Back to Journals » Drug Design, Development and Therapy » Volume 17
The Efficacy and Safety of Remimazolam Besylate Combined with Esketamine for Outpatient Colonoscopy: A Prospective, Randomized, Controlled Clinical Trial
Authors Li W, Zhao J, Hao R, Wang S, Chen M, Liu H, Qi L, Hao Z
Received 3 July 2023
Accepted for publication 10 September 2023
Published 18 September 2023 Volume 2023:17 Pages 2875—2887
DOI https://doi.org/10.2147/DDDT.S425860
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Georgios Panos
Wei Li, Jun Zhao, Ruiping Hao, Shujuan Wang, Ming Chen, Huijun Liu, Le Qi, Zaijun Hao
Department of Anaesthesiology, Ordos Central Hospital, Ordos, Inner Mongolia Autonomous Region, People’s Republic of China
Correspondence: Zaijun Hao, Department of Anesthesiology, Ordos Central Hospital, Ordos, Inner Mongolia Autonomous Region, 017000, People’s Republic of China, Tel/Fax +8615204778880, Email [email protected]
Purpose: Evaluate the efficacy and safety of remimazolam besylate combined with esketamine for outpatient colonoscopy.
Patients and methods: A total of 150 outpatients undergoing colonoscopy were randomized into two groups. A MOAA/S score ≤ 3 was maintained. The primary outcome was the rate of successful colonoscopy completion. Time indicators, hemodynamic parameters, the consumption of lidocaine, esketamine, propofol and remimazolam besylate, MOAA/S scores and bispectral index (BIS) values, the lowest SpO2, body movement, the use of rescue medication, endoscopist and patient satisfaction, recall of the procedure, mini-mental state examination (MMSE), fatigue level and adverse events were recorded.
Results: Procedure completion was equivalent between groups (P > 0.05). Both induction and awakening times were significantly shorter in the P group (P < 0.05). There were no significant differences in colonoscopy time and discharge time (P > 0.05). The lowest SpO2 was significantly lower in the P group, while the level of fatigue was higher (P < 0.05). Patient satisfaction was significantly higher in the R group (P < 0.05). Endoscopist satisfaction was significantly higher in the P group (P < 0.05). There were no significant differences in both systolic and diastolic blood pressure between groups except at T5 and T6 (P > 0.05). Both HR and RR were significantly lower in the P group from T3 to T5 (P < 0.05). BIS values were significantly lower in the P group from T3 to T5, while MOAA/S was significantly lower in the P group at T3 and T4 (P < 0.05). Pain on injection was significantly higher in the P group (P < 0.05).
Conclusion: Remimazolam besylate has a similar efficacy to propofol when combined with subanesthetic doses of esketamine during outpatient colonoscopy. Remimazolam besylate combined with esketamine resulted in less injection pain and more stable hemodynamics, although it prolonged induction and awakening time.
Keywords: remimazolam besylate, propofol, esketamine, colonoscopy
Introduction
As the population ages and human disease changes, endoscopy has become the most widely used and effective diagnostic modality for gastrointestinal diseases.1 Colonoscopy is recommended for early cancer detection and prevention, which can significantly reduce mortality.2 Sedative and analgesic drugs through non-intravenous routes (such as oral anxiolytic medications combined with the use of lidocaine jelly) were previously widely used during colonoscopy. However, this strategy does not inhibit the perioperative stress response, which may hinder functional recovery and delay discharge.3 In order to reduce patient anxiety and discomfort, improve satisfaction and facilitate endoscopic procedures, it is crucial to select an appropriate anesthesia scheme that can achieve a good anesthetic effect while maintaining hemodynamic stability, especially for complex endoscopic gastrointestinal diagnosis and treatment procedures.4,5 However, the sedation rate for gastrointestinal endoscopy was only approximately 50% in China, which is far below that of the United States and Europe.6
The ideal sedative and analgesic drugs should take effect quickly and have a rapid offset, no accumulation, a good safety profile and optimal cost-efficacy.7 Most commonly used sedative and analgesic drugs, such as propofol, midazolam, etomidate, dexmedetomidine and opioids, have their own advantages and disadvantages.8 These drugs are therefore often used in combination during endoscopy. Propofol is the most widely used drug during endoscopic procedures due to its rapid onset of action and brief duration of effect. However, its flaws include dose-dependent cardiorespiratory depression, injection pain, its lack of analgesic properties, the possibility of infection and possible allergic reactions in patients with hypersensitivity to soy, peanut, and egg proteins.9 Propofol combined with opioids is a commonly used regimen because it minimizes the propofol dose and improves the safety of the procedure.10 However, adverse reactions to opioids, such as constipation, nausea, vomiting, and dizziness, can seriously affect patient early recovery.11
Remimazolam besylate, the newest type of water-soluble ultra-short acting benzodiazepine, has been successfully used during endoscopy and general anesthesia induction and maintenance.12 Remimazolam besylate acts on central γ-aminobutyric acid A (GABAA) receptors to stimulate chloride channel opening, induce cell membrane hyperpolarization and interfere with GABA reuptake in a manner similar to midazolam but with a greater potency and maximum effect. Neural activity is inhibited via increased chloride influx to produce sedation and hypnosis.13 With similar structural modifications to remifentanil, remimazolam besylate is metabolized by non-specific cholinesterases into a pharmacologically inactive metabolite in a process that is not affected by liver and kidney function.14 The pharmacokinetic characteristics of remimazolam besylate therefore include rapid onset and recovery, minimal tissue accumulation, early functional exercise and lighter inhibition of circulatory and respiratory function. Importantly, the sedative effects of remimazolam besylate can be reversed with flumazenil.
Esketamine, an N-methyl-D-aspartate receptor (NMDAR) antagonist and the S-enantiomer of ketamine, can provide more effective analgesic, anesthetic, anti-inflammatory and antidepressant effects than ketamine but with fewer dose-dependent side effects. Advantages of esketamine include its ability to maintain spontaneous breathing and its sympathomimetic properties.15 Previous work has concluded that esketamine is a more attractive sedative adjunct to propofol compared with opioids.16 Remimazolam combined with esketamine reduced the incidence of severe hypoxemia during gastrointestinal endoscopy in obese patients compared with propofol combined with esketamine.17 However, few studies have focused on the efficacy and safety of remimazolam besylate combined with esketamine during outpatient colonoscopy.
Methods
Patients
This study was designed according to the CONSORT 2010 statement. Written informed consent was obtained from all patients and their legal guardians. All procedures performed in this study were in accordance with the ethics committee of Ordos Central Hospital (No. 2022–018) and the 1964 Helsinki declaration and its later amendments. This trial was registered at chictr.org (ChiCTR2200066994).
Patients who underwent colonoscopy under sedation at our hospital from December 30, 2022 to March 30, 2023 were recruited for this study. Patients enrolled in this study met the following inclusion criteria: outpatient colonoscopy; age 35–65 years; and American Society of Anesthesiologists (ASA) physical classification I or II. Exclusion criteria were patients with abnormal liver or renal function; known cardiovascular, respiratory or endocrine disease; actively taking a monoamine oxidase inhibitor, sedative, analgesic, hypnotic, antipsychotic, antiemetic or antidepressant; addiction to tobacco or alcohol; known history of drug allergies; an anticipated difficult airway; body mass index (BMI) > 30 kg/m2; high risk of nausea and vomiting; pregnant or breastfeeding; involved in other clinical trials within the past three months; psychosocial disease or cognitive dysfunction; and the inability to cooperate or communicate.
Randomization and Blinding
A block randomization scheme was generated by an independent anesthesiologist who was not participating in this study using Microsoft Excel (Redwood WA, USA). The generated random allocation sequence was placed in a sequentially encoded, sealed, and opaque envelope. After confirming the eligibility of the subjects, the independent anesthesiologist who was also responsible for preparing the drugs required for the experiment opened the envelopes in order and assigned them to the corresponding experimental groups at a 1:1 ratio on the morning of the procedure. Patients and endoscopists were all blinded to group allocation.
Anesthetic Scheme
Patients fasted for 8h and were prohibited from drinking for 4 h prior to the procedure. After entering the room, venous access was established in the patient’s left arm and 250 mL of 0.9% normal saline was intravenously infused. All participants were positioned in the lateral decubitus position and received oxygen at a rate of 3 L/ min via nasal catheter until they woke up. Blood pressure, heart rate (HR), peripheral pulse oxygen saturation (SpO2) and respiratory rate (RR) were monitored routinely. An end-expiratory partial pressure of carbon dioxide (PetCO2) catheter was connected to the patient’s nostril to continuously monitor breathing. Both the bispectral index (BIS) and the modified observer assessment of alertness and sedation (MOAA/S) scales were used to monitor the depth of anesthesia. Penehyclidine 0.2 mg was intravenously administered to reduce salivation before anesthesia induction. 2% lidocaine 0.5 mg/kg and esketamine 0.15 mg/kg were intravenously administered over 30s before the administration of sedative drugs for both groups. One minute later, 0.15–0.25 mg/kg remimazolam besylate (R group) or 1–2 mg/kg propofol (P group) was intravenously injected at a rate of 1 mL/s followed by 1–2 mg/kg/h remimazolam besylate or 2–4 mg/kg/h propofol. The colonoscopy was started when MOAA/S was ≤3. BIS was maintained between 50–70 during the colonoscopy. In cases of anesthesia failure, a 0.02 mg/kg bolus of midazolam was the only allowed rescue sedative while 0.05 mg/kg esketamine was administered as additional analgesia in both groups.
Outcome
The primary outcome was the rate of successful colonoscopy completion. Procedure success was defined as (1) completion of the procedure, and (2) no requirement for an alternative and/or rescue sedative. Demographic data, time indicators (induction time, colonoscopy time, awakening time, discharge time), hemodynamic parameters and MOAA/S scores (recorded at the following time points: T1: arrival at the examination room, T2: after intravenous esketamine, T3: immediately after the start of the colonoscopy, T4: endoscope reached the cecum, T5: at the end of the colonoscopy, T6: 5 min after the colonoscopy, T7: 10 min after the colonoscopy, T8: hospital discharge), BIS values (recorded from T1 to T5), consumption of lidocaine, esketamine, propofol and remimazolam besylate, the lowest SpO2 during the colonoscopy, body movement, the use of rescue medication, endoscopist and patient satisfaction (measured using a 10-mm visual analog scale: 1 = completely dissatisfied and 10 = completely satisfied), recall of the procedure (assessed using the Brice questionnaire score 5 min after the patient was fully alert), Chinese revised version of the mini-mental state examination (MMSE) scale (assessed before sedation and 10 min after the patient was fully alert), fatigue level (graded using a 10-point numerical rating scale 15 min after the patient was fully alert: 1 = no fatigue and 10 = extreme fatigue) and adverse events (including respiratory depression, hypotension, hypertension, bradycardia, tachycardia, arrhythmia, injection pain, dizziness, nausea and vomiting and hallucinations) were also recorded.
Induction time was defined as the time from the first use of sedative drugs to the start of the colonoscopy. Colonoscopy time was defined as the time from the insertion of the endoscope to its removal. Awakening time was defined as the interval from the end of the colonoscopy to the patient being fully alert (MOAA/S = 5). Discharge time was defined as the time from the end of the colonoscopy to the patient achieving a modified postanesthetic discharge scoring system scale ≥ 9. Respiratory depression was defined as SpO2 <90% for more than 10s. Hypertension or hypotension were defined as a fluctuation higher or lower than 20% from the preoperative basal mean blood pressure (MBP) requiring treatment with 10 mg urapidil or 40 μg phenylephrine, respectively. 0.2 mg Atropine was administered if HR was < 50 bpm, while 10 mg esmolol was given if HR was >100 bpm. Hypoxia was treated with airway maneuvers such as chin lift, jaw thrust, adjustment of the patient’s head position, bag-mask ventilation and endotracheal intubation if needed.
Statistical Analysis
Sample size was based previous and our pilot study with PASS 15.0, in which the successful colonoscopy completion rate was 98.5% in the P group vs 96.0% in the R group.12 Each group required 68 patients in each group to achieve α=0.05 and β=0.2. Accounting for a dropout rate of 10%, we ultimately recruited 75 patients into each group.
Statistical analysis was performed using SPSS for Windows version 24.0 (SPSS Inc., Chicago, IL, USA). The Shapiro–Wilk test was used to assess data distribution and the Levene test was used to assess homogeneity of variance. Continuous variables in a normal distribution were presented as mean ± standard deviation (SD) while non-normal data were presented as median and interquartile range. Qualitative data were presented as number and frequency. Between-group comparisons of quantitative data were analyzed using either Student’s t-test or the Mann–Whitney U-test. Hemodynamic parameters, MOAA/S scores and BIS values were compared using repeated measures analysis of variance (ANOVA). Between-group comparisons of qualitative data were analyzed using the χ2 test or Fisher’s exact test. Time-to-event was evaluated using a Log rank test and summarized using Kaplan–Meier survival analysis. P < 0.05 was considered statistically significant.
Results
Patient Demographics and Baseline Characteristics
A CONSORT diagram was used during patient enrollment (Figure 1). A total of 319 outpatients who underwent colonoscopy under sedation from December 30, 2022 to March 30, 2023 were recruited. A total of 169 patients were excluded for the following reasons: 18 patients with abnormal liver or renal function; 21 with cardiovascular, respiratory or endocrine system diseases; 25 were taking a monoamine oxidase inhibitor, analgesic or hypnotic; 45 with addiction to tobacco or alcohol; 8 with a known drug allergy; 5 with an anticipated difficult airway; 14 with a BMI > 30 kg/m2; 19 with a high risk of nausea and vomiting; 3 were involved in other trials within the past three months; 7 had a psychosocial disease or cognitive dysfunction; and 4 were unable to cooperate or communicate. The remaining 150 patients were randomly divided into 75 patients in the R group and 75 patients in the P group. Demographic and baseline characteristics were comparable between the two groups (P > 0.05, Table 1).
 |
Table 1 Demographic and Baseline Characteristics of Patients |
 |
Figure 1 Patient enrollment flow diagram in line with CONSORT guidelines. |
Primary Outcome
There was no statistically significant difference in the successful completion of the colonoscopy procedure between the two groups (93.33%, 70/75 in the R group vs 98.67%, 74/75 in the P group, P = 0.209, Table 2). Two patients in the R group required two remedies while three patients in the R group and one patient in the P group required only one remedy.
 |
Table 2 Primary Outcome Between the Two Groups |
Secondary Outcomes
Compared with patients in the R group, both induction time and awakening time were significantly shorter in the P group (P < 0.05, Table 3, Figures 2 and 3). However, there were no significant differences in colonoscopy time and discharge time between the two groups (P > 0.05, Table 3).
 |
Table 3 Difference of Second Outcomes Between the Two Groups |
 |
Figure 2 Kaplan–Meier curves for time to induction. |
 |
Figure 3 Kaplan–Meier curves for time to wake up. |
Consumption of lidocaine and esketamine were similar between the two groups (P > 0.05, Table 3). Although the frequency of intraoperative body movement was higher in the R group, this difference was not statistically significant (P = 0.588, Table 3). Compared with patients in the R group, the lowest SpO2 was significantly lower in the P group, while the level of fatigue was higher in the P group (P < 0.05, Table 3). Patient satisfaction was significantly higher in the R group (P = 0.021, Table 3), while endoscopist satisfaction was significantly higher in the P group (P = 0.024, Table 3). Pre- and Post-MMSE and the use of both vasoactive drugs and rescue medications were similar between the two groups (P > 0.05, Table 3).
There were no significant differences in both systolic and diastolic blood pressures at any time points except for T5 and T6 (P > 0.05, Figure 4). Both HR and RR were significantly lower in the P group from T3 to T5 (P < 0.05, Figure 4). However, there were no significant differences in SPO2 between the two groups (P > 0.05, Figure 4).
 |
Figure 4 Perioperative hemodynamics. *P < 0.05 vs R group. |
BIS values were significantly lower in the P group from T3 to T5 while MOAA/S was significantly lower in the P group at T3 and T4 (P < 0.05, Figure 5).
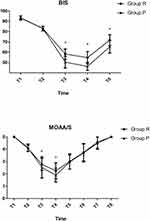 |
Figure 5 Perioperative sedation. *P < 0.05 vs R group. |
Safety Outcomes
The incidence of adverse events was lower in the R group. However, only pain on injection was significantly different between the two groups (P < 0.05; Table 4). All episodes were self-limited and did not require intervention.
 |
Table 4 Difference of Adverse Events Between the Two Groups |
Discussion
This study found that remimazolam besylate has similar efficacy to propofol when combined with subanesthetic doses of esketamine for outpatient colonoscopy. However, remimazolam besylate combined with esketamine had less injection pain and fatigue levels, more stable hemodynamics, and higher patient satisfaction, although it had prolonged induction and awakening times.
Remimazolam besylate exerts a sedative effect by directly acting on the aminobutyric acid type A receptor. It has been used for daytime surgery, precision anesthesia, enhanced recovery after surgery and in other fields such as intensive care medicine because of its unique pharmacologic characteristics that include a relatively high clearance rate, a small steady-state volume of distribution, faster half-life and early restoration of cognitive function.18 Though remimazolam seems to be an ideal sedative drug, it has no analgesic effects. It therefore needs to be combined with other anesthetic drugs.19 A previous study reported that remimazolam can be used in patients with varying degrees of renal impairment without any need for dose adjustment.20 However, logistic regression analysis demonstrated that patients aged > 79 years and who had a plasma albumin concentration of < 3.60 g/dl had increased extubation times, suggesting that lower doses of remimazolam should be administered to these patients during induction and maintenance of general anesthesia.21 Given this finding, we excluded patients with abnormal renal function for safety reasons. A previous study also reported that alcohol use could increase exposure to remimazolam by suppressing one of its major metabolizing enzymes (Carboxylesterase 1: CES1).22 Patients with a BMI > 30 kg/m2 were also at risk of severe hypoxemia.23 We therefore excluded these patients from this study.
Ketamine has positive sympathomimetic effects and can maintain hemodynamic stability and airway reflexes. It has long been considered the ideal choice for anesthesia and sedation. However, the side effects of ketamine, including nausea, vomiting and psychotomimetic effects, limit its use.24 Esketamine, the S+ isomer of ketamine, is about twice as potent as ketamine. It has a rapid onset of action, a strong anesthetic potency, a shorter awakening time and a relatively low risk of side effects (in particular mental complications).25 Esketamine is more suitable for sedation in patients undergoing colonoscopy than opioid drugs because it does not inhibit the μ receptor, thereby avoiding gastrointestinal obstruction.26
Early research reports and manufacturer instructions all suggest that remimazolam should be administered at a standard 5–7 mg initial dose without considering the impact of weight, with a 2.5 mg via intravenous injection over 15s as a rescue dose. However, nearly 30–70% of patients demonstrate obvious body movement reaction even with compound analgesia.27 Increasing the initial dose of remimazolam may be feasible. A previous study has reported that the tolerance of a single intravenous dose of remimazolam is up to 0.4–0.9 mg/kg, which suggests that it has a wide therapeutic window for endoscopic sedation. This indicates that remimazolam is useful for performing various procedures at different depths of anesthesia.28 The initial dose in this study was therefore administered according to the weight standard. A previous study suggested that 0.15mg/kg remimazolam besylate combined with 0.1ug/kg sufentanil adjuvant is better than propofol combined with sufentanil for patients undergoing gastroscopy.29 The ED50 and ED95 concentrations of remimazolam besylate were 0.174 mg/kg and 0.219 mg/kg for patients undergoing endoscopy.30 The initial dose of 0.15–0.25 mg/kg remimazolam besylate used in this study was in line with previous research findings. However, this administration method inevitably produced fluctuations in drug concentration and hemodynamics, especially in patients with moderate to deep sedation levels. Further, the context-sensitive half-time of remimazolam of about 7–8 min appeared to not be affected by infusion duration.31 We therefore opted for continuous sedative infusion in order to maintain a constant level of sedation during endoscopy.
The sedative effects of propofol are achieved through GABA A-mediated inhibition of histamine release in the hypothalamus, blockade of N methyl-D-aspartate receptors and modulation of calcium influx to inhibit postsynaptic neuronal activity.32 A previous study found that the EC50 of propofol was significantly lower in the esketamine 0.5 mg/kg group compared with the esketamine 0, 0.15, and 0.25 mg/kg groups. However, patients in the esketamine 0.5 mg/kg group had a higher incidence of esketamine-related adverse effects such as visual disturbances and significantly longer recovery times.33 Subanesthetic doses (0.15 mg/kg) of esketamine combined with propofol resulted in a significant reduction in propofol consumption compared with alfentanil among patients under sedation during endoscopic retrograde cholangiopancreatography. We therefore used esketamine 0.15 mg/kg in this study. Propofol consumption was significantly lower in this study compared with that reported by prior works, which may be due to the synergistic effects of esketamine and propofol.34 A previous study reported that it may be unreasonable to use BIS monitoring alone during sedation with esketamine.35 We therefore adopted the subjective indicator MOAA/S for this study. In contrast with the results of previous clinical trials, BIS was slightly higher in both groups, which may be due to the effects of esketamine on EEGs.36 In line with the results of previous studies, we found that both BIS and MOAA/S were significantly lower in the P group.37 Considering the undifferentiated success rate of the procedure, the frequency of intraoperative body movement and more stable hemodynamics, we believe that remimazolam besylate combined with esketamine may be the preferred modality for outpatient colonoscopy.
Previous systematic reviews and meta-analyses have reported that the sedative efficiency of remimazolam was significantly higher than that of midazolam, but slightly lower than that of propofol.38 However, we found no statistically significant difference in procedure success between the two experimental groups. In line with previous research results, we did find that hemodynamics differed between the groups. The reason for this may be because the dose of esketamine that was used in this study was lower and that the sympathetic excitatory effects of esketamine may be offset by the cardiovascular depression of remimazolam and propofol.39
Body movement in this study was significantly lower than that reported by prior studies, which may be because esketamine improved the tolerance and smooth placement rate of endoscope insertion.40 In line with the results of previous work, both induction and awakening times were significantly shorter in the P group. Endoscopist satisfaction was therefore significantly higher in the P group, which may lead to reduced endoscopist efficiency.41 Discharge time was similar between the two groups, which means that the turnover rate of endoscopic center beds is unchanged. Patient satisfaction was significantly higher in the R group, which may be due to lower levels of fatigue and injection pain. Complete recovery after discharge from colonoscopy is crucial for outpatients due to patient safety concerns. Future research will focus on the patient’s physical comfort and emotional state.
The most commonly reported adverse events (AEs) during endoscopy include cardiorespiratory depression, body movement, nausea, vomiting and dizziness. However, most of these AEs are mild and require no reversal treatment. There were no significant differences in treatment-related AEs between the two groups in this study.42 The severity of hypoxemia depends on both oxygen reserve capacity and the degree of respiratory center depression during sedation. The use of propofol is usually restricted to anesthetists because of its potential for profound sedative effects, in particular respiratory depression or apnea. Fewer respiratory depression events under deep sedation were recorded among patients who received remimazolam combined with alfentanil during endoscopic retrograde cholangiopancreatography compared with those who received propofol.43 However, our work failed to find a difference between our experimental groups. The reason for this may be related to the reduced consumption of both remimazolam besylate and propofol when combined with different analgesics. Further, the administration rate of esketamine, propofol and remimazolam besylate during induction was slower than in previous works. We also adopted PetCO2 during the colonoscopy, which has been recommended for patients under moderate or deep sedation because it can confirm the existence of apnea and airway obstruction earlier and more easily.44 Finally, it is possible to reduce the use of remedial sedative drugs and the accompanying risk of too deep a sedation by using both BIS and MOAA/S.
A previous cardiac electrophysiology study concluded that remimazolam does not prolong cardiac repolarization, although an increase in QTc interval may occur after bolus administration because of increased HR.45 We also found a difference in HR between our two groups. However, the incidence of tachycardia was similar between the two groups. Propofol induces cardiorespiratory suppression via central chemoreceptor sensitivity, reducing the systemic vascular resistance and myocardial suppression that are particularly common during moderate to severe sedation. It has been reported that ketamine-propofol combination therapy could decrease the rates of respiratory adverse effects and circulatory complications better than fentanyl-propofol.46 The most common airway intervention in this study was to adjust the patient’s head posture. No patients required bag-mask ventilation or endotracheal intubation. The incidence of psychiatric symptoms was also significantly lower, which may be due to the psychotic symptom inhibitory properties of propofol and remimazolam and the lower incidence of psychotropic adverse actions of esketamine.
There was no significant difference between the two groups in this study with respect to both pre- and post-MMSE. There was also no statistically significant difference in post-procedure MMSE scores compared with pre-procedure measurements. This is consistent with the findings of previous work, which reported an impaired memory acquisition stage but unchanged storage and retrieval stages following treatment with either remimazolam or propofol.47
Both lidocaine 0.05 mg/kg and esketamine 0.15 mg/kg were given intravenously before the administration of sedative drugs to reduce the incidence of injection pain. Despite this, the incidence of injection pain was significantly lower in the R group. This may be due to the water-soluble properties of remimazolam, which do not easily cause injection pain. Disagreeing with previous results, postoperative nausea and vomiting were equivalent between groups. This may be due to differences in study design and the lower consumption of propofol.41 We also excluded patients who were at a high risk of nausea and vomiting. Future experiments are necessary to evaluate differences in the incidence of postoperative nausea and vomiting between remimazolam and propofol.
This study has several limitations. First, we only recruited young patients with ASA I–II. However, the efficacy of remimazolam, its rapid onset of sedation and recovery of cognitive function and its lower rate of conscious sedation-related AEs should be warranted in elderly and critically ill patients. Second, this trial was a single-center study. More high-quality large-scale multi-center prospective studies are needed to validate the findings of this study. Third, due to differences in the appearance of the two drugs, this study may be influenced by subjective factors. Finally, this study did not record any relevant information about the cost-effectiveness of these treatments, especially in light of the high price of esketamine and remimazolam.
Conclusion
In conclusion, we found that remimazolam besylate has a similar efficacy to propofol when combined with subanesthetic doses of esketamine during outpatient colonoscopy. However, patients treated with remimazolam besylate combined with esketamine had less injection pain and more stable hemodynamics but longer induction and awakening times.
Data Sharing Statement
The authors intend to share participants’ data and associated documents acquired during the trial (including the study protocol, statistical analysis plan, informed consent forms, and clinical study report). Information will be available on request to the corresponding author by email.
Ethical Approval
All procedures performed on the patients were in accordance with the 1964 Helsinki declaration and its later amendments. The study was approved by the institutional review boards of Ordos Central Hospital.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Key research and development (R&D) Plan Project in Ordos City (NO.YF20232339).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Noh JH, Jung HY. Role of endoscopy in motility disorders of upper gastrointestinal tract. J Neurogastroenterol Motil. 2023;29(1):7–19. doi:10.5056/jnm22170
2. Xu H, Tang RSY, Lam TYT, et al. Artificial intelligence-assisted colonoscopy for colorectal cancer screening: a multicenter randomized controlled trial. Clin Gastroenterol Hepatol. 2023;21(2):337–346.e3. doi:10.1016/j.cgh.2022.07.006
3. Wang X, Zhang M, Sun H, et al. Dexmedetomidine-Oxycodone combination for conscious sedation during colonoscopy in obese patients: a randomized controlled trial. Heliyon. 2023;9(5):e16370. doi:10.1016/j.heliyon.2023.e16370
4. Hung KC, Yew M, Lin YT, et al. Impact of intravenous and topical lidocaine on clinical outcomes in patients receiving propofol for gastrointestinal endoscopic procedures: a meta-analysis of randomised controlled trials. Br J Anaesth. 2022;128(4):644–654. doi:10.1016/j.bja.2021.08.036
5. Janik LS, Stamper S, Vender JS, et al. Pro-con debate: monitored anesthesia care versus general endotracheal anesthesia for endoscopic retrograde cholangiopancreatography. Anesth Analg. 2022;134(6):1192–1200. doi:10.1213/ANE.0000000000005851
6. Zhou S, Zhu Z, Dai W, et al. National survey on sedation for gastrointestinal endoscopy in 2758 Chinese hospitals. Br J Anaesth. 2021;127(1):56–64. doi:10.1016/j.bja.2021.01.028
7. Dossa F, Medeiros B, Keng C, et al. Propofol versus midazolam with or without short-acting opioids for sedation in colonoscopy: a systematic review and meta-analysis of safety, satisfaction, and efficiency outcomes. Gastrointest Endosc. 2020;91(5):1015–1026.e7. doi:10.1016/j.gie.2019.12.047
8. Childers RE, Williams JL, Sonnenberg A. Practice patterns of sedation for colonoscopy. Gastrointest Endosc. 2015;82(3):503–511. doi:10.1016/j.gie.2015.01.041
9. Chen HY, Deng F, Tang SH, et al. Effect of different doses of dexmedetomidine on the median effective concentration of propofol during gastrointestinal endoscopy: a randomized controlled trial. Br J Clin Pharmacol. 2023;89(6):1799–1808. doi:10.1111/bcp.15647
10. Wang LL, Guan ZY, Wang CM, et al. A comparative study on the efficacy and safety of propofol combined with different doses of alfentanil in gastroscopy: a randomized controlled trial. J Anesth. 2023;37(2):201–209. doi:10.1007/s00540-022-03145-5
11. Seo B, Yang MS, Park SY, et al. Incidence and economic burden of adverse drug reactions in hospitalization: a prospective study in Korea. J Korean Med Sci. 2023;38(8):e56. doi:10.3346/jkms.2023.38.e56
12. Wang X, Hu X, Bai N, et al. Safety and efficacy of remimazolam besylate in patients undergoing colonoscopy: a multicentre, single-blind, randomized, controlled, phase III trial. Front Pharmacol. 2022;13:900723. doi:10.3389/fphar.2022.900723
13. Kilpatrick GJ. Remimazolam: non-clinical and clinical profile of a new sedative/anesthetic agent. Front Pharmacol. 2021;12:690875. doi:10.3389/fphar.2021.690875
14. Sheng XY, Liang Y, Yang XY, et al. Safety, pharmacokinetic and pharmacodynamic properties of single ascending dose and continuous infusion of remimazolam besylate in healthy Chinese volunteers. Eur J Clin Pharmacol. 2020;76(3):383–391. doi:10.1007/s00228-019-02800-3
15. Smith-Apeldoorn SY, Vischjager M, Veraart JK, et al. The antidepressant effect and safety of non-intranasal esketamine: a systematic review. J Psychopharmacol. 2022;36(5):531–544. doi:10.1177/02698811221084055
16. Chen J, Zou X, Hu B, et al. Effect of different doses of esketamine compared with fentanyl combined with propofol on hypotension in patients undergoing painless abortion surgery: a prospective, randomized, double-blind controlled clinical trial. BMC Anesthesiol. 2022;22(1):305. doi:10.1186/s12871-022-01848-6
17. Zhang K, Bao Y, Han X, et al. Effects of opioid-free propofol or remimazolam balanced anesthesia on hypoxemia incidence in patients with obesity during gastrointestinal endoscopy: a prospective, randomized clinical trial. Front Med. 2023;10:1124743. doi:10.3389/fmed.2023.1124743
18. Tang Y, Yang X, Yu Y, et al. Remimazolam besylate versus propofol for long-term sedation during invasive mechanical ventilation: a pilot study. Crit Care. 2022;26(1):279. doi:10.1186/s13054-022-04168-w
19. Paton DM. Remimazolam: a short-acting benzodiazepine for procedural sedation. Drugs Today. 2021;57(5):337–346. doi:10.1358/dot.2021.57.5.3264119
20. Stöhr T, Colin PJ, Ossig J, et al. Pharmacokinetic properties of remimazolam in subjects with hepatic or renal impairment. Br J Anaesth. 2021;127(3):415–423. doi:10.1016/j.bja.2021.05.027
21. Shimamoto Y, Sanuki M, Kurita S, et al. Factors affecting prolonged time to extubation in patients given remimazolam. PLoS One. 2022;17(5):e0268568. doi:10.1371/journal.pone.0268568
22. Pesic M, Schippers F, Saunders R, et al. Pharmacokinetics and pharmacodynamics of intranasal remimazolam-A randomized controlled clinical trial. Eur J Clin Pharmacol. 2020;76(11):1505–1516. doi:10.1007/s00228-020-02984-z
23. Kendale SM, Blitz JD. Increasing body mass index and the incidence of intraoperative hypoxemia. J Clin Anesth. 2016;33:97–104. doi:10.1016/j.jclinane.2016.03.020
24. Abdollahpour A, Saffarieh E, Zoroufchi BH. A review on the recent application of ketamine in management of anesthesia, pain, and health care. J Family Med Prim Care. 2020;9(3):1317–1324. doi:10.4103/jfmpc.jfmpc_875_19
25. Wang J, Huang J, Yang S, et al. Pharmacokinetics and safety of esketamine in Chinese patients undergoing painless gastroscopy in comparison with ketamine: a randomized, open-label clinical study. Drug Des Devel Ther. 2019;13:4135–4144. doi:10.2147/DDDT.S224553
26. Han C, Ji H, Guo Y, et al. Effect of subanesthetic dose of esketamine on perioperative neurocognitive disorders in elderly undergoing gastrointestinal surgery: a randomized controlled trial. Drug Des Devel Ther. 2023;17:863–873. doi:10.2147/DDDT.S401161
27. Zhang S, Wang J, Ran R, et al. Efficacy and safety of remimazolam tosylate in hysteroscopy: a randomized, single-blind, parallel controlled trial. J Clin Pharm Ther. 2022;47(1):55–60. doi:10.1111/jcpt.13525
28. Chen SH, Yuan TM, Zhang J, et al. Remimazolam tosilate in upper gastrointestinal endoscopy: a multicenter, randomized, non-inferiority, Phase III trial. J Gastroenterol Hepatol. 2021;36(2):474–481. doi:10.1111/jgh.15188
29. Cao Y, Chi P, Zhou C, et al. Remimazolam tosilate sedation with adjuvant sufentanil in Chinese patients with liver cirrhosis undergoing gastroscopy: a randomized controlled study. Med Sci Monit. 2022;28:e936580. doi:10.12659/MSM.936580
30. Zheng XS, Shen Y, Yang YY, et al. ED50 and ED95 of propofol combined with different doses of esketamine for children undergoing upper gastrointestinal endoscopy: a prospective dose-finding study using up-and-down sequential allocation method. J Clin Pharm Ther. 2022;47(7):1002–1009. doi:10.1111/jcpt.13635
31. Masui K, Stöhr T, Pesic M, et al. A population pharmacokinetic model of remimazolam for general anesthesia and consideration of remimazolam dose in clinical practice. J Anesth. 2022;36(4):493–505. doi:10.1007/s00540-022-03079-y
32. Chen L, Yang ZL, Cheng J, et al. Propofol decreases the excitability of cholinergic neurons in mouse basal forebrain via GABAA receptors. Acta Pharmacol Sin. 2019;40(6):755–761. doi:10.1038/s41401-018-0168-6
33. Feng M, Shi G, Cui W, et al. The median effective concentration of propofol in combination with different doses of esketamine during gastrointestinal endoscopy in adults. Front Pharmacol. 2022;13:1034236. doi:10.3389/fphar.2022.1034236
34. Eberl S, Koers L, van Hooft J, et al. The effectiveness of a low-dose esketamine versus an alfentanil adjunct to propofol sedation during endoscopic retrograde cholangiopancreatography: a randomised controlled multicentre trial. Eur J Anaesthesiol. 2020;37(5):394–401. doi:10.1097/EJA.0000000000001134
35. Herzer G, Mirth C, Illievich UM, et al. Analgosedation of adult patients with elevated intracranial pressure: survey of current clinical practice in Austria. Wien Klin Wochenschr. 2018;130(1–2):45–53. doi:10.1007/s00508-017-1228-5
36. Haaf M, Curic S, Rauh J, et al. Opposite modulation of the NMDA receptor by glycine and S-ketamine and the effects on resting state eeg gamma activity: new insights into the glutamate hypothesis of schizophrenia. Int J Mol Sci. 2023;24(3):1913. doi:10.3390/ijms24031913
37. Yu YH, Han DS, Kim HS, et al. Efficacy of bispectral index monitoring during balanced propofol sedation for colonoscopy: a prospective, randomized controlled trial. Dig Dis Sci. 2013;58(12):3576–3583. doi:10.1007/s10620-013-2833-4
38. Sneyd JR, Rigby-Jones AE. Remimazolam for anaesthesia or sedation. Curr Opin Anaesthesiol. 2020;33(4):506–511. doi:10.1097/ACO.0000000000000877
39. Xu C, He L, Ren J, et al. Efficacy and safety of remimazolam besylate combined with alfentanil in painless gastroscopy: a randomized, single-blind, parallel controlled study. Contrast Media Mol Imaging. 2022;2022:7102293. doi:10.1155/2022/7102293
40. Wang J, Hu W, Zhao X, et al. Sedative effect and safety of different doses of S-ketamine in combination with propofol during gastro-duodenoscopy in school-aged children: a prospective, randomized study. BMC Anesthesiol. 2022;22(1):346. doi:10.1186/s12871-022-01885-1
41. Guo L, Liu T, Zhang Y, et al. Effect of remimazolam versus propofol sedation on the quality of recovery after colonoscopy: a randomised, controlled, noninferiority trial. Eur J Anaesthesiol. 2022;39(12):953–955. doi:10.1097/EJA.0000000000001701
42. Choi JY, Lee HS, Kim JY, et al. Comparison of remimazolam-based and propofol-based total intravenous anesthesia on postoperative quality of recovery: a randomized non-inferiority trial. J Clin Anesth. 2022;82:110955. doi:10.1016/j.jclinane.2022.110955
43. Dong SA, Guo Y, Liu SS, et al. A randomized, controlled clinical trial comparing remimazolam to propofol when combined with alfentanil for sedation during ERCP procedures. J Clin Anesth. 2023;86:111077. doi:10.1016/j.jclinane.2023.111077
44. Skiljic S, Budrovac D, Cicvaric A, et al. Advances in analgosedation and periprocedural care for gastrointestinal endoscopy. Life. 2023;13(2):473. doi:10.3390/life13020473
45. Kleiman RB, Darpo B, Thorn M, et al. Potential strategy for assessing QT/QTc interval for drugs that produce rapid changes in heart rate: electrocardiographic assessment of the effects of intravenous remimazolam on cardiac repolarization. Br J Clin Pharmacol. 2020;86(8):1600–1609. doi:10.1111/bcp.14270
46. Bhattarai R, Hamal PK. Comparison of fentanyl-propofol and ketamine-propofol combination in induction and maintenance with intravenous anesthesia for short surgical procedures at moderate elevations. J Nepal Health Res Counc. 2021;18(4):769–775. doi:10.33314/jnhrc.v18i4.3323
47. Tan Y, Ouyang W, Tang Y, et al. Effect of remimazolam tosilate on early cognitive function in elderly patients undergoing upper gastrointestinal endoscopy. J Gastroenterol Hepatol. 2022;37(3):576–583. doi:10.1111/jgh.15761
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
