Back to Journals » International Journal of General Medicine » Volume 14
The Efficacy and Safety of Liraglutide 3.0 mg for Weight Management in Obese Non-Diabetic Saudi Outpatients
Authors Albaker W, Al Sheikh M , Albakr A, Alkhafaji D, Al Besher E, Al-Hariri M
Received 2 September 2021
Accepted for publication 8 November 2021
Published 23 November 2021 Volume 2021:14 Pages 8643—8650
DOI https://doi.org/10.2147/IJGM.S336904
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Waleed Albaker,1 Mona Al Sheikh,2 Aishah Albakr,3 Dania Alkhafaji,1 Eman Al Besher,4 Mohammed Al-Hariri2
1Department of Internal Medicine, College of Medicine, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia; 2Department of Physiology, College of Medicine, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia; 3Department of Neurology, College of Medicine, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia; 4Department of Family Medicine, Ministry of Health, Dammam, Saudi Arabia
Correspondence: Mohammed Al-Hariri
Department of Physiology, College of Medicine, Imam Abdulrahman Bin Faisal University, P.O. Box 2114-31451, Dammam, Saudi Arabia
Tel +966 50-727-5028
Email [email protected]
Background: Unmanaged cases of obesity might lead to serious conditions and complications, which impair patients’ lives. The aim of this study was to assess the effectiveness and safety of daily 3 mg subcutaneous (s/c) Liraglutide amongst obese non-diabetic patients in Eastern Province, Saudi Arabia.
Methods: A retrospective cohort study of obese non-diabetic Saudi patients with obesity managed with s/c Liraglutide 3.0 mg who visited the outpatient clinic in Al Mashfa Hospital, Al Khobar, KSA during 2019– 2021. We collected patient data from the electronic reporting system for different parameters. Body weight, hemoglobin A1c %, systolic and diastolic blood pressure mmHg were obtained at baseline and after the intervention.
Results: Records of 258 patients who were using a daily dose of Liraglutide 3.0 mg s/c for at least four months have been reviewed. The body weight loss of patients who used Liraglutide for four months was 8.1± 0.8 kg. Moreover, around 204 patients continued for up to six months. Meanwhile, the mean body weight loss was 13 kg. There was a significant reduction of hemoglobin A1c (HbA1c) % by 0.43%. The majority of patients (94.5%) reported satisfaction with the treatment, while adverse events were mainly nausea, vomiting and constipation.
Conclusion: Daily s/c Liraglutide of 3.0 mg is effective in producing significant body weight reduction in obese non-diabetic Saudi patients with tolerable minimal side effects and may provide health benefits in terms of reduced risk of obesity and its related outcomes.
Keywords: obesity, Liraglutide, weight loss, Saudi
Introduction
Obesity is defined as excessive or irregular fat accumulation. It is one of the major chronic health problems increasing day by day and has negative effects on work productivity, quality of life, and health-care costs,1 as well as well-known risk factors for cardiometabolic morbidity2 and overall mortality.3 Furthermore, obesity is associated with up to several years of lost lifespan.3 Recently, obesity has been found to be an accelerator of severe COVID-19 and might adversely influence the efficacy of COVID-19 vaccines.4
Additionally, obesity has become a critical health problem and a cause of concern worldwide.5 The global figure for obesity is growing at an alarming rate; according to a recent estimation, it is approaching 573 million by 2030.6 According to a recent report, the prevalence of obesity was 35% in Saudi Arabia.7
According to the guidelines of obesity treatment, the use of pharmacologic therapy is indicated when the body mass index (BMI) ≥30 kg/m or in patients with a BMI ≥ 27 kg/m with at least one metabolic syndrome component (eg, insulin resistance, dyslipidemia, hypertension, and visceral obesity).8
The treatment regimen for obese patients with or without type 2 diabetes recommends lifestyle interventions (weight loss, increased physical activity and dietary modification) together with early use of metformin for glycemic control. Unfortunately, many clinical trials have demonstrated the failure of this approach and recommended new and more efficacious treatments to control body weight as well as to prevent the disease progression and its associated complications.9
Liraglutide is a glucagon-like peptide-1 receptor agonist that has been successfully reduced blood glucose and body weight. The United States Food and Drug Administration approved Liraglutide 3 mg as a treatment for obesity in December 2014.10
Data demonstrated that Liraglutide can attain weight loss of ≥5–10% from baseline.11 At the same line, in a previous study at single-center, open-label showed that with Liraglutide 3 mg in combination with intensifying behavioural and diet therapy, 28% of subjects were able to achieve 15% weight loss in one year, compared to 12% with the only intensive behavioural therapy.12
Liraglutide is available by subcutaneous injection, averaging a cost of over $1000/month.8 However, Liraglutide treatment is not associated with higher total direct healthcare costs when compared with the clinical effectiveness that is maintained for long term (up to five years), reduced incidence of diabetes and/or its related complications and improvements in health-related quality of life.13,14
Unfortunately, COVID-19 (coronavirus disease 2019) restrictions and protocols (such as limiting physical activity, isolation, reduced access to health care resources) increased depression and anxiety, disruptions in eating habits and frequent snacking have arisen as an extra burden and challenges for obesity control.15 Thus, the management of obesity in Saudi Arabia is considered a public health priority.15 Knowing that the treatment of obesity after it has been established is very difficult, especially when there is some controversy on the available modern treatment of obese patients.16 Therefore, in this study, we aimed to investigate the clinical effectiveness of daily subcutaneous injection of Liraglutide 3.0 mg treatment on weight control among a sample of obese non-diabetic patients in Saudi Arabia.
Materials and Methods
Participants
This is a retrospective cohort study, the patient records-based study that was conducted in endocrine clinics at Al-Mashfa hospital in Al-Khobar city, Saudi Arabia, in 2019–2021. We evaluated 258 patients taking s/c daily Liraglutide 3.0 mg for four-six months.
Inclusion criteria included all Saudi adults who are more than 18 years old, non-diabetic patients with or without complications, BMI more than 30 kg/m2, and are using daily s/c Liraglutide 3.0 mg for more than four months period before enrolment in the study. Patients were excluded from the study if they had previously participated in the current or any other clinical study six months before or 12 months after the first Liraglutide prescription.17
The Ethics Committee of Imam Abdulrahman Bin Faisal University, Institutional Review Board (IRB) approved the proposal (IRB-2021-01-178), which adhered to the Declaration of Helsinki. The informed consent form was obtained from all participants prior to the commencement of the study.
Measurements
We collected patient data from the electronic reporting system for different parameters. Body weight, hemoglobin A1c (HbA1c) %, systolic (SBP) and diastolic (DBP) blood pressure mmHg were obtained at baseline and after the intervention.
Safety was also addressed by collecting adverse events within the patient’s using time and patient satisfaction through a validated questionnaire, which was measured to assess the patient’s satisfactory rate after management.
Data Collection Tools
The assessment of obesity was taken using standardized equipment. Participants were requested to remove their shoes and wear light clothing before taking their measurements. The height of the students was documented to the nearest 0.1 cm, and the weight was calculated to the nearest 0.1 kg (Seca 704; Seca, Hamburg, Germany).2
Venous blood samples were extracted from an antecubital vein under the aseptic condition after overnight fasting of at least 10 hours. Analyses of the blood samples were performed by the hospital laboratory to measure hemoglobin A1c (HbA1c).
Two blood pressure records were taken “after 5 min of rest” using an automatic monitor (Omron M6 Comfort IT), and the mean of the blood pressure recordings was considered for the data analysis.18
The primary endpoint is to study the clinical effectiveness of daily s/c Liraglutide 3.0 mg for a period of four to six months in reducing body weight (mean average weight loss). Secondary endpoints include follow up the change in BMI, HbA1c% and blood pressure (SBP & DBP). Safety aspects were also collected and patients’ satisfaction rate.
Statistical Analysis
Data were analyzed using SPSS (SPSS 26, Inc., Chicago, IL, USA). Descriptive statistics (frequency distribution of nationality, gender, age, body weight, height, BMI, SBP, and DBP) were reported as a percentage or mean ± standard deviation (SD), as appropriate. Comparative statistics of continuous variables were compared using a multivariate generalized linear model repeated measure (GLM-ANOVA). P values below 0.05 were used as an indicator of statistical significance.
Results
Baseline Characteristics
A total of 258 patients were included in the study. Baseline characteristics are shown in Table 1. Most of the recruited sample were females (88%), on average 37.8±10 years old and overly obese (BMI = 34.7±9). Mean baseline SBP and DBP values were 122±1 and 77.2± 0.6, respectively.
 |
Table 1 Baseline Characteristics of the Patients |
Effect of Liraglutide on Body Weight
The majority of patients (n=204, 79%) completed six months of Liraglutide. On average, the body weight loss of patients who used Liraglutide for four months was 8.1±0.8 kg when compared with baseline value (88.8±16 to 80.7±16), and it was significant (p˂0.0001), as presented in Figure 1. Meanwhile, patients on Liraglutide for six months showed a lower significant weight loss (13 kg, p˂0.0001) over the course of six months compared to the baseline (Figure 1).
 |
Figure 1 Mean body weight changes of the study patients through the baseline and the follow-up periods of the study (four and six months). #p˂0.0001. |
BMI mean significantly decreased (34.4±5 to 31.3±5 kg/m2, p<0.0001) from the baseline value in four-month group therapy. BMI was significantly decreased (23.1± 12 kg/m2 p<0.0001) when compared with baseline (Figure 2).
Effect of Liraglutide on Glycemic Control
The analysis showed a significant reduction in HbA1c % (0.4±0.05, p<0.0001) after three months of follow-up (4.5±0.6%) compared to the baseline value (4.9±0.5) as presented in Figure 3.
 |
Figure 3 Mean hemoglobin A1c (HbA1c) % changes of the study patients through the baseline and three months follow-up. #p˂0.0001. |
Effect of Liraglutide on Blood Pressure
Liraglutide 3.0 mg/d was associated with a significant improvement in the SBP values from basal value during the four and six months follow-up (122±14 to 118 and 119 mmHg, respectively) with p˂0.0001. However, Liraglutide 3.0 mg/d slightly increased DBP by nearly 2 mmHg (from 77.5 to 79.8 and 79.7 mmHg, p=0.005) as shown in Figure 4.
 |
Figure 4 Mean systolic (SBP) and diastolic (DBP) blood pressure changes of the study patients through the baseline and periods of the study (four and six months). #p˂0.0001: *p=0.005. |
Differences of the SBP, DBP, BMI and HbA1c by Gender
Comparisons between the basal and final SBP, DBP, BMI and HbA1c showed no statistical differences between female and male participants (p>0.05), as presented in Table 2.
 |
Table 2 Differences of the SBP, DBP, BMI and HbA1c by Gender |
Satisfaction Response
The present study showed that the majority of patients (94.5%) reported satisfaction with the treatment (Figure 5).
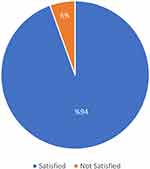 |
Figure 5 Satisfaction rate of the study patients with Liraglutide treatment. |
Reported Side Effects
The reported adverse events were mainly nausea (14%), vomiting (15.3%), and constipation (9.9%). There were no cases of gastroenteritis, H. Pylori or diarrhoea (Figure 6). There were no mood swings, headache, hair loss, or urinary tract infections. Also, no patients have reported vising the emergency care department during the treatment.
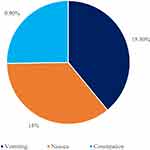 |
Figure 6 Mean reported side effect of Liraglutide. |
Discussion
To the best of our knowledge, this is the first study to assess the efficacy of Liraglutide for weight management in obese non-diabetic Saudi patients. The results of this retrospective study supported the expected hypothesis. Our findings showed improvement in body weight loss as well as glucose control by using 3 mg of liraglutide s/c injection.
The results from this study seem consistent with other results done testing similar subjects.19,20 Data support the potential benefit of Liraglutide among obese non-diabetic Saudi patients in a dose-dependent manner with once-daily doses of 3.0 mg, as patients have experienced a significant weight loss from baseline. In a recent study, Liraglutide 3.0 mg has shown a significant weight reduction in participants with obesity and a poor response to only lifestyle therapy.21 Another clinical study has had a follow-up of one hundred and seventeen post-bariatric surgery patients, over seven months taking Liraglutide 3.0 mg. Patients lost a statistically significant amount of weight regardless of the type of surgery they had. This significant decrease in weight was sustained after one year of taking Liraglutide 3.0 mg.22
The increase in the body visceral fat is not only strongly associated with lipotoxicity (deposition of ectopic lipids in vital organs, such as the arteries, heart, pancreas, skeletal muscle, and liver) but also enhances the release of fatty acids into the circulation, dysregulated adipokine, subclinical inflammation, which in turn can contribute to the pathophysiology of cardiometabolic disorders.23
Liraglutide likely induces satiety by preserving free leptin levels, as well as activating some areas in the hindbrain24 that improve the cardiometabolic as well as atherosclerotic vascular profiles.25 Furthermore, many recent randomized controlled trial studies showed a significant reduction in body weight compared to those in the placebo group using Liraglutide.21,26 Alternatively, Liraglutide has been suggested to protect against the pre-diabetes state.24 Meanwhile, despite the clinical effectiveness in the control of body weight and glucose, a local study could not find a significant effect on the body weight control by Liraglutide, which could be attributed to the smaller sample size16 or weight gain effect of the insulin and Sulphonylurea.27
Liraglutide acts to restore glucose-dependent insulin secretion,28 thus the reported findings in our sample on the improvement of the body weight have reflected positively on the glycemic control. After accounting for baseline HbA1c, the study showed a significant reduction in HbA1c % value after three months of follow-up. Several studies have reported a significant glycemic control success.17,29 On the other hand, the collected data disagree with other studies that have been conducted on a small sample with available HbA1c values,16,22 that cannot be extrapolated to every patient.
The present results reported a significant reduction in the SBP values, similar to what was observed in previous studies.22,30 Meanwhile, our findings found a small significant increase in the DBP. This is in accordance with another clinical trial on the effects of Liraglutide on DBP.29 Furthermore, a meta-analysis of randomized controlled trials evaluated the effect of Liraglutide on blood pressure found no consistent pattern on DBP.31 The same author attributed the bias risk of these studies to the limitation of sample size and lack of clinical trials on the effect of Liraglutide on blood pressure in patients with and without hypertension.31 However, this effect could be attributed to an increase in heart rate effect induced by liraglutide.32
The study results revealed that most of the reported adverse effects were mainly vomiting, followed by nausea and constipation. Overall, Liraglutide is well tolerated than other hypoglycemic agents, and the main adverse effects were related to the gastrointestinal system.33 However, the most frequently reported adverse events were mild or moderate nausea and diarrhea.34
Limitation
This study was a retrospective cohort study, collected from already existing patient data using the electronic reporting system; the data included more females and did not provide complete HbAlc % data after six months for study participants. Data regarding body fat changes during the study period were not collected.
Conclusion
Daily subcutaneous injection of Liraglutide 3.0 mg was an effective and safe therapy in reducing body weight among the adult obese Saudi non-diabetic population and may provide health benefits in terms of reduced risk of obesity and its related outcomes.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Acknowledgment
We thank the support of the Al-Mashfa hospital in Al-Khobar city, Saudi Arabia, for making this research possible.
Author Contributions
All authors made a significant contribution to the work reported, whether in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
No funding to declare.
Disclosure
The authors declare no conflicts of interest in this work and that there is no conflict of interest regarding the publication of this article.
References
1. Chooi YC, Ding C, Magkos F. The epidemiology of obesity. Metabolism. 2019;92:6–10. doi:10.1016/j.metabol.2018.09.005
2. Albaker W, El-Ashker S, Baraka MA, El-Tanahi N, Ahsan M, Al-Hariri M. Adiposity and cardiometabolic risk assessment among university students in Saudi Arabia. Sci Prog. 2021;104(1):003685042199853. doi:10.1177/0036850421998532
3. Bhaskaran K, dos-Santos-Silva I, Leon DA, Douglas IJ, Smeeth L. Association of BMI with overall and cause-specific mortality: a population-based cohort study of 3·6 million adults in the UK. Lancet Diabetes Endocrinol. 2018;6(12):944–953. doi:10.1016/S2213-8587(18)30288-2
4. Stefan N, Birkenfeld AL, Schulze MB. Global pandemics interconnected — obesity, impaired metabolic health and COVID-19. Nat Rev Endocrinol. 2021;17(3):135–149. doi:10.1038/s41574-020-00462-1
5. Al-Hariri MT, Elkilany AM, Alkahtani SA. Effects of potentially modifiable risk factors on the health of adults in the Eastern Province of KSA. J Taibah Univ Med Sci. 2018;13(1). doi:10.1016/j.jtumed.2017.08.0026
6. Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes. 2008;32(9):1431–1437. doi:10.1038/ijo.2008.102
7. Habbab RM, Bhutta ZA. Prevalence and social determinants of overweight and obesity in adolescents in Saudi Arabia: a systematic review. Clin Obes. 2020;10(6):e12400. doi:10.1111/cob.12400
8. Nuffer WA, Trujillo JM. Liraglutide: a new option for the treatment of obesity. Pharmacotherapy. 2015;35(10):926–934. doi:10.1002/phar.1639
9. Deacon CF. Potential of Liraglutide in the treatment of patients with type 2 diabetes. Vasc Health Risk Manag. 2009;5(1):199–211. doi:10.2147/vhrm.s4039
10. Monami M, Dicembrini I, Marchionni N, Rotella CM, Mannucci E. Effects of glucagon-like peptide-1 receptor agonists on body weight: a meta-analysis. Exp Diabetes Res. 2012;2012:672658. doi:10.1155/2012/672658
11. Davies MJ, Bergenstal R, Bode B, et al. Efficacy of Liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. JAMA. 2015;314(7):687–699. doi:10.1001/jama.2015.9676
12. Wadden TA, Walsh OA, Berkowitz RI, et al. Intensive behavioral therapy for obesity combined with Liraglutide 3.0 mg: a randomized controlled trial. Obesity. 2019;27(1):75–86. doi:10.1002/oby.22359
13. Kolotkin RL, Gabriel Smolarz B, Meincke HH, Fujioka K. Improvements in health-related quality of life over 3 years with Liraglutide 3.0 mg compared with placebo in participants with overweight or obesity. Clin Obes. 2018;8(1):1–10. doi:10.1111/cob.12226
14. Wilke T, Mueller S, Fuchs A, Kaltoft MS, Kipper S, Cel M. Diabetes-related effectiveness and cost of Liraglutide or insulin in German patients with type 2 diabetes: a 5-year retrospective claims analysis. Diabetes Ther. 2020;11(10):2357–2370. doi:10.1007/s13300-020-00903-0
15. Alfaris N. Management of obesity in Saudi Arabia during the era of COVID-19: a clash of two pandemics. Obesity. 2021;29(1):18. doi:10.1002/oby.23055
16. Albarkah Y, Tourkmani A, Bin Rsheed A, Al Harbi T, Ebeid Y, Bushnag R. Effects of Liraglutide addition to multiple diabetes regimens on weight and risk of hypoglycemia for a cohort with type 2 diabetes followed in primary care clinics in Saudi Arabia. J Fam Med Prim Care. 2019;8(6):1919–1924. doi:10.4103/jfmpc.jfmpc_372_19
17. Price H, Blüher M, Prager R, Phan TM, Thorsted BL, Schultes B. Use and effectiveness of a fixed-ratio combination of insulin degludec/liraglutide (IDegLira) in a real-world population with type 2 diabetes: results from a European, multicentre, retrospective chart review study. Diabetes Obes Metab. 2018;20(4):954–962. doi:10.1111/dom.13182
18. El-Ashker S, Pednekar MS, Narake SS, Albaker W, Al-Hariri M. Blood pressure and cardio-metabolic risk profile in young Saudi males in a university setting. Med. 2021;57(8):755. doi:10.3390/medicina57080755
19. Clements JN, Shealy KM. Liraglutide: an injectable option for the management of obesity. Ann Pharmacother. 2015;49(8):938–944. doi:10.1177/1060028015586806
20. Grabarczyk TR, Wissman NK. Weight outcomes with empagliflozin as compared with Liraglutide in veterans with type 2 diabetes mellitus. Ann Pharmacother. 2020;49(8):938–944. doi:10.1177/1060028020915791
21. Kelly AS, Auerbach P, Barrientos-Perez M, et al. A randomized controlled trial of Liraglutide 3.0 mg for weight management in adolescents with obesity. Horm Res Paediatr. 2020;93(10):164. doi:10.1159/000508500
22. Wharton S, Liu A, Pakseresht A, et al. Real-world clinical effectiveness of Liraglutide 3.0 mg for weight management in Canada. Obesity. 2019;27(6):917–924. doi:10.1002/oby.22462
23. Stefan N. Causes, consequences, and treatment of metabolically unhealthy fat distribution. Lancet Diabetes Endocrinol. 2020;8(7):616–627. doi:10.1016/S2213-8587(20)30110-8
24. Iepsen EW, Torekov SS, Holst JJ. Liraglutide for type 2 diabetes and obesity: a 2015 update. Expert Rev Cardiovasc Ther. 2015;13(7):753–767. doi:10.1586/14779072.2015.1054810
25. Ussher JR, Drucker DJ. Cardiovascular actions of incretin-based therapies. Circ Res. 2014;114(11):1788–1803. doi:10.1161/CIRCRESAHA.114.301958
26. Pi-Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of Liraglutide in weight management. N Engl J Med. 2015;373(1):11–22. doi:10.1056/nejmoa1411892
27. Cheng V, Kashyap SR. Weight considerations in pharmacotherapy for type 2 diabetes. J Obes. 2011;2011:984245. doi:10.1155/2011/984245
28. Juhl CB, Hollingdal M, Sturis J, et al. Bedtime administration of NN2211, a long-acting GLP-1 derivative, substantially reduces fasting and postprandial glycemia in type 2 diabetes. Diabetes. 2002;51(2):424–429. doi:10.2337/diabetes.51.2.424
29. Marso SP, Poulter NR, Nissen SE, et al. Design of the Liraglutide effect and action in diabetes: evaluation of cardiovascular outcome results (LEADER) trial. Am Heart J. 2013;166(5):823–830. doi:10.1016/j.ahj.2013.07.012
30. Russo GT, Labate AM, Giandalia A, et al. Twelve-month treatment with Liraglutide ameliorates visceral adiposity index and common cardiovascular risk factors in type 2 diabetes outpatients. J Endocrinol Invest. 2015;38(1):81–89. doi:10.1007/s40618-014-0163-9
31. Zhao X, Huang K, Zheng M, Duan J. Effect of Liraglutide on blood pressure: a meta-analysis of Liraglutide randomized controlled trials. BMC Endocr Disord. 2019;19(1):4. doi:10.1186/s12902-018-0332-5
32. Kumarathurai P, Sajadieh A, Anholm C, Kristiansen OP, Haugaard SB, Nielsen OW. Effects of Liraglutide on diastolic function parameters in patients with type 2 diabetes and coronary artery disease: a randomized crossover study. Cardiovasc Diabetol. 2021;20(1):12. doi:10.1186/s12933-020-01205-2
33. Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, Phase III, double-blind, parallel-treatment trial. Lancet. 2009;373(9662):473–481. doi:10.1016/S0140-6736(08)61246-5
34. Foster GD, Sanders MH, Millman R, et al. Obstructive sleep apnea among obese patients with type 2 diabetes. Diabetes Care. 2009;32(6):1017–1019. doi:10.2337/dc08-1776
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

