Back to Journals » Infection and Drug Resistance » Volume 16
The Efficacy and Influencing Factors of Polymyxin B in High-Level Carbapenem-Resistant Klebsiella pneumoniae Infections
Authors Jia X , Yin Z, Zhang W, Du S
Received 21 March 2023
Accepted for publication 30 May 2023
Published 27 June 2023 Volume 2023:16 Pages 4177—4187
DOI https://doi.org/10.2147/IDR.S409090
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Suresh Antony
Xuedong Jia,1,2 Zhao Yin,1,2 Wan Zhang,1,2 Shuzhang Du1,2
1Department of Pharmacy, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, People’s Republic of China; 2The Precision Clinical Pharmacy Key Laboratory of Henan Province, Zhengzhou, People’s Republic of China
Correspondence: Xuedong Jia; Shuzhang Du, Department of Pharmacy, The First Affiliated Hospital of Zhengzhou University, No. 1 Jianshe Dong Road, ErQi District, Zhengzhou, Henan, 450052, People’s Republic of China, Email [email protected]; [email protected]
Background: Polymyxin B (PMB) is a remedial treatment for carbapenem-resistant Klebsiella pneumoniae (CRKP) infection; however, there is a paucity of reports on the treatment of high-level CRKP infections with polymyxin B. Studies are needed to explore its treatment efficacy and associated influencing factors.
Methods: Patients with high-level CRKP infections treated with PMB during hospitalization from June 2019 to June 2021 in a hospital were retrospectively studied, and risk factors affecting the efficacy were explored by subgroup analysis.
Results: A total of 92 patients were enrolled, and the results showed that the PMB-based regimen had a bacterial clearance rate of 45.7%, an all-cause discharge mortality rate of 22.8%, and an incidence of acute kidney injury (AKI) of 27.2% for high-level CRKP treatment. The combination of β-lactams other than carbapenems facilitated bacterial clearance, and the combination of electrolyte disturbances and higher APACHE II scores was detrimental to microbial clearance. Risk factors for all-cause discharge mortality were advanced age, concomitant antifungal drugs, concomitant tigecycline and incidence of AKI.
Conclusion: PMB-based regimens are an effective option for the treatment of high-level CRKP infections. However, the optimal dose of treatment and the choice of combination regimens need to be explored in further studies.
Keywords: efficacy, influencing factors, polymyxin B, carbapenem-resistant Klebsiella pneumoniae
Introduction
Carbapenem-resistant Enterobacteriaceae (CRE) infections have broken out globally and have become epidemic in several countries.1 A meta-analysis of 50 original studies showed that CRE-infected patients have a higher risk of death than those infected with the corresponding sensitive organisms,2 so aggressive countermeasures against CRE infection are important to reduce mortality from infection in patients. Carbapenem-resistant Klebsiella pneumoniae (CRKP) infections are the most common among CRE infections, and data from the China CRE Network showed that 73.9% of CRE isolates were CRKP.3 From 2007–2018, the rate of CRKP increased from 0.9% to 19.9% in 19 tertiary hospitals in China.4
Infections caused by CRKP pose a major public health threat and are strongly associated with high mortality rates. Adherent villi, capsules, lipopolysaccharides (LPS) and glycosylated lipids or iron carriers constitute the main virulence factors leading to CRKP pathogenicity, and multiple mechanisms of drug resistance exist, of which carbapenemase (KPC) and metallo-β-lactamase (MBL) production are the most common.5 Previous studies6–8 showed that when the MIC of CRKP to carbapenems is less than 16 mg/L, infection treatment can be achieved through appropriate dose increases of carbapenems, longer infusion times, and shorter dosing intervals. However, for high-level CRKP strains (MIC ≥ 16 mg/L), carbapenems are no longer available for the treatment of this type of infection.9 Although ceftazidime-avibactam, aztreonam-avibactam, cefiderocol, imipenem-relebactam, and meropenem-vaborbactam have been reported to be active against CRKP in recent years,10,11 most of these drugs exert antimicrobial activity by inhibiting the action of KPC, and clinical isolates of CRKP strains often harbor multiple resistance mechanisms simultaneously, greatly limiting the choice of therapeutic agents.
Polymyxin B (PMB) has gained renewed interest in recent years because in vitro drug sensitivity tests have shown that it maintains high antimicrobial activity against CRE.12 PMB exerts antibacterial effects by interacting with lipopolysaccharide (LPS) on the surface of bacterial cell walls, leading to changes in the permeability of bacterial cell membranes.12 The unique mechanism of action results in minimal cross-resistance with commonly used clinical antibiotics.13 It was relaunched for use in China in September 2017 and is currently recommended as a remedial treatment for CRKP infection.13–15 The current use in China has still been relatively short, so the clinical outcomes of PMB-based regimens for high-level CRKP infections have been reported relatively infrequently, and the factors affecting their effectiveness have not been systematically explored. China’s Henan province is generally consistent with the national situation, with CRKP accounting for 77% of CRE isolates.16 With a large number of patients with CRKP infection, it is important to explore appropriate treatment options for the prevention and control of CRKP infection, and previous studies have shown the emergence of colistin-resistant KP strains,17–19 so exploring the effectiveness of PMB in treating high-level CRKP and its influencing factors is of great reference value to promote the rational use of PMB and to delay the emergence of drug-resistant strains. In this study, we retrospectively analyzed cases of high-level CRKP infections treated with PMB and explored the factors influencing the treatment effect of PMB to provide some reference for the clinical response to high-level CRKP infection.
Methods
Study Design
The study was conducted at the First Affiliated Hospital of Zhengzhou University (Zhengzhou, China), a more than 8500-bed large tertiary teaching and four-district hospital, between June 2019 and June 2021. The inclusion criteria were as follows: patients treated with intravenous PMB monotherapy or in combination and bacterial culture results confirming CRKP infection. The exclusion criteria were as follows: younger than 18 years old or received PMB treatment for less than 7 days. This study was approved by the Research Ethics Commission of Zhengzhou Hospital (2021-KY-0063-002). Because this study was retrospective and information about the patients was withheld, the ethics committee approved our application for exemption from informed consent.
All demographic (age, sex, underlying disease), infection-related indicators (axillary temperature, white blood cell (WBC), procalcitonin (PCT), C-reactive protein (CRP)), microbiological data (before and after drug administration), PMB treatment information (dose, frequency, duration, combination drugs) and survival status at discharge were retrospectively extracted.
Definition of Related Indicators
The Vitek® 2 automated system (France Biomerieux) was used for bacterial identification and drug sensitivity testing. The Clinical and Laboratory Standards Institute (CLSI) 2017 standards were used for the interpretation of the drug sensitivity test results. Imipenem and meropenem were used to screen CRKP, and a minimum inhibitory concentration (MIC) ≥ 16 mg/L was considered a high level of resistance to carbapenems. Isolates with MIC ≤ 2 mg/L were considered sensitive to PMB (colistin breakthrough point of Enterobacteriaceae).20
Microbial clearance and patient hospital all-cause mortality were the primary outcomes, and the occurrence of acute kidney injury (AKI) was the secondary outcome. Bacterial clearance was considered if CRKP was not detected in all of the infected sites after treatment, and antimicrobial treatment failure was considered if CRKP was still detected in any infected site after treatment. All-cause discharge mortality was defined as all-cause death during hospitalization. A creatinine increase of 1.5 times the baseline level was defined as AKI.21
Statistical Methods
All data were statistically analyzed using IBM SPSS 24.0 software (IBM, United States). Count data were described by the number of patient cases and the percentages, and differences were compared using Pearson’s chi-square test (χ2) or Fisher’s exact test. Normally distributed data were expressed as the mean ± standard deviation for measurement data and compared using the t-test; nonnormally distributed measures were expressed as median (interquartile range [IQR]) and compared using the Mann‒Whitney U-test. Factors influencing the microbial clearance rate and all-cause discharge mortality of PBM were analyzed by logistic regression in the subgroup analysis. All variables with a p ≤ 0.1 were further used in a multivariate logistic regression model in a backward stepwise manner. Differences were considered statistically significant at p < 0.05.
Results
Characteristics
A total of 92 patients treated with PMB for ≥7 days for high-level CRKP infection were included in this study (Figure 1). The general information of the patients is listed in Table 1. The overall median age was 63.5 years, and 81.5% for male patients (Table 1). The median number of hospital days was 35 (Table 1), and the number of ICU admission days was 25. Two or more sites of infection were present in 40 (43.5%) patients, of which 85 (92.4%) were pulmonary and 28 (30.4%) were predominantly bloodstream infections. There were 38 (41.3%) patients with coinfection with other pathogens, including 20 (21.7%) with Pseudomonas aeruginosa and 17 (18.5%) with Acinetobacter baumannii (Table 1). The drug sensitivity results showed that the isolates were resistant to most of the tested drugs, and most of the isolates were sensitive to polymyxin (90/92, 91.8%) and tigecycline (85/92, 92.4%), of which 40 (43.5%) isolates had MICs ≤0.5 mg/L for polymyxin, as detailed in Table 2.
 |
Table 1 Clinical Characteristics of Patients and Univariate Analysis of Factors Associated with Microbiological Clearance and All-Cause Discharge Mortality |
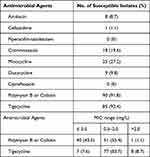 |
Table 2 Susceptibility Profile and Minimal Inhibitory Concentration (MIC) of CRKP to Various Antibiotics |
 |
Figure 1 Schematic diagram of the patient screening process. |
Medications and Outcomes
All patients included in the statistical analysis were given PMB by intravenous drip. PMB was administered at doses of 100, 150, and 200 mg/day in 44.6%, 45.2%, and 17.4% of the patients, respectively (Table 1). A total of 85 (92.4%) patients were treated with other antimicrobial drugs in combination, mainly with carbapenem (58 patients, 63.0%) and tigecycline (43 patients, 46.7%) (Table 1). During treatment, 25 patients (27.2%) developed AKI, and the overall discharge mortality rate of the patients was 22.8% (21/92). The microbial clearance rate was 45.7% (42/92). Overall, the patients improved after treatment with PMB. The patients had significant decreases in temperature, leukocytes, CRP, PCT, and APACHE II levels (Table 3). Two patients with PMB-resistant infections were unsuccessfully treated, with a discharge mortality rate of 100% (2/2), and 7 patients with infections with bacteria that were tigecycline-resistant or intermediate (including 1 with concurrent PMB resistance) had a discharge mortality rate of 28.6 (2/7), which was higher than the overall mortality.
 |
Table 3 Comparison of Patient Conditions Before and After Therapy |
Factors Related to Microbiological Clearance
The microbial clearance rate was 45.7% for PMB. The characteristics of the failure and clearance groups are shown in Table 1. The results in the table show that the APACHE II score, electrolyte disturbance, concomitant cephalosporins and PMB 200 WIU/day had statistically significant differences (Table 1). The results of the binary logistic regression analysis indicated that APACHE II score [OR=0.888 (0.799, 0.986), p=0.027], concomitant β-lactams other than carbapenems (including cefoperazone-sulbactam, piperacillin-tazobactam, ceftazidime and cefepime) [OR=15.757 (2.528, 98.215), p=0.003] and electrolyte disturbance (OR=0.127 (0.021, 0.754), p=0.023) were independently associated with a lower rate of microbiological clearance (Table 4).
 |
Table 4 Multivariate Analysis of Factors Associated with Microbiological Clearance |
Factors Related to All-Cause Discharge Mortality
The all-cause discharge mortality was 22.8% for PMB. The characteristics of the survivor and nonsurvivor groups are shown in Table 3. The results in the table show that patient age, the course of PMB use, concomitant anti-positive bacterial drugs, concomitant antifungal drugs, concomitant tigecycline, and the incidence of AKI had statistically significant differences (Table 1). The results of the binary logistic regression analysis indicated that age [OR=1.708 (1.025, 1.135), p=0.004], concomitant antifungal drugs [OR=4.226 (1.129, 15.821), p =0.032], concomitant tigecycline [OR=4.253 (1.157, 15.628), p=0.029] and incidence of AKI [OR=3.197 (1.056, 14.523), p=0.041] were independently associated with all-cause discharge mortality (Table 5).
 |
Table 5 Multivariate Analysis of Factors Associated with All-Cause Discharge Mortality |
Factors Related to the Incidence of AKI
The results of the univariate analysis of this study showed that combined liver disease, concomitant anti-positive bacteria drugs and concomitant antifungal drugs had statistically significant differences (detailed results are available in the supplementary materials, Table S1). The binary logistic regression analysis indicated that combined liver disease [OR=17.149 (1.651, 178.158), p=0.017] and concomitant antifungal drugs [OR=3.916 (1.361, 11.269), p =0.011] were independently associated with all-cause discharge mortality (detailed results are available in the supplementary materials, Table S2).
Discussion
This study retrospectively analyzed the clinical data of 92 patients treated with PMB for high-level CRKP infections, and the results showed that 45.7% of the patients achieved bacterial clearance. Also, the results showed that the combination of β-lactams other than carbapenems facilitated bacterial clearance, and the combination of electrolyte disturbances and higher APACHE II scores in the patients was detrimental to microbial clearance. The all-cause mortality rate of patients discharged from the hospital was 22.8%, and risk factors contributing to all-cause discharge mortality were advanced patient age, concomitant antifungal drugs, concomitant tigecycline and incidence of AKI, indicating that PMB may be an effective therapeutic agent for the treatment of high-level CRKP infections. While 27.2% of the patients experienced AKI during treatment, monitoring of patients’ renal function needs to be enhanced during treatment with PMB.
The results of previous studies22–31 showed that the overall bacterial clearance rate of PMB for CRO infections was 39%-42%, and only the results of a multicenter retrospective cohort study conducted by Zhang et al25 showed a bacterial eradication rate of 77.65% using PMB for carbapenem-resistant bacterial infections. The results of the present study were generally consistent with those of a previous study (45.7%), suggesting that the bacterial clearance rate of PMB in the treatment of high-level CRKP infections may be independent of its degree of resistance to carbapenems and may be used as one of the basic drugs in the treatment of high-level CRKP infections.
Based on the PK/PD characteristics of PMB, it is hypothesized that an appropriate increase in the dose of PMB is beneficial in improving the clinical efficacy of the drug,32 and the results of existing studies also show that an appropriate increase in the dose of PMB can reduce the all-cause mortality of patients.29,33 The results of our study showed that the bacterial clearance group used high-dose PMB (200 WIU/day) more than the noncleared group, but the multifactorial analysis did not find any therapeutic advantage of high-dose PMB on microbial clearance and all-cause mortality of patients. This may be related to the small number of patients included in our study, and the small sample size may have affected the statistical efficacy of the study, so future studies with large samples are needed for further exploration.
The results of Medeiros et al31 showed that receiving a combination of two in vitro appropriate antimicrobials based on bacterial sensitivity (mainly PMB plus amikacin) had a higher patient survival rate than treatment with a single appropriate drug. However, our findings did not show an advantage of the combination in reducing patient mortality. This may be because the vast majority of the patients included in our study were infected with fully or panresistant CRKP and had very few appropriate antibiotics in their sensitivity tests. Although the vast majority of the patients were given combination therapy, most of the drugs combined were drugs that showed resistance patterns in vitro. Wistrand-Yuen et al evaluated the synergistic effect of PMB with 13 commonly used antibiotics (including minocycline, amikacin, cefepime, ciprofloxacin, fosfomycin, meropenem, minocycline, etc.) against CRKP (MIC>16 mg/L) and showed that only the combination with minocycline, rifampicin or fosfomycin showed a synergistic antibacterial effect.34 Therefore, further studies are needed to explore the effectiveness of the PMB combination regimen on high-level CRKP infections.
A previous study showed that clinical isolates of CRKP have high rates of heteroresistance to PMB and tigecycline,27 and in vitro studies have shown that the combination of PMB and tigecycline can kill resistant bacteria at lower drug doses.22,27 Therefore, it is hypothesized that a combination regimen of PMB and tigecycline may be a potentially effective treatment strategy for patients infected with CRKP. Combination regimens of polymyxin and tigecycline are also recommended in the CRE treatment guidelines.9,35 However, our results showed that the combination of tigecycline did not improve bacterial clearance and was a risk factor for increased all-cause mortality, similar to the results of a multicenter retrospective study by Chang et al.26 Therefore, the effectiveness of PMB in combination with tigecycline in the treatment of high-level CRKP infections needs to be explored in further prospective studies.
The results of this study showed that the incidence of AKI during the use of PMB was 27.2%, which was similar to the results of a previous study.12,36 Since the incidence of AKI is an independent risk factor for all-cause mortality in patients, monitoring of renal function needs to be improved during the use of PMB.
The results of this study showed that the combination of PMB with antifungal drugs was an independent risk factor for increased all-cause mortality in patients, so in the clinical use of PMB for CRKP treatment, the indications for the use of antifungal drugs should be strictly grasped in patients and should not blindly cover antifungal treatment without any basis. Since this study is a retrospective study and the sample size of the study is small, further attention needs to be paid to the effect of PMB combined with antifungal drugs on all-cause mortality in patients in the future.
The results of previous studies37,38 showed that polymyxin exposure is an important risk factor for polymyxin resistance, so optimizing the use of PMB should be a key strategy to stop the spread of polymyxin resistance. The treatment of polymyxin-resistant strains is even more challenging, and the two patients with polymyxin-resistant infections included in this study were not clinically successful, so in the future, there is a need to improve the control of the clinical use of PMB and more strictly use PMB clinically to minimize the production of PMB-resistant bacteria. Shein and team showed that colistin-EDTA combination therapy reduced bacterial load and serum creatinine in the visceral organs of colistin-resistant Klebsiella pneumoniae-infected mice as well as reduced mortality in mice,17–19 so future studies could be conducted to explore whether similar effects exist for PMB and to explore new pathways for the treatment of PMB-resistant bacteria.
The limitations of this study are as follows: (i) This study was retrospective, and the exact timing of PMB use could not be clarified. The results of a previous study by Liang et al30 showed that for patients with sepsis, early use of PMB resulted in significantly higher bacterial clearance compared with delayed administration (65. 22% versus 29.41%, P=0.025; OR=0.533) and may have an impact on research studies. (ii) The sample size included in this study was small, and the data were obtained from a single center, which has some limitations in the generalization of the findings.
Conclusion
PMB may be a potential therapeutic agent for high-level CRKP infections and can provide an important reference for the treatment of these infections. Since this study is a retrospective small-sample exploratory study, the risk factors and dosing strategy of PMB for high-level CRKP infections need to be further explored through multicenter, large-scale prospective studies.
Ethical Approval
This study was approved (Approval number: 2021-KY-0063-002) and supervised by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University and was conducted in strict accordance with the Declaration of Helsinki.
Acknowledgments
We would like to thank the pharmacy interns Conghui Guo and Feng Chen for their help in collecting and verifying some of the data in this study during their internship.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study is part of a research team effort and receives no direct or indirect financial support.
Disclosure
We declare that we have no conflicts of interest.
References
1. Jean SS, Harnod D, Hsueh PR. Global threat of carbapenem-resistant gram-negative bacteria. Front Cell Infect Microbiol. 2022;12:823684. doi:10.3389/fcimb.2022.823684
2. Zhou R, Fang X, Zhang J, et al. Impact of carbapenem resistance on mortality in patients infected with Enterobacteriaceae: a systematic review and meta-analysis. BMJ Open. 2021;11(12):e054971. doi:10.1136/bmjopen-2021-054971
3. Zhang Y, Wang Q, Yin Y, et al. Epidemiology of carbapenem-resistant Enterobacteriaceae infections: report from the China CRE network. Antimicrob Agents Chemother. 2018;62(2). doi:10.1128/AAC.01882-17
4. Gao L, Lv Y, Li Y. Analysis of the drug resistance of carbapenem-resistant Klebsiella pneumoniae in the China antimicrobial resistance surveillance trial program, 2007–2018. Microb Drug Resist. 2020;26(8):944–950. doi:10.1089/mdr.2019.0299
5. Karampatakis T, Tsergouli K, Behzadi P. Carbapenem-Resistant Klebsiella pneumoniae: Virulence Factors, Molecular Epidemiology and Latest Updates in Treatment Options. Antibiotics. 2023;12(2):234.
6. Eisert A, Lanckohr C, Frey J, et al. Comparison of two empirical prolonged infusion dosing regimens for meropenem in patients with septic shock: a two-center pilot study. Int J Antimicrob Agents. 2021;57(3):106289. doi:10.1016/j.ijantimicag.2021.106289
7. Yu Z, Pang X, Wu X, Shan C, Jiang S, Miyamoto A. Clinical outcomes of prolonged infusion (extended infusion or continuous infusion) versus intermittent bolus of meropenem in severe infection: a meta-analysis. PLoS One. 2018;13(7):e0201667. doi:10.1371/journal.pone.0201667
8. Zhao YC, Zou Y, Xiao YW, et al. Does prolonged infusion time really improve the efficacy of meropenem therapy? A prospective study in critically ill patients. Infect Dis Ther. 2022;11(1):201–216. doi:10.1007/s40121-021-00551-2
9. Bassetti M, Peghin M, Pecori D. The management of multidrug-resistant Enterobacteriaceae. Curr Opin Infect Dis. 2016;29(6):583–594. doi:10.1097/QCO.0000000000000314
10. Tompkins K, van Duin D. Treatment for carbapenem-resistant Enterobacterales infections: recent advances and future directions. Eur J Clin Microbiol Infect Dis. 2021;40(10):2053–2068. doi:10.1007/s10096-021-04296-1
11. Tilahun M, Kassa Y, Gedefie A, Ashagire M. Emerging carbapenem-resistant Enterobacteriaceae infection, its epidemiology and novel treatment options: a review. Infect Drug Resist. 2021;14:4363–4374. doi:10.2147/IDR.S337611
12. Jia X, Guo C, Yin Z, Zhang W, Du S, Zhang X. Risk factors for acute kidney injury induced by intravenous polymyxin B in Chinese patients with severe infection. Infect Drug Resist. 2022;15:1957–1965. doi:10.2147/IDR.S363944
13. Rigatto MH, Falci DR, Zavascki AP. Clinical use of polymyxin B. Adv Exp Med Biol. 2019;1145:197–218.
14. Falagas ME, Kyriakidou M, Voulgaris GL, Vokos F, Politi S, Kechagias KS. Clinical use of intravenous polymyxin B for the treatment of patients with multidrug-resistant Gram-negative bacterial infections: an evaluation of the current evidence. J Global Antimicrob Resist. 2021;24:342–359. doi:10.1016/j.jgar.2020.12.026
15. Nang SC, Azad MAK, Velkov T, Zhou QT, Li J. Rescuing the last-line polymyxins: achievements and challenges. Pharmacol Rev. 2021;73(2):679–728. doi:10.1124/pharmrev.120.000020
16. Yan WJ, Jing N, Wang SM, et al. Molecular characterization of carbapenem-resistant Enterobacteriaceae and emergence of tigecycline non-susceptible strains in the Henan province in China: a multicentrer study. J Med Microbiol. 2021;70(3). doi:10.1099/jmm.0.001325
17. Shein AMS, Hongsing P, Abe S, et al. Will there ever be cure for chronic, life-changing colistin-resistant Klebsiella pneumoniae in urinary tract infection? Front Med. 2021;8:806849. doi:10.3389/fmed.2021.806849
18. Shein AMS, Wannigama DL, Higgins PG, et al. Novel colistin-EDTA combination for successful eradication of colistin-resistant Klebsiella pneumoniae catheter-related biofilm infections. Sci Rep. 2021;11(1):21676. doi:10.1038/s41598-021-01052-5
19. Shein AMS, Wannigama DL, Higgins PG, et al. High prevalence of mgrB-mediated colistin resistance among carbapenem-resistant Klebsiella pneumoniae is associated with biofilm formation, and can be overcome by colistin-EDTA combination therapy. Sci Rep. 2022;12(1):12939. doi:10.1038/s41598-022-17083-5
20. Satlin MJ, Lewis JS, Weinstein MP, et al. Clinical and Laboratory Standards Institute and European Committee on antimicrobial susceptibility testing position statements on polymyxin B and colistin clinical breakpoints. Clin Infect Dis. 2020;71(9):e523–e529. doi:10.1093/cid/ciaa121
21. Venkataraman R, Kellum JA. Defining acute renal failure: the RIFLE criteria. J Intensive Care Med. 2007;22(4):187–193. doi:10.1177/0885066607299510
22. Lu Q, Li GH, Qu Q, et al. Clinical efficacy of polymyxin B in patients infected with carbapenem-resistant organisms. Infect Drug Resist. 2021;14:1979–1988. doi:10.2147/IDR.S312708
23. Qu J, Qi TT, Qu Q, et al. Polymyxin B-based regimens for patients infected with carbapenem-resistant gram-negative bacteria: clinical and microbiological efficacy, mortality, and safety. Infect Drug Resist. 2022;15:1205–1218. doi:10.2147/IDR.S357746
24. Xia GL, Jiang RL. Efficacy and safety of polymyxin B in carbapenem-resistant gram-negative organisms infections. BMC Infect Dis. 2021;21(1):1034. doi:10.1186/s12879-021-06719-y
25. Zhang X, Qi S, Duan X, et al. Clinical outcomes and safety of polymyxin B in the treatment of carbapenem-resistant Gram-negative bacterial infections: a real-world multicenter study. J Transl Med. 2021;19(1):431. doi:10.1186/s12967-021-03111-x
26. Chang K, Wang H, Zhao J, et al. Polymyxin B/Tigecycline Combination vs Polymyxin B or Tigecycline Alone for the treatment of hospital-acquired pneumonia caused by carbapenem-resistant Enterobacteriaceae or carbapenem-resistant Acinetobacter baumannii. Front Med. 2022;9:772372. doi:10.3389/fmed.2022.772372
27. Tian Y, Zhang Q, Wen L, Chen J, Van Tyne D. Combined effect of polymyxin B and tigecycline to overcome heteroresistance in carbapenem-resistant Klebsiella pneumoniae. Microbiol Spectr. 2021;9(2):e0015221. doi:10.1128/Spectrum.00152-21
28. Lu Q, Zhu HH, Li GH, et al. A comparative study of the microbiological efficacy of polymyxin B on different carbapenem-resistant gram-negative bacteria infections. Front Med. 2021;8:620885. doi:10.3389/fmed.2021.620885
29. Cai Y, Leck H, Tan RW, et al. Clinical experience with high-dose polymyxin B against carbapenem-resistant gram-negative bacterial infections-A cohort study. Antibiotics. 2020;9(8):451. doi:10.3390/antibiotics9080451
30. Liang Q, Huang M, Xu Z. Early use of polymyxin B reduces the mortality of carbapenem-resistant Klebsiella pneumoniae bloodstream infection. Braz J Infect Dis. 2019;23(1):60–65. doi:10.1016/j.bjid.2018.12.004
31. Medeiros GS, Rigatto MH, Falci DR, Zavascki AP. Combination therapy with polymyxin B for carbapenemase-producing Klebsiella pneumoniae bloodstream infection. Int J Antimicrob Agents. 2019;53(2):152–157. doi:10.1016/j.ijantimicag.2018.10.010
32. Jia X, Yin Z, Zhang W, Guo C, Du S, Zhang X. Effectiveness and nephrotoxicity of intravenous polymyxin B in carbapenem-resistant gram-negative bacterial infections among Chinese children. Front Pharmacol. 2022;13:902054. doi:10.3389/fphar.2022.902054
33. Elias LS, Konzen D, Krebs JM, Zavascki AP. The impact of polymyxin B dosage on in-hospital mortality of patients treated with this antibiotic. J Antimicrob Chemother. 2010;65(10):2231–2237. doi:10.1093/jac/dkq285
34. Wistrand-Yuen P, Olsson A, Skarp KP, et al. Evaluation of polymyxin B in combination with 13 other antibiotics against carbapenemase-producing Klebsiella pneumoniae in time-lapse microscopy and time-kill experiments. Clin Microbiol Infect. 2020;26(9):1214–1221. doi:10.1016/j.cmi.2020.03.007
35. Tsuji BT, Pogue JM, Zavascki AP, et al. International consensus guidelines for the optimal use of the polymyxins: endorsed by the American College of Clinical Pharmacy (ACCP), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Society of America (IDSA), International Society for Anti-infective Pharmacology (ISAP), Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases Pharmacists (SIDP). Pharmacotherapy. 2019;39(1):10–39. doi:10.1002/phar.2209
36. Hou SY, Wu D, Feng XH. Polymyxin monotherapy versus polymyxin-based combination therapy against carbapenem-resistant Klebsiella pneumoniae: a systematic review and meta-analysis. J Global Antimicrob Resist. 2020;23:197–202. doi:10.1016/j.jgar.2020.08.024
37. Teo JQ, Chang CW, Leck H, et al. Risk factors and outcomes associated with the isolation of polymyxin B and carbapenem-resistant Enterobacteriaceae spp.: a case-control study. Int J Antimicrob Agents. 2019;53(5):657–662. doi:10.1016/j.ijantimicag.2019.03.011
38. Macesic N, Nelson B, McConville TH, et al. Emergence of polymyxin resistance in clinical Klebsiella pneumoniae through diverse genetic adaptations: a genomic, retrospective cohort study. Clin Infect Dis. 2020;70(10):2084–2091. doi:10.1093/cid/ciz623
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
