to improve the reliability of outcomes.
Conclusions
This study is a prospective, multicenter, randomized controlled trial that aims to assess the efficacy and safety of integrated rehabilitation for PSA. It is expected that its subsequent findings will promote a comprehensive treatment scheme incorporating various rehabilitation modalities, thereby improving the therapeutic strategy of PSA.
Data Sharing Statement
Upon the completion of the study, supporting data will be available from the corresponding author by request.
Ethics Statement
Ethical approval (number: ZSLL-KY-2021-009-01) has been obtained from the Ethics Committee of The Third Affiliated Hospital of Zhejiang Chinese Medical University.
Acknowledgments
The authors appreciate the support from all participants who have been or will be included in this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The trial is financially funded by the 2021 Special Project for Modernization of Chinese Medicine in Zhejiang Province (No. 2021ZX010) and Zhejiang Provincial Famous Traditional Chinese Medicine Experts Inheritance Studio Construction Project (grant no. GZS2021027).
Disclosure
The authors declare that they have no competing interests.
References
1. Mozaffarian D, Benjamin EJ, Go AS, et al. Executive summary: heart disease and stroke statistics–2016 update: a report from the American Heart Association. Circulation. 2016;133(4):447–454. doi:10.1161/CIR.0000000000000366
2. Feigin VL, Nguyen G, Cercy K, et al. Global, regional, and country-specific lifetime risks of stroke, 1990 and 2016. N Engl J Med. 2018;379(25):2429–2437.
3. Wu S, Wu B, Liu M, et al. Stroke in China: advances and challenges in epidemiology, prevention, and management. Lancet Neurol. 2019;18(4):394–405. doi:10.1016/S1474-4422(18)30500-3
4. Wang W, Jiang B, Sun H, et al. Prevalence, incidence, and mortality of stroke in China: results from a nationwide population-based survey of 480 687 adults. Circulation. 2017;135(8):759–771. doi:10.1161/CIRCULATIONAHA.116.025250
5. Sanner Beauchamp JE, Casameni Montiel T, Cai C, et al. A Retrospective study to identify novel factors associated with post-stroke anxiety. J Stroke Cerebrovasc Dis. 2020;29(2):104582. doi:10.1016/j.jstrokecerebrovasdis.2019.104582
6. Almhdawi KA, Alazrai A, Kanaan S, et al. Post-stroke depression, anxiety, and stress symptoms and their associated factors: a cross-sectional study. Neuropsychol Rehabil. 2021;31(7):1091–1104. doi:10.1080/09602011.2020.1760893
7. Ryan BJ, Clunne SM, Baker CJ, Shiggins C, Rose ML, Kneebone II. A systematic review of non-drug interventions to prevent and treat anxiety in people with aphasia after stroke. Disabil Rehabil. 2021;1–10. doi:10.1080/09638288.2021.1925752
8. Knapp P, Campbell Burton CA, Holmes J, et al. Interventions for treating anxiety after stroke. Cochrane Database Syst Rev. 2017;5(5):Cd008860. doi:10.1002/14651858.CD008860.pub3
9. Golding K, Fife-Schaw C, Kneebone I. Twelve month follow-up on a randomised controlled trial of relaxation training for post-stroke anxiety. Clin Rehabil. 2017;31(9):1164–1167. doi:10.1177/0269215516682820
10. MacPherson H, Peters D, Zollman C. Closing the evidence gap in integrative medicine. BMJ. 2009;339:b3335. doi:10.1136/bmj.b3335
11. Fang J, Chen L, Ma R, et al. Comprehensive rehabilitation with integrative medicine for subacute stroke: a multicenter randomized controlled trial. Sci Rep. 2016;6:25850. doi:10.1038/srep25850
12. Lee MJ, Yun YJ, Yu SA, et al. Integrative medicine rehabilitation for children with cerebral palsy: a study protocol for a multicenter pragmatic randomized controlled trial. Trials. 2020;21(1):723. doi:10.1186/s13063-020-04639-x
13. Yang XY, Yang NB, Huang FF, Ren S, Li ZJ. Effectiveness of acupuncture on anxiety disorder: a systematic review and meta-analysis of randomised controlled trials. Ann Gen Psychiatry. 2021;20(1):9. doi:10.1186/s12991-021-00327-5
14. Tong QY, Liu R, Zhang K, Gao Y, Cui GW, Shen WD. Can acupuncture therapy reduce preoperative anxiety? A systematic review and meta-analysis. J Integr Med. 2021;19(1):20–28. doi:10.1016/j.joim.2020.10.007
15. Kwon CY, Lee B, Chung SY, Kim JW. Herbal medicine for post-stroke anxiety: a systematic review and meta-analysis of randomized controlled trials. Complement Ther Clin Pract. 2019;35:237–252. doi:10.1016/j.ctcp.2019.02.015
16. Yang S, Xu Y, Peng W, et al. Chinese herbal medicine for symptoms of depression and anxiety in chronic obstructive pulmonary disease: a systematic review and meta-analysis. Complement Ther Clin Pract. 2021;45:101470. doi:10.1016/j.ctcp.2021.101470
17. Cirillo P, Gold AK, Nardi AE, et al. Transcranial magnetic stimulation in anxiety and trauma-related disorders: a systematic review and meta-analysis. Brain Behav. 2019;9(6):e01284. doi:10.1002/brb3.1284
18. Kozel FA. Clinical repetitive transcranial magnetic stimulation for posttraumatic stress disorder, generalized anxiety disorder, and bipolar disorder. Psychiatr Clin North Am. 2018;41(3):433–446. doi:10.1016/j.psc.2018.04.007
19. Chan AW, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. 2013;158(3):200–207. doi:10.7326/0003-4819-158-3-201302050-00583
20. Wang Y, Han S, Qin H, et al. Chinese Stroke Association guidelines for clinical management of cerebrovascular disorders: executive summary and 2019 update of the management of high-risk population. Stroke Vasc Neurol. 2020;5(3):270–278. doi:10.1136/svn-2020-000385
21. Zhang T, Zhao J, Li X, et al. Chinese Stroke Association guidelines for clinical management of cerebrovascular disorders: executive summary and 2019 update of clinical management of stroke rehabilitation. Stroke Vasc Neurol. 2020;5(3):250–259. doi:10.1136/svn-2019-000321
22. Chinese Psychiatric Society. The Chinese Classification of Mental Disorders.
23. Maier W, Buller R, Philipp M, Heuser I. The Hamilton anxiety scale: reliability, validity and sensitivity to change in anxiety and depressive disorders. J Affect Disord. 1988;14(1):61–68. doi:10.1016/0165-0327(88)90072-9
24. Mikami K, Jorge RE, Moser DJ, et al. Prevention of post-stroke generalized anxiety disorder, using escitalopram or problem-solving therapy. J Neuropsychiatry Clin Neurosci. 2014;26(4):323–328. doi:10.1176/appi.neuropsych.11020047
25. Kimura M, Tateno A, Robinson RG. Treatment of poststroke generalized anxiety disorder comorbid with poststroke depression: merged analysis of nortriptyline trials. Am J Geriatr Psychiatry. 2003;11(3):320–327. doi:10.1097/00019442-200305000-00009
26. Bruss GS, Gruenberg AM, Goldstein RD, Barber JP. Hamilton Anxiety Rating Scale Interview guide: joint interview and test-retest methods for interrater reliability. Psychiatry Res. 1994;53(2):191–202. doi:10.1016/0165-1781(94)90110-4
27. Dunstan DA, Scott N, Todd AK. Screening for anxiety and depression: reassessing the utility of the Zung scales. BMC Psychiatry. 2017;17(1):329. doi:10.1186/s12888-017-1489-6
28. Dunstan DA, Scott N. Norms for Zung’s self-rating anxiety scale. BMC Psychiatry. 2020;20(1):90. doi:10.1186/s12888-019-2427-6
29. Han G, Maruta M, Ikeda Y, et al. Relationship between performance on the mini-mental state examination sub-items and activities of daily living in patients with Alzheimer’s disease. J Clin Med. 2020;9(5):1537. doi:10.3390/jcm9051537
30. Okabe K, Nagata T, Shinagawa S, et al. Effects of neuropsychiatric symptoms of dementia on reductions in activities of daily living in patients with Alzheimer’s disease. Geriatr Gerontol Int. 2020;20(6):584–588. doi:10.1111/ggi.13918
31. Feng Y, Zhang J, Zhou Y, Chen B, Yin Y. Concurrent validity of the short version of Montreal Cognitive Assessment (MoCA) for patients with stroke. Sci Rep. 2021;11(1):7204. doi:10.1038/s41598-021-86615-2
32. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi:10.1111/j.1532-5415.2005.53221.x
33. Petkus AJ, Reynolds CA, Wetherell JL, Kremen WS, Gatz M. Temporal dynamics of cognitive performance and anxiety across older adulthood. Psychol Aging. 2017;32(3):278–292. doi:10.1037/pag0000164
34. Hijikata N, Kawakami M, Ishii R, et al. Item difficulty of Fugl-Meyer assessment for upper extremity in persons with chronic stroke with moderate-to-severe upper limb impairment. Front Neurol. 2020;11:577855. doi:10.3389/fneur.2020.577855
35. See J, Dodakian L, Chou C, et al. A standardized approach to the Fugl-Meyer assessment and its implications for clinical trials. Neurorehabil Neural Repair. 2013;27(8):732–741. doi:10.1177/1545968313491000
36. Mollayeva T, Thurairajah P, Burton K, Mollayeva S, Shapiro CM, Colantonio A. The Pittsburgh Sleep Quality Index as a screening tool for sleep dysfunction in clinical and non-clinical samples: a systematic review and meta-analysis. Sleep Med Rev. 2016;25:52–73. doi:10.1016/j.smrv.2015.01.009
37. Xiao M, Huang G, Feng L, et al. Impact of sleep quality on post-stroke anxiety in stroke patients. Brain Behav. 2020;10(12):e01716. doi:10.1002/brb3.1716
38. Liu F, Yang Y, Wang S, et al. Impact of sleep duration on depression and anxiety after acute ischemic stroke. Front Neurol. 2021;12:630638. doi:10.3389/fneur.2021.630638
39. Schmid AA, Kroenke K, Hendrie HC, Bakas T, Sutherland JM, Williams LS. Poststroke depression and treatment effects on functional outcomes. Neurology. 2011;76(11):1000–1005. doi:10.1212/WNL.0b013e318210435e
40. Ford CE, Malley D, Bateman A, Clare IC, Wagner AP, Gracey F. Selection and visualisation of outcome measures for complex post-acute acquired brain injury rehabilitation interventions. NeuroRehabilitation. 2016;39(1):65–79. doi:10.3233/NRE-161339
41. Naghavi FS, Koffman EE, Lin B, Du J. Post-stroke neuronal circuits and mental illnesses. Int J Physiol Pathophysiol Pharmacol. 2019;11(1):1–11.
42. Villain M, Cosin C, Glize B, et al. Affective prosody and depression after stroke: a pilot study. Stroke. 2016;47(9):2397–2400. doi:10.1161/STROKEAHA.116.013852
43. Adomavičienė A, Daunoravičienė K, Kubilius R, Varžaitytė L, Raistenskis J. Influence of new technologies on post-stroke rehabilitation: a comparison of Armeo Spring to the Kinect system. Medicina. 2019;55(4):98. doi:10.3390/medicina55040098
44. Wu P, Liu S. Clinical observation on post-stroke anxiety neurosis treated by acupuncture. J Tradit Chin Med. 2008;28(3):186–188. doi:10.1016/S0254-6272(08)60043-6
45. Aidar FJ, Jacó de Oliveira R, Gama de Matos D, et al. A randomized trial of the effects of an aquatic exercise program on depression, anxiety levels, and functional capacity of people who suffered an ischemic stroke. J Sports Med Phys Fitness. 2018;58(7–8):1171–1177. doi:10.23736/S0022-4707.17.07284-X
46. Rao V, Bergey A, Rosenberg P. Sertraline for treatment of post-stroke anxiety. J Neuropsychiatry Clin Neurosci. 2012;24(2):E22. doi:10.1176/appi.neuropsych.11060134
47. Golding K, Kneebone I, Fife-Schaw C. Self-help relaxation for post-stroke anxiety: a randomised, controlled pilot study. Clin Rehabil. 2016;30(2):174–180. doi:10.1177/0269215515575746
48. Zhang SH, Wang YL, Zhang CX, et al. Effect of interactive dynamic scalp acupuncture on post-stroke cognitive function, depression, and anxiety: a multicenter, randomized, controlled trial. Chin J Integr Med. 2022;28(2):106–115. doi:10.1007/s11655-021-3338-1
49. Amorim D, Amado J, Brito I, et al. Acupuncture and electroacupuncture for anxiety disorders: a systematic review of the clinical research. Complement Ther Clin Pract. 2018;31:31–37. doi:10.1016/j.ctcp.2018.01.008
50. Ma CH, Li LSW, Kwok LP, et al. A retrospective, cross sectional controlled study of Chinese medicine for post-stroke rehabilitation. J Neurol Sci. 2017;381:923. doi:10.1016/j.jns.2017.08.2595
51. Lefaucheur JP, Andre-Obadia N, Poulet E, et al. French guidelines on the use of repetitive transcranial magnetic stimulation (rTMS): safety and therapeutic indications. Neurophysiol Clin. 2011;41(5–6):221–295. doi:10.1016/j.neucli.2011.10.062
52. Sagliano L, Atripaldi D, De Vita D, D’Olimpio F, Trojano L. Non-invasive brain stimulation in generalized anxiety disorder: a systematic review. Prog Neuropsychopharmacol Biol Psychiatry. 2019;93:31–38. doi:10.1016/j.pnpbp.2019.03.002
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.