Back to Journals » Infection and Drug Resistance » Volume 15
The Effects of Multi-Donor Fecal Microbiota Transplantation Capsules Combined with Thalidomide on Hormone-Dependent Ulcerative Colitis
Authors Guo XH, Zhu YL, Yang L, Li WJ, Du XF
Received 8 August 2022
Accepted for publication 14 November 2022
Published 19 December 2022 Volume 2022:15 Pages 7495—7501
DOI https://doi.org/10.2147/IDR.S385485
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Xiao-He Guo, Yan-Li Zhu, Lu Yang, Wen-Jing Li, Xue-Fang Du
Department of Gastroenterology, the First Affiliated Hospital of Xinxiang Medical University, Weihui, 453100, People’s Republic of China
Correspondence: Yan-Li Zhu, Department of Gastroenterology, the First Affiliated Hospital of Xinxiang Medical University, No. 88 of Jiankang Road, Weihui District, Henan, 453100, People’s Republic of China, Tel +86 15036615840, Email [email protected]
Objective: This study aimed to assess the effects of multi-donor fecal microbiota transplantation (FMT) capsules combined with thalidomide on hormone-dependent ulcerative colitis (UC).
Methods: A total of 59 patients with steroid-dependent UC treated at the Gastroenterology Department of the First Affiliated Hospital of Xinxiang Medical University between January 2017 and January 2019 were enrolled in this study. Using a random number table, the patients were divided into two groups: a group treated with FMT capsules (the FMT group) and a group treated with FMT capsules and thalidomide (the FMT+S group). Multi-donor FMT capsules were prepared, and all subjects and stool donors followed the FMT pathway for FMT transplantation. Each patient’s Mayo score, C-reactive protein (CRP) level, and level of fecal calprotectin before FMT treatment and at week 1 and week 13 after treatment were recorded. All patients were followed up for 15 weeks.
Results: A total of 56.7% of the patients (34/59) achieved a therapeutic response at the end of the research period. Compared with the FMT group, the FMT+S group had better clinical benefit (P < 0.05). In the comparison of efficacy at week 1 and week 13 after treatment, the Mayo scores, calprotectin levels, and CRP indexes in the FMT+S group were better than those in the FMT group (P < 0.05). There were no serious adverse events in the treatment process or during follow-up.
Conclusion: A combination of FMT capsules and thalidomide provides a treatment choice for patients with hormone-dependent UC, and it can be used as an adjuvant therapy. However, large-scale, multi-center, and prospective trials are required to further verify the reliability of this treatment.
Keywords: fecal microbiota transplantation capsules, thalidomide, hormone-dependent ulcerative colitis
Introduction
Ulcerative colitis (UC) is a chronic non-specific inflammatory bowel disease characterized by persistent chronic inflammation of the mucosa. Treatment with corticosteroid hormones is a highly effective method for moderating the severity of the condition. However, about 20% of patients do not achieve the expected efficacy, and long-term treatment with steroid hormones may lead to prolonging the course of the disease.1
The induction and maintenance of remission on a long-term basis is the primary goal of treatment for refractory ulcerative colitis (RUC). Although some biological and immunological agents alleviate the clinical curability of RUC,2 the high economic costs limit the efficacy of its clinical outcomes. The emergence of fecal microbiota transplantation (FMT) and thalidomide has offered a solution to this predicament, but this combination treatment is only used conservatively for hormone-dependent UC, and trial results have been unstable The combination of thalidomide and salicylic acid in the treatment of RUC reduces the application of hormones and the associated side effects. Multi-center, randomized controlled studies have also demonstrated the efficacy of FMT in the treatment of multiple digestive diseases, including inflammatory bowel disease.3,4 However, there are few studies on the application of FMT capsules combined with thalidomide in the treatment of corticosteroid-dependent UC. Therefore, the present study assessed the clinical efficacy and safety of FMT capsules combined with thalidomide in the treatment of hormone-dependent UC.
Data and Methods
General Data
The clinical baseline data of 59 patients with steroid refractory moderate to severe UC (ineffective or dependent on glucocorticoid therapy) treated with FMT at the Gastroenterology Department of the First Affiliated Hospital of Xinxiang Medical University between January 2017 and January 2019 were retrospective analyzed. The patients were divided into two groups using a random number table: a group treated with FMT capsules (the FMT group; n = 29) and a group treated with FMT capsules combined with thalidomide (the FMT+S group; n = 30). This study was a single-center retrospective analysis and was approved by the Ethics Committee of the First Affiliated Hospital of Xinxiang Medical University (QN-2017-B019).
The sample consisted of 28 male patients (47.46%) and 31 female patients (52.54%), aged (32.43 ± 7.31) years. The course of disease was 91 (9–187) months. A total of 11 cases were of primary type (18.64%), and 48 were of chronic relapse type (81.36%). All were of extensive colon type. Three cases were moderate (5.08%), and 56 cases (94.92%) were severe. There were no statistically significant differences between the two groups in general baseline data, including gender, age, body weight, and course of disease (P > 0.05), indicating comparability.
Methods
Inclusion and Exclusion Criteria
Based on symptoms, signs, and relevant auxiliary examination results, the patients who met the diagnostic criteria for steroid refractory UC in the Consensus Opinion on Diagnosis and Treatment of Inflammatory Bowel Disease (2018, Beijing) were included as study subjects.5
Inclusion criteria: Adult inpatients and outpatients who had at least three UC episodes and received complete FMT treatment at the Gastroenterology Department of the First Affiliated Hospital of Xinxiang Medical University within the research period.
Exclusion criteria: (1) Montreal rating below S2; (2) patient suffered from other severe diseases, including other intestinal diseases—such as Clostridium difficile infection—diabetes mellitus, or cancer; (3) follow-up period was less than three months; (4) patient had a history of drug use or a medical history (especially the use of antibiotics, laxatives, or diet pills in the past three months), used immunomodulators, or received chemotherapy; (5) patient had a history of infection, morbid obesity, diabetes, irritable bowel syndrome, colorectal polyps or cancers, immunity impairment, allergies, or chronic fatigue syndrome; (6) patient otherwise not suitable for the study.
All patients underwent laboratory examinations, including blood and stool examination, during hospitalization. Patients with laboratory abnormalities were excluded. Regarding medication history, many patients have complicated medical histories, and some have tried a variety of treatments. Most of the drugs selected by patients are mesalazine, osalazine, sulfasalazine, baluazine, various probiotics, traditional Chinese medicine, hormones, folk prescription, acupuncture, moxibustion and so on.
The Preparation of Single-Donor FMT Microbiota Frozen Capsules
A total of 14 healthy stool donors provided stool samples for all patients participating in the trial. The inclusion and exclusion criteria, screening, and testing for donors followed international standards.6 The laboratory treated each fresh stool within 12 hours upon approval of the sample, and the average sample weight was 83 (71–99) g. Stool samples from each volunteer were treated separately.
Each sample was mixed with 200 mL 0.9% normal saline, and the stool protoplasm was obtained by stomach bag filtering. The protoplasm liquid was fully mixed with 100% glycerol at a ratio of 10:1 and refrigerated at –80°C for more than a month. The frozen mixed liquid was defrosted overnight at 4°C within 24 h before the preparation of the capsules, and 160 mL 0.9% normal saline was used to prepare the suspension again. The stool suspension was mixed with 100% glycerol at a ratio of 5:1 and then centrifuged (25°C, 400 g, 20 min). After the supernate was discarded, the suspension was centrifuged again (4°C, 10,000 g, 30 min). The sediment was added to the residual liquid to count the colonies, and the target liquid was prepared again. The concentration of microorganisms in the capsules was 1012/mL. A separate hand-held semi-capsule was used, and gelatin capsule no. 1 was filled. Capsule no. 0 and capsule no. 00 were wrapped twice, frozen quickly in a dry ice environment of –55°C, and stored in a liquid nitrogen environment of –80°C. In order to restore the activity of the bacterial microbiota and achieve the ideal efficacy, capsules were heated to room temperature and stay overnight before being used in the trial.7
The Application of FMT Capsules and Thalidomide
All patients refused any form of antibiotic application within one week before the trial, and all patients discontinued steroid drugs before the trial. Both groups received oral administration of capsules (one capsule three times a day), and the FMT+S group was given thalidomide for three days (one 50 mg capsule before sleep). The oral time window had to be completed within 40–60 min after taking FMT capsules. Each treatment cycle lasted for seven days, and the interval between cycles was 14 days. The treatment period was regarded as ending after the completion of four cycles.
Efficacy Evaluation Criteria
The clinical efficacy of the two groups was evaluated at three time points: before FTM treatment, after FTM treatment, and at week 13 after the completion of treatment. The evaluation criteria referred to the Consensus on the Diagnosis and Treatment of Inflammatory Bowel Disease in China.8 Based on experience with FMT and ethical considerations, this study did not require every patient to undergo a colonoscopy three months after FMT treatment, so an enteroscopy was arranged for week 13 after the completion of treatment. Overall response rate = (number of cases with clinical response + number of cases with moderate response)/total number of cases × 100%. Clinical response rate = number of cases with clinical response/total number of cases × 100%. Response rate = number of cases with moderate response/total number of cases × 100%. Clinical indicators, including Mayo score and fecal calprotectin and C-reactive protein (CRP) levels were compared between the two groups.
Statistical Analysis
The SPSS 24.0 software was used for data analysis. The measurement data conforming to normal distribution were expressed as (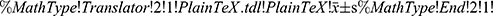 ± s), and non-normally distributed measurement data were expressed as (M[Q1, Q3]). A rank-sum test was used for rating data comparison. The enumeration data were expressed as the number of cases or as a percentage. A χ2 test measured repeatedly was used to compare the indicators before and after treatment. A two-tailed test was performed for P-value, and P < 0.05 indicated a statistically significant difference.
± s), and non-normally distributed measurement data were expressed as (M[Q1, Q3]). A rank-sum test was used for rating data comparison. The enumeration data were expressed as the number of cases or as a percentage. A χ2 test measured repeatedly was used to compare the indicators before and after treatment. A two-tailed test was performed for P-value, and P < 0.05 indicated a statistically significant difference.
Results
Evaluation of the Clinical Efficacy in the Two Groups
During efficacy evaluation at week 13, the overall response rate, clinical response rate, mucosal healing rate, and clinical non-response rate in the FMT+S group were significantly better than those in the FMT group (P < 0.05; see Table 1).
 |
Table 1 Evaluation of Clinical Efficacy at Week 13 of Treatment in the Two Groups |
Modified Mayo Score Changes in the Two Groups
At week 1 after treatment, the modified Mayo score in the FMT+S group was (10.57 ± 2.99) points, and there was no significant difference with the FMT group ([11.56 ± 3.38] points), but the modified Mayo scores in both groups were significantly lower than those before treatment (z = –1.049, P < 0.05). At week 13 after treatment, the modified Mayo score in the FMT+S group was (6.35 ± 1.57) points, and there was a significant statistical difference compared with the FMT group ([9.96 ± 2.09] points). The modified Mayo scores at week 13 were significantly lower than those before treatment in both groups (P < 0.01; see Table 2).
 |
Table 2 Mayo Score Changes at Different Time Nodes in Two Groups |
Baseline Changes in Fecal Calprotectin and CRP Levels in Both Groups at Week 13
The levels of CRP and fecal calprotectin in both groups at week 13 were lower than those before treatment (P < 0.05). Before treatment and at week 1 after treatment, the differences in CRP and fecal calprotectin levels between both groups were statistically significant (P < 0.05; see Table 3).
 |
Table 3 Baseline Changes of Indicators in Both Groups at Week 13, Compared with Those Before Treatment |
Adverse Reactions
There were no serious adverse events in either group during or after FMT treatment. In the FMT+S group, two cases (6.67%) had a fever within 6 h after FMT treatment, and two cases (6.90%) showed an increase in the frequency of transient diarrhea within 24 h after FMT treatment, but there were no short-term adverse reaction events in the FMT group. All symptoms were alleviated spontaneously within a day without any drug intervention. No long-term adverse reactions occurred in the two groups.
Discussion
The pathogenesis and pathophysiological process of hormone-dependent RUC have not yet been clarified, but the prevailing view is that the pathogenesis may be associated with glucocorticoid receptor dysfunction, the effects of various immune-inflammatory factors, and the intestinal microenvironment.9 Hormone tolerance means that this population has to undergo surgery or other open treatments, causing a significant clinical economic burden.
Previous reports on RUC have found a potential effect of thalidomide in the treatment of the disease.10 In the present study, the effect of thalidomide combined with FMT capsules in corticosteroid-dependent UC was further investigated. It was found that 57.6% of patients with RUC achieved clinical symptom relief and sustainable steroid drug independence after taking FMT capsules, and nearly 30% of patients maintained a long-term response. These results indicate that FMT capsules are an effective measure for improving or alleviating non-steroidal clinical symptoms in patients with steroid-dependent UC.
Although these patients have a risk of low immunity when receiving FMT treatment,11 no severe or significant long-term adverse events were reported in the present study. To avoid the impact on the trial results of repeated use of cortisol hormones in patients with steroid-dependent RUC, all patients in this study discontinued hormone treatments three days before FMT treatment, further verifying that patients with hormone-dependent RUC can undergo FMT capsule transplantation safely. De Leon et al12 also reported that FMT had no remission induction effects on patients with UC in the chronic active stage, but the reason for the unstable results is still unclear. The root cause may be that it is difficult for colonoscopic administration to retain the infusion of microbiota suspension. Therefore, the present study implemented FMT based on a certain volume of purified fecal microbiota according to the established protocols for standardized laboratories. The target microbiota were colonized by preparing the capsules and degrading them through the esophagus, stomach, and duodenum, and the overall response rate of FMT in treating hormone-dependent RUC increased. The overall response rate in the present study of 57.6% also verifies that oral administration of capsules can ensure the overall response rate better than colonoscopy lavage. However, this conclusion remains to be supported by more rigorous trials.
In the present study, the proportion of patients benefiting from FMT capsules was significantly lower than that in the FMT+S group, verifying that thalidomide has good efficacy in the improvement of the intestinal microenvironment of patients with hormone-dependent RUC. The mucosal healing indicator in the FMT+S group was also significantly higher than that in the FMT group. The comparison of CRP levels and other inflammatory indicators in the corresponding period also verified that thalidomide can reduce the degree of inflammatory activity and alleviate the mucosal damage caused by stress factors, with good efficacy.13 Unlike with the use of thalidomide for other indications, no malignant side effects caused by thalidomide were reported in the present study, suggesting that its use combined with FMT capsules is a very effective adjuvant therapy.
The comparison at week 1 and week 13 after treatment in the present study found that there were no significant statistical differences between the FMT+S group and the FMT group at week 1. Furthermore, various indicators, including fecal calprotectin levels, were not significantly improved, indicating a delayed effect of FMT. Since the drug delivery method of self-degrading capsules was adopted, this finding may indicate that the degree of microbiota reconstruction in patients with hormone-dependent UC is related to clinical efficacy or to the inability of colonized microbiota to reach an effective concentration in the early stage.14 However, due to the single-center research restrictions and ethical requirements in the present study, enteric microflora recombination was not tested after FMT treatment, and the association between microbiological concentrations in FMT capsules and long-term clinical benefits could not be evaluated. Furthermore, due to equipment limitations, the analysis of microflora composition absence could not be conducted for the patients and their corresponding donors, which could affect the reliability of the research conclusions. This should therefore be further verified in subsequent studies.
No long-term adverse reaction events were observed within the 13-week follow-up period, indicating the high safety of thalidomide combined with FMT capsules in the treatment of hormone-dependent RUC within the study period. In terms of compliance, oral medication is more beneficial to a patient’s long-term follow-up and cooperation in the treatment and the reduction of economic costs. There is currently no consensus on the clinical efficacy of thalidomide in the treatment of hormone-dependent RUC in related studies,15 and multiple treatment measures, including immunomodulators, biological targeted therapy, and surgery, can be used to alleviate the induction and maintenance of steroid-dependent UC. Due to hormone-dependent ulceration, the treatment effect of conventional drugs is poor. Relevant literature reports believe that intestinal microbiota imbalance plays an important role in the pathogenesis of ulceration, and microbiota transplantation has a certain effect in this aspect. Mizuno et al16 treated 10 patients with active UC (5 cases of moderate and 5 cases of severe) with FMT, evaluated the Mayo score changes of the patients 12 weeks after operation, and analyzed the fecal microbiota of the patients and healthy donors. The results showed that none of the 10 patients had serious adverse reactions related to FMT. There was no significant difference in the Mayo score of most patients 12 weeks after surgery compared with that before surgery, and the diversity of fecal microbiota of patients was slightly increased, but the difference was not statistically significant, indicating that single FMT is safe in the treatment of UC, but the efficacy is limited. Nishida et al17 treated 41 refractory UC patients who failed drug treatment with single FMT, and no adverse reactions occurred in all patients, but the clinical remission rate was low, and the intestinal microbiota of patients did not return to normal function. Rossen et al18 confirmed that the clinical remission rate of FMT in the treatment of UC is 0% ~ 68%, indicating that FMT has a certain degree of efficacy on UC. Therefore, FMT combined with thalidomide was chosen to treat hormone dependent ulcerative colitis.
The present study showed that for patients with hormone-dependent RUC—a challenging target group—thalidomide combined with FMT capsules may be an additional adjuvant therapy.19 However, multiple restrictions limit the reliability of this conclusion, including the small number of cases, the lack of a control group, and insufficient endoscopic assessment of each patient during follow-up. Multi-center randomized clinical trials with a larger sample size and a longer follow-up period should therefore be conducted to provide more reliable conclusions.
Conclusion
Not every patient with hormone-dependent RUC benefits from FMT alone. The present study showed that a treatment regimen of FMT capsules combined with thalidomide is feasible for these patients. It is also suggested that treatment with FMT capsules is a forward-looking and innovative concept, as it can be combined not only with hormones but also with other potential treatment methods. Based on this progressive strategy, relevant studies will be reported in the future.
Ethics Approval and Consent to Participate
The study was conducted in accordance with the Declaration of Helsinki (as was revised in 2013). The study was approved by Ethics Committee of the First Affiliated hospital of Xinxiang Medical University. Written informed consent was obtained from all participants.
Acknowledgments
We are particularly grateful to all the people who have given us help on our article.
Funding
Medical Science and Technology Project of Henan Province, LHGJ20210539; Youth Foundation of the First Affiliated Hospital of Xinxiang Medical University, QN-2017-B019.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Harbord M, Eliakim R, Bettenworth D., et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. part 2: current management. J Crohn's Colitis. 2017;11(7):769–784. doi:10.1093/ecco-jcc/jjx009
2. Hoffmann P, Krisam J, Stremmel W, Gauss A. Real-world outcomes of vedolizumab therapy in ulcerative colitis and crohn’s disease at a tertiary referral center. Dig Dis. 2019;37(1):33–44. doi:10.1159/000492322
3. Costello SP, Hughes PA, Waters O, et al. Effect of fecal microbiota transplantation on 8-week remission in patients with ulcerative colitis: a randomized clinical trial. JAMA. 2019;321(2):156–164. doi:10.1001/jama.2018.20046
4. Rossen NG, Fuentes S, van der Spek MJ, et al. Findings from a randomized controlled trial of fecal transplantation for patients with ulcerative colitis. Gastroenterology. 2015;149(1):110–118.e4. doi:10.1053/j.gastro.2015.03.045
5. Yahui G, Weiwei N. Inflammatory bowel disease group, digestive branch, Chinese Medical Association Consensus on the diagnosis and treatment of inflammatory bowel disease (2018, Beijing). Chin J Digestion. 2018;38(5):292–311.
6. Kelly CR, Kahn S, Kashyap P, et al. Update on fecal microbiota transplantation 2015: indications, methodologies, mechanisms, and outlook. Gastroenterology. 2015;149(1):223–237. doi:10.1053/j.gastro.2015.05.008
7. Cui B, Li P, Xu L, et al. Step-up fecal microbiota transplantation strategy: a pilot study for steroid-dependent ulcerative colitis. J Transl Med. 2015;13:298. doi:10.1186/s12967-015-0646-2
8. Xiaonan L, Fengrong Y, Xiaolan Z. Interpretation of the consensus on diagnosis and management of inflammatory bowel disease (Beijing, 2018) from the perspective of diagnosis of ulcerative colitis First. Clinical Focus. 2018;33(11):987–990.
9. Paramsothy S, Paramsothy R, Rubin DT, et al. Faecal microbiota transplantation for inflammatory bowel disease: a systematic review and meta-analysis. J Crohns Colitis. 2017;11(10):1180–1199. doi:10.1093/ecco-jcc/jjx063
10. Shen ZH, Zhu CX, Quan YS, et al. Relationship between intestinal microbiota and ulcerative colitis: mechanisms and clinical application of probiotics and fecal microbiota transplantation. World J Gastroenterol. 2018;24(1):5–14. doi:10.3748/wjg.v24.i1.5
11. Kelly CR, Ihunnah C, Fischer M, et al. Fecal microbiota transplant for treatment of Clostridium difficile infection in immunocompromised patients. Am J Gastroenterol. 2014;109(7):1065–1071. doi:10.1038/ajg.2014.133
12. De Leon LM, Watson JB, Kelly CR. Transient flare of ulcerative colitis after fecal microbiota transplantation for recurrent Clostridium difficile infection. Clin Gastroenterol Hepatol. 2013;11(8):1036–1038. doi:10.1016/j.cgh.2013.04.045
13. Zhu J, Zhang F, Zhou J, Li H. Assessment of therapeutic response in Crohn’s disease using quantitative dynamic contrast enhanced MRI (DCE-MRI) parameters: a preliminary study. Medicine. 2017;96(32):e7759. doi:10.1097/MD.0000000000007759
14. Manichanh C, Borruel N, Casellas F, Guarner F. The gut microbiota in IBD. Nat Rev Gastroenterol Hepatol. 2012;9(10):599–608. doi:10.1038/nrgastro.2012.152
15. Hui-jun SHU, Hong YANG, Zheng WANG. Efficacy and safety of thalidomide in the treatment of adult refractory ulcerative colitis. Chin J Practical Int Med. 2018;38(3):223–226.
16. Mizuno S, Nanki K, Matsuoka K, et al. Single fecal microbiota transplantation failed to change intestinal microbiota and had limited effectiveness against ulcerative colitis in Japanese patients. Intest Res. 2017;15(1):68–74. doi:10.5217/ir.2017.15.1.68
17. Nishida A, Imaeda H, Ohno M, et al. Efficacy and safety of single fecal microbiota transplantation for Japanese patients with mild to moderately active ulcerative colitis. J Gastroenterol. 2017;52(4):476–482. doi:10.1007/s00535-016-1271-4
18. Rossen NG, MacDonald JK, de Vries EM, et al. Fecal microbiota transplantation as novel therapy in gastroenterology: a systematic review. World J Gastroenterol. 2015;21(17):5359–5371. doi:10.3748/wjg.v21.i17.5359
19. Zheng YY, Wang X, Si JT, et al. Randomized clinical trials of traditional Chinese medicines for treating ulcerative colitis: a scoping review. World J Tradit Chin Med. 2021;7:326–331. doi:10.4103/wjtcm.wjtcm_22_21
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
