Back to Journals » International Journal of General Medicine » Volume 17
The Efficacy and Safety of Nirmatrelvir/Ritonavir Against COVID-19 in Elderly Patients
Authors Xiang Z, Wang Y , Qu Y, Lv B, Han J, Xu D, Fan K, Su C, Shen Z
Received 24 October 2023
Accepted for publication 9 January 2024
Published 30 January 2024 Volume 2024:17 Pages 297—304
DOI https://doi.org/10.2147/IJGM.S446335
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Zheng Xiang,* Yueyuan Wang,* Yuchen Qu, Bo Lv, Junping Han, Delai Xu, Kai Fan, Cunjin Su, Zhu Shen
Department of Pharmacy, The Second Affiliated Hospital of Soochow University, Suzhou, 215004, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Cunjin Su; Zhu Shen, Email [email protected]; [email protected]
Objective: To assess the key factors influencing the effectiveness of nirmatrelvir/ritonavir in treating elderly patients with COVID-19.
Methods: This study was conducted on patients aged ≥ 60 who were admitted to the Second Affiliated Hospital of Soochow University for COVID-19 infection and were treated with nirmatrelvir/ritonavir. Clinical information was collected from patients and steady-state blood concentrations of nirmatrelvir and ritonavir were measured. Factors associated with treatment effects were searched by univariate and multivariate analysis.
Results: A total of 68 (51 males and 17 females) patients had a median age of 80 (73.0– 84.8) years were enrolled in this study. The blood concentration measurements (trough concentrations) of nirmatrelvir and ritonavir were 5.1 (2.6– 7.1) and 0.4 (0.2– 0.9) μg/mL, respectively. Adverse drug reaction was reported in 4 (5.9%) patients. Univariate analysis showed that age, clinical classification, APACHE II score, total bilirubin (TBil), aspartate transaminase (AST), lactate dehydrogenase (LDH), and total cholesterol (TC) were significantly associated with the effectiveness of treatment (P value < 0.05). Concentration of nirmatrelvir was also associated with treatment outcome (P value < 0.1). Based on the results of univariate analysis, the above factors were introduced into the multiple linear regression equation as independent variables, and the results showed that clinical classification was included in the regression equation model and was the most important factor affecting the treatment outcome. By receiver operating characteristic curve analysis, the area under curve of age + biochemical indicators + APACHE II score + clinical classification was 0.968 (95% CI = 0.919– 1.000; P < 0.0001). Among the 68 patients included in the study, 4 cases experienced adverse drug reactions.
Conclusion: Age, clinical classification, APACHE II score, TBil, AST, LDH, and TC were significantly associated with the effectiveness of treatment in elderly patients with COVID-19.
Keywords: nirmatrelvir/ritonavir, COVID-19, elderly patients, effectiveness
Introduction
Coronavirus disease 2019 (COVID-19) is an infectious coronavirus disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1 As of November 2023, there have been over 63 million confirmed COVID-19 cases, and more than 6 million COVID-19–related deaths worldwide.2 Although a total of 1.2 billion vaccine doses have been administered, COVID-19 is still a huge threat to life due to the lack of effective medical treatment. Paxlovid (nirmatrelvir/ritonavir) is a novel oral antiviral medication developed by Pfizer for the treatment of COVID-19.3,4 This medication comprises nirmatrelvir, a protease inhibitor that specifically targets the SARS-CoV-2 3-chymotrypsin–like cysteine protease enzyme, and ritonavir, a CYP3A4 inhibitor that serves to inhibit the metabolism of nirmatrelvir, thereby extending its effective concentration in the body.5 On December 22, 2021, the Food and Drug Administration granted emergency use authorization for nirmatrelvir/ritonavir to be used in the treatment of mild-to-moderate COVID-19 cases in individuals with a high risk of progression to severe illness. These high-risk groups include individuals who are elderly (≥60 years of age), overweight or obese (BMI≥25 kg/m2), afflicted with chronic kidney disease, diabetes, immunosuppressive conditions, cardiovascular disease, hypertension, cancer, and other relevant comorbidities.6,7 Clinical data have shown that patients with risk factors are prone to progressing toward severe or potentially life-threatening conditions if they do not receive timely and effective treatment after being infected with COVID-19. Statistical data reveals an elevated mortality rate among elderly patients and those with critical illness.8–10 Consequently, timely and effective treatment administration is crucial in augmenting the survival rate of high-risk patients. To achieve this goal, measures must be implemented to ensure patients receive effective medication dosages while minimizing drug interactions and adverse reactions during treatment.
Therapeutic drug monitoring is a widely utilized clinical practice for monitoring the concentrations of drugs in patients’ bloodstream. It serves as a valuable tool in evaluating the correlation between drug concentrations and therapeutic effectiveness, thereby mitigating the occurrence of adverse drug reactions resulting from elevated drug concentrations.11 This approach assumes a critical role in facilitating precise drug administration and individualized medication strategies. However, due to the relatively short period since the introduction of nirmatrelvir/ritonavir to the market and the limited availability of clinical data, there remains insufficient information concerning its effectiveness and the incidence of adverse drug reactions during patient usage. Moreover, there is a lack of guidelines or studies elucidating the correlation between blood drug concentration and both effectiveness and adverse drug reactions.
To investigate whether blood drug concentration affects the therapeutic efficacy of nirmatrelvir/ritonavir in elderly patients with COVID-19 and identify the primary factors influencing treatment outcomes, we conducted a study involving the collection of clinical information from 68 hospitalized elderly patients diagnosed with COVID-19. Blood drug concentrations of nirmatrelvir and ritonavir were determined using our established methodology. Subsequently, we conducted an analysis to identify the primary factors impacting treatment effectiveness. Furthermore, we discussed the influence of these factors on the treatment of elderly patients with COVID-19 and the adverse drug reactions in this study.
Materials and Methods
Patients
This study was conducted with the approval of the medical science research ethics committee of the second affiliated hospital of Soochow University. All participants gave written informed consent, and the study was conducted according to the Declaration of Helsinki. According to the diagnostic criteria outlined in the “Diagnosis and Treatment of COVID-19 (Trial Version 10)”, all enrolled patients exhibited relevant clinical manifestations indicative of novel coronavirus infection, along with positive findings in pathogenic and serological examinations.12 Prior to enrollment, written informed consent was obtained from all elderly patients (age ≥60 years) who had been receiving nirmatrelvir/ritonavir therapy for a minimum of 48 hours to reach a steady state and underwent concentration measurements during the treatment period. Patients were excluded from the study if they had incomplete data, which hindered the assessment of outcome events; if the clinical diagnosis was unclear; if they had impaired kidney function with an estimated glomerular filtration rate (eGFR) < 60 mL/min; if they failed to adhere to the prescribed medication regimen; if they use contra-indicated medication, which has high dependency on CYP3A for clearance or potent CYP3A inducers; or if they did not adhere to the prescribed blood sample collection schedule. Following admission, all patients received regular treatment with nirmatrelvir/ritonavir. The medication was administered orally every 12 hours at a dose of 300 mg (150 mg × 2 tablets) in combination with ritonavir 100 mg (100 mg × 1 tablet) for a duration of 2 days until steady-state plasma concentrations were achieved. Approximately 2–3 mL of venous blood was drawn 30 minutes before the administration of the morning dose to measure the trough concentration (Cmin).
Acute Physiology and Chronic Health Evaluation II (APACHE II) score was calculated according to the physiological parameters and chronic health status of the patients at admission, and used to evaluate the severity of the patients’ clinical conditions at admission. Furthermore, we assessed the clinical classification of enrolled patients based on the criteria outlined in the ‘Diagnosis and Treatment of COVID-19 (Trial Version 10).12
Measurement of Nirmatrelvir/Ritonavir Concentration
Nirmatrelvir/ritonavir concentrations were quantified using a validated liquid chromatography tandem mass spectrometry (LC-MS/MS) method.13 Saquinavir was employed as the internal standard for this analysis. The separation of the analyte was achieved using a Thermo Hypersil GOLD C18 column (2.1 mm × 100 mm, 3 μm), and detection was performed using electrospray ionization in positive mode with full mass monitoring. The specific ions monitored for nirmatrelvir, ritonavir, and saquinavir were m/z 500.24792, 721.32004, and 671.39155, respectively. The lower limit of quantification was established at 78.1 ng/mL for nirmatrelvir and 15.6 ng/mL for ritonavir. The inter-run precision was found to be less than 15%, and the accuracy ranged from 87.45% to 104.63%.13
Outcome Definition
Treatment effectiveness classification: according to the “Diagnosis and Treatment Protocol for Novel Coronavirus Infection” issued by the Ministry of Health, patients were categorized into two groups based on clinical diagnosis and treatment outcomes. The treatment-effective group comprised patients who exhibited a notable improvement in their condition and tested negative for SARS-CoV-2 nucleic acid within 28 days after receiving the nirmatrelvir/ritonavir.
On the other hand, the treatment-ineffective group comprised patients with persistent or worsening symptoms with a hospital stay of more than 28 days, those who discontinued treatment and were discharged, and patients who succumbed to the disease. Definition of adverse reactions: adverse reactions related to nirmatrelvir/ritonavir were assessed by reviewing the original medical records of relevant patients and analyzing pre- and post-application test results. Management of drug adverse reactions: For patients with mild symptoms within their tolerance range, the dosage of the medication may be reduced or discontinued as advised by the physician. If necessary, appropriate medications can be administered to alleviate symptoms. For patients experiencing moderate to severe symptoms, immediate discontinuation of the drug is recommended, followed by targeted treatment and management as directed by the physician.
Statistical Analysis
Continuous variables were first determined using the normal distribution test homogeneity of variance test to determine whether the data conformed to a normal distribution and homogeneity of variance, with conformity expressed as mean ± standard deviation and non-conformity expressed as median (IQR). Categorical variables were expressed as frequencies and percentages. Univariate and multifactorial logistic regression models were used to analyze the potential factors affecting the prognosis of hospitalized patients. ROC curves were used to determine the efficacy of the screened potential factors for efficacy judgments. All statistical tests were two-sided, and P values less than 0.05 were considered statistically significant. GraphPad Prism 9+, version 9.2.0 (GraphPad Software, LLC., San Diego, USA) and IBM SPSS Statistics for Windows, version 26.0 (IBM Corp., Armonk, N.Y., USA) were used in our study.
Results
Patient Characteristics
A total of 124 patients were infected with SARS-CoV-2 and hospitalized during the study period, 68 of whom met the study eligibility criteria. The other 51 patients were excluded because of impaired kidney function with eGFR less than 45 mL/min, missing clinical data, discontinuation of medication for various reasons, age less than 60 years old, or nirmatrelvir concentration below the detection limit of 78.1 ng/mL. The demographic and clinical characteristics of the included patients were listed in Table 1. There were 51 males and 17 females, aged 61 to 93 years, with a mean age of 80 years. Major comorbid diseases included: obesity, diabetes, hypertension, hepatic insufficiency, and cancer. 4 cases of adverse drug reactions occurred. The blood concentration measurements (trough concentrations) of nirmatrelvir and ritonavir were 5.1 (2.6–7.1) and 0.4 (0.2–0.9) μg/mL, respectively. Of these 68 individuals, 48 had an outcome of discharge or remission and were classified in the effective group, while 20 had an outcome of exacerbation and death in 28 days (including discharge from treatment) and were classified in the ineffective group, the overall cure rate was about 70.6%. Clinical data between the groups were shown in Table 1.
 |
Table 1 Baseline Demographic and Clinical Characteristics of the Included Patients |
Factors Associated with Effectiveness
Logistic regression analysis was used to identify independent factors influencing effectiveness. The results of the univariate and multivariate analyses were presented in Table 2. The results of univariate analysis showed that age, clinical classification, APACHE II score, total bilirubin (TBil), aspartate transaminase (AST), lactate dehydrogenase (LDH), and total cholesterol (TC) were significantly associated with the effectiveness of treatment (P value <0.05). Cmin of nirmatrelvir was also associated with treatment outcome (P value <0.1). Based on the results of the univariate analysis, the above factors were introduced into the multiple linear regression equation as independent variables, and the results showed that clinical classification was included in the regression equation model and was the most important factor affecting the treatment outcome.
 |
Table 2 Univariate and Multivariate Analyses of Factors Influencing Effectiveness |
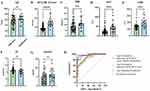
Figure 1A–G shows the distribution of age, APACHE II score, TBil, AST, LDH, TC and nirmatrelvir concentration in the effective and ineffective groups. The mean values of age, APACHE II score, TBil, AST, LDH and nirmatrelvir concentration in the effective group were significantly lower than those in the ineffective group. And the mean value of TC in the effective group was higher than that in the ineffective group. ROC curves are widely used to evaluate the sensitivity and specificity of biomarkers, and the relationship between sensitivity and specificity can be visualized.
Using the ROC curve (Figure 1H), the area under the curve (AUC) of biochemical indicators with significant differences (including Tbil, AST, LDH and TC) was 0.859 (95% confidence interval (95% CI) = 0.762–0.956; P < 0.0001), the AUC of age and biochemical indicators was 0.882 (95% CI = 0.803–0.962; P < 0.0001), the AUC of age + biochemical indicators + APACHE II score was 0.946 (95% CI = 0.885–1.000; P < 0.0001), the AUC of age + biochemical indicators + APACHE II score + clinical classification was 0.968 (95% CI = 0.919–1.000; P <0.0001), indicating that the combination of age, biochemical indicators (including Tbil, AST, LDH and TC), APACHE II score and clinical classification can well predict the treatment outcome of nirmatrelvir/ritonavir in elderly patients with COVID-19. Overall, the results of the ROC curve analysis showed that the use of clinical biochemical assays alone was less useful for the determination of nirmatrelvir/ritonavir efficacy than the addition of the patient’s APACHE II score and clinical staging.
Cases of Adverse Drug Reaction
Among the 68 patients included in the study, 4 cases experienced adverse drug reactions, and their clinical information is presented in Table S1. These four patients had comorbid conditions, including hepatic insufficiency, cancer, hypertension, and diabetes. The primary manifestations of adverse drug reactions observed were diarrhea, dizziness, sweating, and vomiting. Blood concentration measurements of nirmatrelvir and ritonavir were found to be low in all three patients except for Case 3. Diarrhea was the predominant adverse drug reaction symptom reported in Cases 1 and Cases 4. We hypothesized that the occurrence of diarrhea increased gastrointestinal motility, leading to rapid drug excretion without sufficient absorption, resulting in lower blood levels. Conversely, Case 3 exhibited a significantly higher than normal blood concentration, and the underlying cause for this elevated concentration remains unidentified. It is speculated that the elevated blood concentration in this case might have triggered gastrointestinal reactions and subsequent vomiting.
Discussion
Although WHO declares end to COVID-19 as a global health emergency on May 5, 2023, the novel coronavirus known as SARS-CoV-2 still exists in the population for a long time. During this study, the predominant variant strain was identified as the Omicron variant. This variant is characterized by rapid transmission, strong concealment, high infectivity, and a short incubation period, leading to widespread infections. A considerable number of elderly patients were hospitalized due to viral infection.14,15 Nirmatrelvir/ritonavir is extensively utilized as a first-line treatment for patients with mild-to-moderate COVID-19. This study is to explore whether blood drug concentration affects the therapeutic efficacy of nirmatrelvir/ritonavir in elderly patients with COVID-19 and identify the primary factors influencing treatment outcomes. Through the utilization of logistic regression, we identified age, clinical classification, APACHE II score, TBil, AST, LDH, and TC as risk factors affecting the treatment outcome of nirmatrelvir/ritonavir in elderly patients with COVID-19, with clinical classification being independent risk factors. In addition, through the ROC curve analysis, we found that these risk factors are helpful for the clinical efficacy of nirmatrelvir, especially the clinical test indicators combined with APACHE II score and clinical staging. The weakened immune system in most elderly patients, coupled with age-related metabolic and diminished repair functions, results in impaired drug absorption and metabolism, leading to relatively poorer treatment outcomes in the elderly population. The clinical classification of COVID-19 and the APACHE II score at admission are crucial factors influencing treatment outcomes. Clinical classification includes mild, moderate, severe, and critical cases. Different clinical classification has a significant impact on disease severity and the prognosis for patients.16,17 Older patients may be more likely to progress to critical cases, which might necessitate treatments like nirmatrelvir. However, treatment outcomes can vary depending on the patient’s immune status and other underlying health issues. Higher APACHE II scores are typically associated with more severe illness and higher mortality rates. Older patients often have more chronic health problems, potentially resulting in higher APACHE II scores.18 The concentration of LDH can reflect the extent of cell damage or inflammation. Elevated LDH levels in the treatment-resistant group may be attributed to the severity of the patients’ condition, indicating more cell damage and inflammation. It could also be associated with concurrent pulmonary infections, cardiac issues, or other comorbidities in the patients. Additionally, studies have shown that elevated LDH levels may be linked to liver dysfunction, hindering efficient drug metabolism, and ultimately leading to suboptimal therapeutic effects.19 TBil and AST are commonly used to assess liver function. In the ineffective group, higher levels of TBil and AST may result from the more severe COVID-19 infection, leading to impaired liver function.20 TC levels can be associated with various factors, including dietary habits, genetic predisposition, medication use, chronic diseases, and liver function. Lower TC levels in the ineffective group may reflect the patients’ nutritional status or liver dysfunction. Malnutrition or underlying liver issues in patients could potentially affect drug metabolism and efficacy.21
Surprisingly, the ineffective group exhibited higher nirmatrelvir concentrations compared to the effective group. We speculate that the ineffective group’s higher age and poorer liver function resulted in slower drug metabolism after administration, ultimately leading to higher drug concentrations. However, higher drug concentrations did not lead to improved treatment outcomes. We hypothesize several reasons for this. Firstly, varying viral loads: patients in the ineffective group may have had higher viral loads at the start of treatment, meaning that a higher drug concentration is needed to effectively suppress viral replication. In such cases, even with a higher blood drug concentration of nirmatrelvir, it may not reach the necessary level for effective viral suppression. Secondly, Disease Duration: overall, patients in the ineffective group exhibited a longer disease duration. Prolonged disease duration is associated with alterations in hepatic and renal function, protein binding rates, and intestinal absorption and permeability. These dynamic changes have the potential to impact blood drug trough concentrations. Thirdly, differential drug distribution: variations in patients’ physiological conditions can result in different drug distributions. Although the blood drug concentration is higher in the ineffective group, if the concentration at the site of infection is insufficient, it may not effectively inhibit viral replication. Lastly, the virus may develop resistance to nirmatrelvir. If the virus becomes resistant to the drug during treatment, even with a higher blood concentration, the drug may not effectively inhibit viral proliferation. Therefore, we believe that the higher blood drug concentration of nirmatrelvir in the ineffective group is a result of treatment outcomes, rather than the cause. Consequently, monitoring only the blood drug concentration of nirmatrelvir is insufficient for predicting treatment efficacy in patients. However, in cases of severe or critical clinical classification, combining it with other physiological and biochemical indicators can help predict the effectiveness of nirmatrelvir.
Common adverse reactions documented in the drug label for nirmatrelvir/ritonavir included diarrhea and taste disturbance, with occasional reports of indigestion, vomiting and muscle pain. Notably, all four reported cases of ADR in this study resulted in a complete recovery, with three of them having relatively low drug concentrations. Moreover, most patients with high drug concentrations did not experience ADR, suggesting the relatively high safety profile of nirmatrelvir/ritonavir. The medication regimen employed in this study strictly adhered to the instructions provided in the drug guide, ensuring compliance with drug combinations. None of the enrolled patients received concomitant administration of drugs known for their high dependency on CYP3A for clearance or potent CYP3A inducers. Nevertheless, caution must be exercised regarding potential drug interactions when using nirmatrelvir/ritonavir.22,23
This study is a single-center cohort study conducted in a real-world setting, and it is important to acknowledge the limitations of the study: (1) The study included a relatively small number of patients, which may restrict the generalizability of the findings. Therefore, further confirmation in a larger and independent population is warranted to enhance the external validity of the results. (2) Although patients were compared at baseline, the assessment of comorbid underlying conditions relied on crude statistics without considering the matching of disease severity scores to evaluate the severity of the disease. This approach may introduce bias and impact the accuracy of the results. (3) The study did not adequately account for the potential influence of non-drug factors on the outcomes or the impact of drug combinations. It is essential to consider these factors in future research to ensure a more comprehensive understanding of the results. To address these limitations and provide more robust evidence, future studies should involve large-scale clinical trials supported by valid and reliable data. Furthermore, prospective studies and stratified analyses considering different prognoses should be conducted to guide clinical practice.
Conclusion
The present study contributes evidence supporting the favorable therapeutic effectiveness of nirmatrelvir/ritonavir in elderly patients with COVID-19. The results reveal a correlation between advanced age, clinical classification, APACHE II score, TBil, AST, LDH, and TC were significantly associated with the effectiveness of treatment, underscoring the importance of closely monitoring clinical indicators in elderly patients with COVID-19. Additionally, the study findings suggest a relatively high level of safety associated with nirmatrelvir/ritonavir, as no severe adverse drug reactions were observed even in patients with high blood drug concentrations.
Data Sharing Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author Zhu Shen.
Funding
This work is supported by the National Natural Science Foundation of China (No. 82102486), Gusu Talent Program (GSWS2021022), and Jiangsu Research Hospital Association for Precision Medication (JY202032).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi:10.1038/s41586-020-2012-7
2. COVID-19 knowledge & data hub. Available from: https://www.geodoi.ac.cn/covid-19/index.aspx.
3. Harris E. FDA Grants full approval to paxlovid, COVID-19 antiviral treatment. JAMA. 2023;2023:1.
4. Hashemian SMR, Sheida A, Taghizadieh M, et al. Paxlovid (Nirmatrelvir/Ritonavir): a new approach to Covid-19 therapy? Biomed Pharmacother. 2023;162:114367. doi:10.1016/j.biopha.2023.114367
5. Marzi M, Vakil MK, Bahmanyar M, et al. Paxlovid: mechanism of Action, Synthesis, and In Silico Study. Biomed Res Int. 2022;2022:7341493. doi:10.1155/2022/7341493
6. Paxlovid. European medicines agency; 2022, Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/paxlovid.
7. US Food and Drug Administration. Emergency use authorization 105; 2022. Available from: https://www.fda.gov/media/155049/download.
8. Sun F, Lin Y, Wang X, et al. Paxlovid in patients who are immunocompromised and hospitalised with SARS-CoV-2 infection. Lancet Infect Dis. 2022;22(9):1279. doi:10.1016/S1473-3099(22)00430-3
9. Najjar-Debbiny R, Gronich N, Weber G, et al. Effectiveness of paxlovid in reducing severe coronavirus disease 2019 and mortality in high-risk patients. Clin Infect Dis. 2023;76(3):e342–e349. doi:10.1093/cid/ciac443
10. World Health Organization. WHO Coronavirus (COVID-19) Dashboard; 2022. Available from: https://covid19.who.int/.
11. Kang JS, Lee MH. Overview of therapeutic drug monitoring. Korean J Intern Med. 2009;24(1):1–10. doi:10.3904/kjim.2009.24.1.1
12. National Health Commission of the People’s Republic of China. Diagnosis and Treatment of COVID-19 (Trial Version 10). Chin J Rational Drug Use. 2023;20(01):1–11.
13. Zhao F, Xiang Z, Han J, et al. Simultaneous quantification of nirmatrelvir/ritonavir in human serum by LC-HRMS. J Pharm Biomed Anal. 2023;237:115796. doi:10.1016/j.jpba.2023.115796
14. Mohseni Afshar Z, Tavakoli Pirzaman A, Karim B, et al. SARS-CoV-2 Omicron (B.1.1.529) Variant: a Challenge with COVID-19. Diagnostics (Basel). 2023;13:3.
15. Ren SY, Wang WB, Gao RD, et al. Omicron variant (B.1.1.529) of SARS-CoV-2: mutation, infectivity, transmission, and vaccine resistance. World J Clin Cases. 2022;10(1):1–11. doi:10.12998/wjcc.v10.i1.1
16. Yan X, Han X, Peng D, et al. Clinical characteristics and prognosis of 218 patients with COVID-19: a retrospective study based on clinical classification. Front Med Lausanne. 2020;7:485. doi:10.3389/fmed.2020.00485
17. Suma LS, Anand HS, Vinod Chandra SS. Nature inspired optimization model for classification and severity prediction in COVID-19 clinical dataset. J Ambient Intell Humaniz Comput. 2023;14(3):1699–1711. doi:10.1007/s12652-021-03389-1
18. Mehryar HR, Yarahmadi P, Anzali BC. Mortality predictive value of APACHE II Scores in COVID-19 patients in the intensive care unit: a cross-sectional study. Ann Med Surg Lond. 2023;85(6):2464–2468. doi:10.1097/MS9.0000000000000641
19. Ergenc I, Capar E, Erturk SB, et al. Diagnostic performance of lactate dehydrogenase (LDH) isoenzymes levels for the severity of COVID-19. J Med Biochem. 2023;42(1):16–26. doi:10.5937/jomb0-37234
20. Yashashwini A, Vedavathi R. The study of de ritis (Ast/Alt) ratio in comparison with other parameters for predicting poor prognosis in covid 19 patients. J Assoc Physicians India. 2022;70(4):11–12.
21. Ressaire Q, Dudoignon E, Moreno N, et al. Low total cholesterol blood level is correlated with pulmonary severity in COVID-19 critical ill patients. Anaesth Crit Care Pain Med. 2020;39(6):733–735. doi:10.1016/j.accpm.2020.07.015
22. Arbel R, Wolff Sagy Y, Hoshen M, et al. Nirmatrelvir use and severe covid-19 outcomes during the omicron surge. N Engl J Med. 2022;387(9):790–798. doi:10.1056/NEJMoa2204919
23. Owen DR, Allerton CMN, Anderson AS, et al. An oral SARS-CoV-2 M(pro) inhibitor clinical candidate for the treatment of COVID-19. Science. 2021;374(6575):1586–1593. doi:10.1126/science.abl4784
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.