Back to Journals » Journal of Pain Research » Volume 14
The Effectiveness of Thermal Neuromodulation Using Precise Heat in the Treatment of Chronic Low Back Pain Over 60 Days: An In-Home User Trial
Authors Hapgood JE, Chabal C , Dunbar PJ
Received 22 April 2021
Accepted for publication 16 August 2021
Published 7 September 2021 Volume 2021:14 Pages 2793—2806
DOI https://doi.org/10.2147/JPR.S316865
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Dawood Sayed
Jenny E Hapgood, Charles Chabal, Peter J Dunbar
Soovu Labs Inc., Seattle, WA, USA
Correspondence: Charles Chabal
Soovu Labs Inc., Seattle, WA, USA
Tel +1 206-579-4910
Email [email protected]
Purpose: Two previous independent double-blind randomized studies demonstrated that thermal neuromodulation using high temperature pulsed heat reduced pain in subjects with chronic low back pain. The present study examined the effects of high temperature pulsed heat via an experimental device in a real-world In-Home Use Trial (IHUT) over a sixty-day period.
Materials and Methods: This in-home study recruited 34 subjects with chronic low back pain, provided them with an experimental device that delivered treatment session of high temperature pulsed heat up to 45°C, and followed them for eight weeks. Subjects were allowed to use the device as needed. Primary outcome was pain rating as measured by the 11-point Numeric Pain Scale at baseline, four and eight weeks of treatment. The secondary outcome measures were the interference with daily living components of the Brief Pain Inventory at baseline versus eight weeks of treatment.
Results: Thirty-two subjects completed the study. Pain levels were 5.81 at baseline, 2.79 at four weeks and 2.25 at eight weeks. All changes in pain levels between baseline and four weeks, baseline, and eight weeks and between four and eight weeks were statistically significant (p < 0.05). At eight weeks, the seven components of pain interference with activities of daily living and pain interference with walking were statistically reduced (P < 0.05). About 72% of subjects reported a single 30-minute treatment session produced over 3 hours of pain relief.
Conclusion: An eight-week in-home trial of high-temperature thermal modulation devices produced significant reductions in pain and pain interference with activities of daily living, an important measure of function. Efforts were made to control and reduce study contamination. This study provides important initial data for long-term outcome studies of thermal neuromodulation using high temperature pulsed heat to treat low back pain and to improve subject function and demonstrated that individuals with chronic pain can effectively self-manage pain.
Keywords: thermal analgesia, heat, pulsed heat, chronic low back pain
Plain Language Summary
Two earlier studies showed that high-temperature pulsed heat at 45°C reduced pain in those with chronic low back pain in a short-term, highly controlled clinical study. This Trial builds on previous studies and tests the same experimental device in people with chronic low back pain but in their own home over a period of eight weeks. Study subjects (N=34) reported their pain at baseline and after both four and eight weeks of device use. The way pain interfered with their activities of daily living such as sleeping, interacting with others and mood was measured at baseline and after eight weeks of treatment. Finally, study subjects reported how long a heat treatment session of up to 30 minutes relieved their pain. The goal of the study was to determine if the experimental device was effective in reducing low back pain in people in a real world out of medical clinic setting.Thirty-two subjects completed the IHUT, and the results showed that the experimental device significantly reduced subjects’ pain at both four and eight weeks of treatment compared to baseline. Pain interference with activities of daily living was also reduced after 8 weeks of device use. Improvements were reported in sleep, mood, walking, enjoyment of life, normal work, general activities, and relationships. These improvements are thought to contribute to an enhanced quality of life. Finally, 72% of the subjects reported that a single high temperature pulsed heat treatment reduced pain for 3 or more hours. While this type of real-world study has limitations, the results offer chronic pain sufferers hope of a drug-free, self-management method to manage chronic pain at home. The device offers pain sufferers a mobile highly effective method of pain management and may represent a new form of pain management therapy.
Introduction
A recent publication by the United States Center for Disease Control estimated that over 20% of the US population (50 million) suffers from chronic pain and 8%, or over 20 million individuals, suffers from high impact chronic pain.1 High-impact chronic pain is defined as chronic pain that limited life or work activities on most days or every day during the past 6 months.2 The costs of chronic pain are staggering and are estimated at $560 billion per year.3 Looking at just chronic low back pain, a recent study reported that the point incidence of low back pain was 13.1% in individuals aged 20–69, as defined by having a history of pain located between the rib margin and gluteal folds nearly every day over a three-month period.4 The authors also reported that those with chronic low back pain were socioeconomically disadvantaged with lower incomes, less education, and multiple comorbid medical conditions.4
Compounding the severity and incidence of chronic pain is the understanding that many commonly used treatments are associated with potential major side effects and complications. Increasing rates of opioid use disorder and opioid-related overdose deaths have made the use of prescription opioids for the treatment of chronic pain problematic with recent guidelines recommending their avoidance.5 The side effects and potential problems of common pain-relieving medications such as nonsteroidal anti-inflammatory (NSAID) drugs are now better recognized with the FDA issuing guidelines related to the potential of significant unwanted cardiovascular effects from these drugs.6 Given these circumstances, not surprisingly, there has been a greater focus on nonpharmacological interventions and therapies to manage chronic pain with new clinical guidelines recommending the use of nondrug treatments as one of the first options in pain management, and the American College of Physicians lists superficial heat as one of the initial treatments.7
Against this background, the authors recently published two independent randomized controlled blinded studies on the use of heat in the treatment of chronic low back pain.8,9 These studies were done in a clinical laboratory under tightly controlled conditions and compared thermal neuromodulation with high temperature precise heat between 41°C and 45°C8,9 versus lower temperature steady heat delivered by an identical experimental device. The term precise is used as the temperature to desensitize human TRPV-1 channels without tissue damage is estimated to range between 41°C and 45°C through skin and epidermal tissues.10 In the initial trial, the high temperature precise heat was delivered at approximately 1.4 pulses per minute. The second trial used the identical temperature 45° C but pulsed the heat at twice the rate of the initial trial. The results in subjects who suffered from chronic longstanding back pain (mean 10 years) showed that high temperature pulsed heat in both experimental conditions produced significantly faster and better pain relief than the control condition of lower-temperature steady heat. In addition, 30 minutes of treatment by both high temperature precise heat interventions produced pain relief that lasted for several hours longer than the initial 30-minute treatment.
Given the background that chronic low back pain is public health problem,4 guidelines generally recommend the use of non-drug options7 and studies support the effectiveness of heat in the management of low back pain, this study was proposed and serves as a logical extension of the two published and highly controlled short-term clinical trials.8,9 This longer term in-home user trial (IHUT) was designed as an out of clinic, real-world test. Subjects with chronic low back pain were provided with the experimental device and allowed to use the device for at least two months. While the limitations of this design and efforts to minimize contamination of results are discussed in later sections of this manuscript, the IHUT focused on long term out of clinic results in the subjects’ actual home setting. More importantly, efforts were made to assess the effect of the intervention on important aspects of quality of life. Pain relief without improvement of function or quality of life would have limited clinical applications. In addition while many may personally acknowledge the comfort of a hot shower, hot soak, or a heating pad, these provide no user mobility. Chemical hot packs while allowing mobility do not allow for user customization of temperature and take a relatively long time to achieve effect. The device tested in this IHUT study offers users mobility and customization and therefore could represent an advancement in practical pain management.
The primary objective of this study was to examine the effect of the experimental intervention on chronic low pain over an eight-week period. In addition, pain interference with activities of life and effective duration of a treatment session were measured.
Methods
Study Design
Between May 2020 – January 2021, Soovu Labs, Inc. conducted an experimental In-Home Use Trial (“IHUT”) of its Soovu Wearable Pain Relief System (Figure 1) in thirty-four individuals with chronic low back pain. The IHUT study was conducted with IRB approval from the Western Institutional Review Board and registration on ClinicalTrials.gov (NCT04407884). Procedures followed were in accordance with the Declaration of Helsinki and all subjects gave written informed consent. All subjects enrolled in the IHUT received a complete system which included two experimental devices; a charging cradle and charging cable; printed instructional materials; a 60-day supply of adhesive rings; and access to the companion mobile app. Subjects were instructed to use the experimental device on an as-needed basis for temporary relief of chronic pain and to follow the indications for use and printed instructions. Subjects were instructed to keep the devices charged and to use the mobile app to operate the devices. If they needed assistance or had questions, subjects were instructed to call, email, or text message the study coordinator, a contractor of Soovu Labs.
 |
Figure 1 Pictures of the experimental device. Left shows the pods resting in the charging cradle inside the carrying case. Right shows pods, charging cradle, and two adhesive rings. |
Data Collection
Data collection occurred throughout the trial using multiple methods: 1) online quantitative surveys via Survey Monkey® to collect subjects’ feedback at baseline and at four weeks and eight weeks; 2) qualitative interviews via video conference/phone call at one week after the subject’s first use of the experimental system and after the completion of the eight-week quantitative survey to collect subjects’ opinions on the overall ease of use of the system and its components; and 3) passive automatic collection of each subject’s actual usage of each device. Device usage data was collected via Bluetooth and stored in Soovu Labs’ AWS secure cloud database. Usage data collected included the following for each heat session that was run: date and time, duration, heat profile and temperature. In addition to these data collection methods, a small amount of qualitative, ad-hoc questions about device operations were collected and responded to via email and text message by the study coordinator.
Compensation
Subjects were compensated in stages of up to $620 for their ongoing participation in the IHUT. The compensation schedule was as follows: first payment of $200 issued upon completion of the quantitative survey four weeks after the subject’s first use of the device, second payment of $200 issued upon completion of the quantitative survey eight weeks after the subject’s first use of the device; final payment of $200 plus a $20 Amazon® gift card issued upon the successful return of the device at the end of the trial period.
Recruitment
Subjects were recruited from a list of individuals who had participated in past marketing focus groups with Soovu Labs and from a recruitment database maintained by Fieldworks®, a market research firm in Seattle, WA. All subjects met the following inclusion criteria: 21–70 years old; must have had non-radiating low back pain at least 3 days a week on average in the last 30 days; must have had this low back pain for at least 6 months; must rate pain a “5” or greater on scale of 0–10; must have an iPhone version 6.0 or newer; and must run mobile apps at least “a few times” or “some days” a week. In addition, all subjects had to respond that they were “motivated”, “very motivated”, or “extremely motivated” to “do something to help relieve your low back pain”; had to respond that they were in “fair health”, “moderate health”, “good health” or “excellent health”; and had to respond that they had none of the listed chronic conditions. The selection of one or more of the following chronic conditions resulted in exclusion from the trial: “Presently have or had clinically significant, severe, progressive, or uncontrolled renal, hepatic, hematological, immunologic, gastrointestinal, endocrine, pulmonary, cardiac, neurologic, cerebrovascular or psychiatric disease”; “Presently have or had sciatica where the sciatic component of the pain is greater than the non-radiating pain located in the low back”; “Presently have or had skin diseases and disorders (eg, psoriasis, eczema, scars, wounds and other dermatological lesions)”; “Currently receiving treatment for abnormal tissue growth”; “Currently pregnant, planning to become pregnant, or lactating”; “Presently have or had a hypersensitivity to/issues with adhesive products on the skin”; “Presently have or had a hypersensitivity to/issues with products that are used for heat therapy (eg, heating pads)”. Finally, any individuals who responded “yes” to “taking opioid medications” were excluded from participation in the trial.
Study Process
Enrollment
Subjects who screened into the IHUT received a pre-enrollment call from the study coordinator. During the pre-enrollment call, the study coordinator described the expectations of the IHUT including duration of the trial, required feedback mechanisms and timepoints, and compensation. The study coordinator also provided an overview of the experimental system and answered any questions the subjects had. Finally, the study coordinator reviewed the IRB informed consent documentation with the subjects and provided an online link for their review and signature of the documentation.
After the on-boarding call and successful completion of the informed consent documentation, subjects were officially enrolled in the IHUT. A complete experimental system was sent to each subject’s home, and the subject was instructed to download the mobile app that controls the experimental devices. Each subject was then responsible for setting up the devices on his or her own which included setting up an account in the mobile app, charging the devices, and pairing the devices with the mobile app. A consort diagram of the recruitment and enrollment process is shown in Figure 2.
In this self-reporting study, prior to each assessment it was acknowledged that even though they may have used the device on other parts of the body, the assessment was specific for use on their low back.
System Use
As the IHUT took place in a real-world setting, each subject was encouraged to use the experimental devices on an as-needed basis and as she or he saw fit for the temporary relief of chronic low back pain. As a result, subjects used the experimental devices in a variety of settings including at home, at work, and while driving. Subjects were instructed to place the devices on or near where they had pain in their low back. Subjects were encouraged to experiment with placement of the devices as well as the settings of each heat session they ran. For study purposes, only the effect of the experimental device on subjects’ low back pain was evaluated. To adhere the devices to the body, subjects were provided with hypoallergenic and biocompatible adhesive rings. When subjects finished their allotment of adhesives, they were provided an additional supply.
The mobile app controlling the experimental devices allowed for the customization of each heat session to suit the subject’s personal preferences. With the customization controls, subjects were able to set the maximum temperature of the devices selecting from low (42°C), medium (43°C), high (44°C), or max (45°C) and the duration of the heat session, selecting 10 minutes, 20 minutes, or 30 minutes. Finally, subjects were able to select the heat profile for the session, selecting from “Deep”, “Soothe” or “Breathe.” The heat profiles are differentiated by the rate at which the devices’ temperature rises, peaks, falls, and repeats the cycle. Each heat profile produces a different warming sensation and is highly subject to personal preference. Subjects in the IHUT were encouraged to try each profile throughout the trial period.
Safety Assessment
Subjects were asked to inform the study coordinator about any concerns from using the device or adhesive rings. They were told that the device is FDA registered, the adhesive rings are latex free and biocompatible, and that mild erythema (skin redness) after using the device was normal. Any concern throughout the study was documented into a study log, and concerning events were shared with the study physician. In addition, used data for each device was collected in real time and stored in the study database.
Outcome Assessments
- The primary outcome of this study was the changes in pain level as rated by the numeric pain scale (NPS) comparing baseline pain levels to pain levels after four and eight weeks of use.11
- Secondary outcomes were the interference with daily life scale of the Brief Pain Inventory comparing baseline with levels after eight weeks of treatment.12
- The duration of pain relief after a single treatment was also measured after eight weeks of treatment.
- Subjects’ preferences for the duration of each treatment session (10, 20 or 30 minutes), preferences for the maximum treatment temperature (42, 43, 44, or 45°C), and treatment program (“deep” profile where the heat pulses at the rate of about 2.8 times per minute, “soothe” 1.4 times per minute and “breathe” one pulse per minute) were collected.
Statistical Analysis
Study data were collected and entered into a spreadsheet and statistical analysis was performed using JPM version 15.2.0 (SAS Institute Inc.) with a two-tailed alpha set to 0.05 for all analyses. The primary outcome measure of changes in pain scores measured by the NPS and was assessed using a one-way repeated measures ANOVA. The secondary outcome of changes in pain interference, as measured by the BPI, was assessed with paired t-tests, and checked with a Wilcoxon signed rank test in case the data were non-parametric.
Results
Thirty-four subjects were recruited into the study. Two subjects dropped out of the study during the trial. Thirty subjects completed all assessments, and 2 subjects completed all assessments except for the final questionnaire on the BPI pain interference with life. For data analysis, all 32 subjects were used except for the final questions on pain interference with life, for which there were 30 responses. These numbers are reflected on the consort diagram (Figure 2).
Of the 32 subjects, 14 were males and 18 were females with the age grouping of 9 subjects 21–34 years of age, 18 subjects 35–49 years of age, and 5 subjects 50–70 years of age. All subjects’ primary pain complaint was chronic low back pain.
The primary study outcome was the change in pain scores from baseline to week 8 using the 11-point NPS measure. The baseline pain score was 5.81, standard error SE ± 0.34 (N=32). At the end of eight weeks, the pain score was 2.25 SE ± 0.32 (N=32). Figure 3 shows the reduction in pain from baseline to 4 weeks, and from baseline to 8 weeks. In all time periods, the reduction in pain was statistically significant. Figure 4 shows the changes in pain levels over time for each subject. Pain levels continued to fall throughout the study for many subjects.
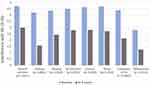
Seven questions from the BPI measuring “pain interference with life” and an additional question rating their “pain interference with walking” were asked at baseline and at the end of 8 weeks. All measures of pain interference with life and interference with walking showed a statistical improvement by 8 weeks (Figure 5).
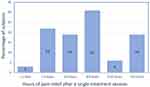
At week 8, all subjects were asked to report how long relief lasted after a heat session with the experimental devices. Nineteen percent reported a treatment session reduced pain for over 12 hours, 3% reported a treatment session reduced pain for 9–12 hours, 31% reported a treatment session reduced pain for 6–8 hours, 19% reported a treatment session reduced pain for 3–5 hours, and 22% reported a treatment session reduced pain for 1–2 hours (Figure 6).
Subjects’ preferences for treatment temperature, treatment duration and how often the device would generate pulses of heat per minute were collected. These results displayed graphically in Figure 7 show that 83% of subjects chose the hottest temperatures for the pulsed heat either 44 or 45°C and only 1% selected the lowest setting of 42 °C. Eighty-three percent of subjects chose a treatment duration of 20 or 30 minutes with 17% choosing a duration of 10 minutes. Finally, nearly 69% of subjects chose the fastest rate of heat pulses per minute at approximately 2.8 times per minute (“deep”), 23% chose a pulse rate of 1.4 times per minute (“soothe”), and about 10% chose the slowest rate of once per minute (“breathe”).
Concerns regarding potential side effects from the device were solicited from each of the 32 subjects. If subjects reported a concern they were contacted via telephone. Overall, the experimental device was used for a total of 1303.5 hours (summary use of all subjects). Over the 8-week study period, 11 subjects reported some temporary erythema upon removing a heating pad after a treatment session. In every case, the erythema completely resolved in less than 6 hours and no subjects repeated this concern a second time. Two subjects had minor discomfort removing the adhesive pads but reported this as a minor concern. No subjects required a call from the study physician because of side effects.
Discussion
This IHUT was designed as an experimental trial for several reasons. We had published two independently run, double-blind, randomized controlled trials assessing the technology in subjects with chronic low back pain.7,8 These trials demonstrated the effectiveness of high temperature pulsed heat to reduce pain. The IHUT was a next logical step to assess the technology in long-term use in subjects living in a real-world setting. A randomized controlled study design was considered but had several deficiencies when considering some key elements of our initial controlled trials. For instance, in the clinical trials, we used an active placebo device, which was identical to the experimental device except for the temperature of heat. While the experimental device pulsed to 45 °C, the placebo device provided constant heat at 37 °C. The placebo control device was noticeably hot to the touch, and subjects were never told whether they received an experimental or placebo device. Subjects were asked at the various points in the study whether they felt they had the placebo or experimental device. Despite using identical devices and both devices being noticeably hot, some subjects were able to correctly guess which device they had received in the trial.8 When looking at a longer-term, real-life trial it was felt that even an active placebo would not be able to successfully function as an effective placebo device, particularly over the two-month trial. In addition, prior to embarking on a complicated long-term blinded controlled trial it was felt that a pilot type study such as the present one should be done to test the methodology and provide a basis for future sample size calculations and guide a study design that used a comparison group such as usual care or alternative device as a control condition. Therefore, this in-home user trial study was selected.
This study clearly had some limitations. Subjects were compensated for their time, they were not blinded, and they likely had treatment expectations when they entered the study. Despite the limitations of this study, efforts were made to attain an accurate as possible testing paradigm with minimal bias. For instance, although all potential subjects were screened to assure that they met study criteria, no effort was made to select subjects who were more likely to respond to the study device. For example, some long-term trials may pretest subjects or use a “run in” period and only select those who have a favorable response to the intervention. In this case, no pretest or use of the experimental device was done before the subject entered the study. If the subject met the study criteria, they were entered into the study with no bias based on any study run-in or pre-study testing. This was done to reduce the chances of having an enriched subject sample that would favorably bias the results. If subjects did not find the device useful for pain relief during the study, it was expected that the assessment would demonstrate that. Likewise, if subjects were not benefiting from the study device, it was reasonably expected that they potentially would stop the study or drop off. In fact, two subjects did not complete the study and are counted as treatment failures regardless of the reason for drop out.
In an additional effort to reduce bias, subjects were paid in over 3 different time epochs. Subjects were paid upon completion of a quantitative survey four weeks after the subject’s first use of the device; again, upon completion of the quantitative survey eight weeks after the subject’s first use of the device; and final payment upon the successful return of the devices to Soovu Labs at the end of the trial period. Subjects would receive payment over these periods as long as they participated in the data collection activities (eg, quantitative surveys) regardless of whether or not they used the devices. It was hoped that by offering payment at three different times there would be less incentive to continue the trial compared to offering a single payment at its conclusion. Nevertheless, payment of the subjects even as installments may have added a bias to the results. Based on these outcomes, a long-term follow-up study with a wait list control, crossover design, or an active alternative device group such as transcutaneous electrical nerve stimulator (TENS) is being considered.
Efforts were made to minimize subject contact with the study personnel. After subjects were oriented to the use of the device, they independently enrolled the experimental devices using the phone app on their own personal smart phone. The phone app could offer support and directions and subjects were also provided with a printed instructional booklet which covered all operations of the device. In addition, at one week of treatment, subjects were contacted via internet-based video conferencing to answer any questions or concerns after which study personnel did not intervene with subjects unless subjects requested support. This occurred only very rarely and was usually technically related. Subjects completed an online assessment at study start, and after 4 and 8 weeks of treatment. Because of both the trial protocol and COVID-19 pandemic no subject ever met any study personnel in person except for video orientation.
Our previous studies demonstrated that under tightly controlled experimental conditions the device offered rapid and significantly more effective pain relief than the lower temperature, steady-heat control device.8,9 A logical question was how these laboratory results translate into real-world use. The IHUT was designed to help answer this question. In the real world, pain is not static but is dynamic.13,14 Pain is episodic with peaks and valleys sometimes related to the individual’s mood, stresses, and biological variables such as everyday activity.15,16 Short-term laboratory observations cannot easily capture these variables. Pain was assessed using the 11-point Numeric Pain Scale, a well-accepted and validated measure.11,17 While pain relief was the primary outcome variable, perhaps more importantly, this study attempted to measure the impact of the experimental device on how pain negatively affects and interferes with important components of life. For the IHUT, the pain interference component of the brief pain inventory (BPI) was selected.18,19 The BPI has good correlation across cultures,12 and with other accepted measures such as the PROMIS Pain Interference metric20 and the Oswestry Disability Index (ODI).21 The “pain interference with life” section of the BPI has seven components that measure how pain interferes with quality of life and includes measures on relationships with others, enjoyment of life, mood, quality of sleep, walking, general activity, and interference with work. The BPI is a well-validated and accepted instrument that has shown validity in multiple cultures and has good sensitivity to change.22,23 In addition, subjects were asked to rate how their pain affected their ability to exercise. This question was added since physical therapy, which usually involves some form of exercise, is a typical component of a comprehensive approach to pain management.
The results of this IHUT showed highly statistical improvement in each of the seven components of the BPI and in the ability to exercise with p values often less than 0.001. These findings reinforce the concept that pain affects the individual in a multi-modal fashion and proper treatment of pain can directly impact many of these important components of quality of life. In some respects, the interference of pain on the components measured by the BPI may have a greater impact on the individual’s life than the pain level itself. The ability to reduce pain and its negative impact on the quality of life should not be discounted. Better pain control can be a critical component of getting patient acceptance and participation in other facets of pain management including self-care non-drug strategies such as physical therapy and psychological-based services and approaches. One of the barriers to this healthier and more comprehensive approaches to pain management can be patient acceptance and compliance with recommendations. While not well studied, better pain control can be an important step towards this more comprehensive approach.
Another somewhat remarkable finding in this study was that over time the effectiveness of the experimental treatment tended to increase. This is displayed in Figures 3 and 4. Compared to the pain level at the start of the study, at week four, there was a statistical reduction in pain that further increased by week eight. It is not known why this occurred. There are a couple of potential hypotheses. One hypothesis is that with better pain control subjects were able to increase activity and perhaps get better sleep as demonstrated by the results in Figure 5. Perhaps these activities and healthier lifestyle had an additive effect on the experimental device, thereby producing better pain relief. An alternative explanation is that the heat produced an underlying biological effect that was beneficial for their pain disorder. This could involve relaxation of muscles or ligaments, causing greater flexibility and range of motion or may be related to increased blood flow in the subcutaneous structures.24 Other experimental studies on heat demonstrated that heat could produce muscle, tendon, and ligament relaxation.24 In addition, percutaneous heat measured by implanted temperature probes may increase underlying muscle temperature and blood flow significantly.25 Heat applied to the skin can cause temperature increases up to 2.5cm deep into the quadriceps mass.25 In the IHUT, no physiologic measurements were done. A final hypothesis for the high level of analgesia near the end of the study may be related to the subjects’ perception of having better control of their pain. For some chronic pain sufferers, they may have fear and frustration related to the lack of control over their pain level. This in turn can lead to increased anxiety and for some a feeling of hopelessness.26,27 In the case of the experimental device, subjects could activate their device with the touch of a button providing some semblance of self-control over their pain somewhat analogous to a patient-controlled analgesia device (PCA).28 While not measured in this IHUT, our previous clinical studies demonstrated that the onset of pain relief was rapid with significant pain relief achieved by the first assessment at 5 minutes of treatment.8,9 This onset of pain relief is even faster than the onset of most oral analgesics29 and may help reduce some of the subjects’ anxiety and fear over loss of control related to their pain. As such, these changes may have contributed to the greater analgesic response as subjects gained confidence in the experimental device.
An unusual finding was that the duration of analgesia with a single treatment lasted significantly longer than the actual treatment. In fact, as Figure 6 demonstrates, 31% of subjects experienced 6 to 8 hours of pain relief after a single treatment and 19% experienced greater than 12 hours of relief following a single treatment session. Our previous clinical studies demonstrated a similar finding of prolonged pain relief after a single treatment.7,8 This was seen both with the current experimental device in the IHUT and was also reported by other groups following prolonged treatment with chemical hot packs.30,31 The reason for the prolonged pain relief is not clear but may be related to established research that shows pulsed heat at certain temperatures can result in prolonged deactivation of TRPV1 calcium channels.32 While that research was not done on humans, it is known that capsaicin, a selective TRPV1 channel agent, can cause prolonged desensitization and inactivation of the TRPV1 channel.33,34 In addition, some of the prolonged TRPV1 response may be secondary to change occurring in the dorsal horn or central nervous system as prolonged effects can sometimes be noted with acupuncture like TENS causing a sustained effect that can be reversed by naloxone.35 The present study was not intended to examine potential mechanisms of action, therefore naloxone was given.
User preferences were tracked by the system during the trial. During and after introduction to the device, subjects were never told how long to use the device, what temperature setting to use or what pulse rate of heat to select. Perhaps not surprising subjects preferred hotter temperatures and longer duration of treatment. Interestingly, only 1% of subjects preferred the lowest setting of 42°C which is still two degrees hotter than most chemical hot packs. In terms of pulsed heat profile, subjects preferred by far the “deep” setting of 2.8 pulses per minute. This setting delivered the most thermal energy to the skin as compared to the other slower pulsed heat settings of 1.4 and 1 thermal pulse per minute. At present, since there is no active cooling of the device, the maximum rate of pulsing is dependent on how rapidly the skin dissipates the applied heat as a new pulse will not start until the skin returns to about 40 °C topping out at about 2.8 pulses per minute.
Previously, Soovu Labs conducted a formal clinical study with an independent consultant clinic affiliated with the University of California Davis which demonstrated product safety (results available at www.Soovu.com). The current advanced experimental device is designed to track in real time each heating session and advises users to immediately stop the device and discontinue treatment if they experience discomfort or sustained redness and will warn users to move the devices at pre-determined thresholds (to avoid sustained redness and/or discomfort). It was assuring that with over 1300 hours of use in an unsupervised setting there were no serious side effects. The initial concerns about redness were related to hyperemia from the applied heat and resolved relatively quickly. Once subjects understood the cause, no subject repeated concerns over hyperemia after use.
This study represents an advance in the understanding and potential clinical benefits of a relatively unique form of heat therapy. While humans have recognized the benefits of heat and used heat for centuries and there have been some very well-designed studies funded by Thermacare® to independent universities starting around the year 200024,25,30,36,37 it was felt that a better understanding of some of the neurophysiologic properties of pain relief via heat, and the optimization of the thermal–analgesic relationship could produce a more effective non-drug pain device. To that end we published two independent well controlled short-term trials and a paper discussing potential mechanisms of action.8,9 While the studies were scientifically rigorous, the question remained as how 30-minute studies translated to real-life clinical care. This background formed the basis of this exploratory study. A literature review did not find any previous study that examined the use of heat over a two-month period in a home-based setting. In addition, while there are some studies that look at the use of chemical hot packs over several days, a limitation of this concept is that because of the potential dangers of an oxidation reaction in a hot pack, the temperature must kept at a relatively low range (39–40° C) and cannot be adjusted by the user. Our presumed mechanism of action (MOA) is “defunctionalization” of the TRPV1 channel, and the temperatures required in humans is likely 43–44° C or greater, much higher than the 39–40° C of chemical hot packs.10 In addition, as our user preference data indicate, one single temperature or one pulsation rate does not meet the needs of different individuals with different body habitus and temperature tolerances. Admittedly, hotter temperatures sometimes hot enough to cause serious burns can be obtained with plugged in heating blankets but the danger and lack of portability hampers the practicality of their application. Heat as an effective, easy to use, portable therapy could be used as an integral part of conservative non-interventional pain management. For example, if integrated into a comprehensive pain management approach, including physical therapy, would the addition of a device that could offer fast onset on-demand pain relief improve adherence to therapy is interesting but unknown. Hopefully, this current study can form the base of other clinical studies in which heat is a component.
The present IHUT was designed as a follow-up study to our previously independently run randomized double blinded controlled trials in subjects with chronic low back pain.7,8 This was an attempt to determine whether some of the positive findings demonstrated in the clinical trials could translate into positive results in the longer-term complex outpatient environment. The results of the in-home trial demonstrated that pain relief from the high-temperature precise-heat experimental device could be sustained over 60 days and that a single treatment session could produce hours of pain relief in many subjects. This prolonged response to heat has been reported by others using chemical hot packs.30,32 In addition, measures of pain interference with aspects of life were also followed. In all seven domains of the components of the brief pain inventory, statistical improvement occurred after 8 weeks of treatment. This improvement in functional assessment may be more significant than the mere reduction of pain levels. Multiple efforts in the trial design and implementation were made to try to reduce contamination. As acknowledged, this in-home trial does come with some inherent limitations; however, it is expected that the IHUT is a baseline study that will provide useful information for additional long-term well-controlled studies using either a control wait-list or separate intervention with a crossover design. The use of heat, in particular high temperature pulsed heat, has not been well studied in humans and this in-home trial appears to be the first one examining the effects of this intervention on both pain level and quality of life over the long term.
Conclusion
Low back pain is a major clinical problem, and efforts are underway to develop more effective nondrug options. Heat has been used for many years for pain relief, and research is exploring how heat interacts with the nervous system and how this interaction can be modified to increase clinical effectives. To that end, an eight-week real-world in-home test of high temperature thermal modulation device produced significant reductions in pain and pain interference with activities of daily living, an important measure of function and quality of life. In addition, 72% of subjects reported that a single treatment produced over three hours of pain relief. Efforts were made to control and reduce study contamination, nevertheless there remained a potential for outcome bias. This study provides important initial data for long-term outcome studies of thermal neuromodulation using high temperature pulsed heat to treat low back pain and to improve subject function.
Data Sharing Statement
The datasets generated during the current study are available from the corresponding author on reasonable request for 3 years after publication.
Funding
This study was funded by Soovu Labs Inc. Seattle, WA.
Disclosure
The authors Dr. Chabal and Ms. Hapgood are employees and shareholders of Soovu Labs Inc. In addition, Dr Charles Chabal has a patent 20210052869 issued to Soovu labs, a patent 20200345537 issued to Chabal, a patent 10603208 issued to Chabal, a patent 20200008973 issued to Chabal; and Drs. Chabal and Dunbar are shareholders in Soovu. Dr. Dunbar is a shareholder of Soovu Labs Inc. In addition, Dr Peter J Dunbar has a patent 7871427 licensed to Soovu Labs, a patent 8702775 licensed to Soovu Labs, a patent 8579953 licensed to Soovu Labs, a patent 8702775 licensed to Soovu Labs, a patent 9937072 licensed to Soovu Labs, a patent 10188547 licensed to Soovu Labs, a patent MODULAR STIMULUS APPLICATOR SYSTEM AND METHOD licensed to Soovu Labs.
References
1. Dahlhamer J, Lucas J, Zelaya C, et al. Prevalence of chronic pain and high-impact chronic pain among adults — United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67:1001–1006. doi:10.15585/mmwr.mm6736a2
2. Interagency Pain Research Coordinating Committee. National pain strategy: a comprehensive population health-level strategy for pain. Washington, DC: US Department of Health and Human Services, National Institutes of Health; 2016.
3. Institute of Medicine. Relieving pain in America: a blueprint for transforming prevention, care, education, and research. Washington, DC: National Academies Press; 2011.
4. Shmagel A, Foley R, Ibrahim H. Epidemiology of chronic low back pain in US adults: data from the 2009–2010 National Health and Nutrition Examination Survey. Arthritis Care Res. 2016;68(11):1688–1694. doi:10.1002/acr.22890
5. Chaparro LE, Furlan AD, Deshpande A, et al. Opioids compared to placebo or other treatments for chronic low-back pain. Cochrane Database Syst Rev. 2013;27:49–59.
6. FDA Drug Safety Communication. FDA strengthens warning that non-aspirin nonsteroidal anti-inflammatory drugs (NSAIDs) can cause heart attacks or strokes. 2015.
7. Qaseem A, Wilt TJ, McLean RM, Forciea MA. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166(7):514–530. doi:10.7326/M16-23672
8. Chabal C, Dunbar PJ, Painter I, Young D, Chabal DC. Properties of thermal analgesia in a human chronic low back pain model. J Pain Res. 2020;13:2083–2092. doi:10.2147/JPR.S260967
9. Chabal C, Dunbar P, Painter I. Is thermal analgesia, exploring the boundary between pain relief and nociception using a novel pulsed heating device. Anesth Pain Res. 2020;4(2):1–7. doi:10.33425/2639-846X.1042
10. Chabal C. Fundaments of thermal analgesia in humans: exploring new methods of pain relief. Anesth Pain Res. 2021;5(1):1–8.
11. Jensen MP, Karoly P, Braver S. The measurement of clinical pain intensity: a comparison of six methods. Pain. 1986;27:117–126. doi:10.1016/0304-3959(86)90228-9
12. Cleeland CS, Ryan KM. Pain assessment: global use of the brief pain inventory. Ann Acad Med Singapore. 1994;23(2):129–138.27.
13. Winger JG, Plumb Vilardaga JC, Keefe FJ. Indices of pain variability: a paradigm shift. Pain. 2019;160(11):2411–2412. doi:10.1097/j.pain.0000000000001627
14. Hoy D, Brooks P, Blyth F, Buchbinder R. The epidemiology of low back pain. Best Pract Res Clin Rheumatol. 2010;24(6):769–781. doi:10.1016/j.berh.2010.10.002
15. Sturgeon JA, Hah JM, Sharifzadeh Y, et al. Predictors of daily pain medication use in individuals with recurrent back pain. Int J Behav Med. 2018;25(2):252–258. doi:10.1007/s12529-017-9686-8
16. Burns JW, Bruehl S, France CR, et al. Psychosocial factors predict opioid analgesia through endogenous opioid function. Pain. 2017;158(3):391–399. doi:10.1097/j.pain.0000000000000768
17. Hjermstad MJ, Fayers PM, Haugen DF, et al.; European Palliative Care Research Collaborative (EPCRC). Studies comparing numerical rating scales, verbal rating scales, and visual analogue scales for assessment of pain intensity in adults: a systematic literature review. J Pain Symptom Manage. 2011;41(6):1073–1093. doi:10.1016/j.jpainsymman.2010.08.016
18. Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105–121. doi:10.1016/j.jpain.2007.09.005
19. Mendoza TR, Chen C, Brugger A, et al. The utility and validity of the modified brief pain inventory in a multiple-dose postoperative analgesic trial. Clin J Pain. 2004;20(5):357–362. doi:10.1097/00002508-200409000-00011
20. Cook KF, Schalet BD, Kallen MA, Rutsohn JP, Cella D. Establishing a common metric for self-reported pain: linking BPI pain interference and SF-36 bodily pain subscale scores to the PROMIS pain interference metric. Qual Life Res. 2015;24(10):2305–2318. doi:10.1007/s11136-015-0987-6
21. Song CY, Lin SF, Huang CY, Wu HC, Chen CH, Hsieh CL. Validation of the brief pain inventory in patients with low back pain. Spine. 2016;41(15):E937–E942. doi:10.1097/BRS.0000000000001478
22. Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the brief pain inventory for chronic nonmalignant pain. J Pain. 2004;5(2):133–137. doi:10.1016/j.jpain.2003.12.005
23. Kumar SP. Utilization of brief pain inventory as an assessment tool for pain in patients with cancer: a focused review. Indian J Palliat Care. 2011;17(2):108–115. doi:10.4103/0973-1075.84531
24. Petrofsky JS, Laymon M, Lee H. Effect of heat and cold on tendon flexibility and force to flex the human knee. Med Sci Monit. 2013;19:661–667. doi:10.12659/MSM.889145
25. Petrofsky JS, Laymon M, Berk L, et al. Effect of ThermaCare HeatWraps and Icy Hot cream/patches on skin and quadriceps muscle temperature and blood flow. J Chiropr Med. 2016;15:9–18. doi:10.1016/j.jcm.2015.12.002
26. Hülsebusch J, Hasenbring MI, Rusu AC. Understanding Pain and depression in back pain: the role of catastrophizing, help-/hopelessness, and thought suppression as potential mediators. Int J Behav Med. 2016;23(3):251–259. doi:10.1007/s12529-015-9522-y
27. Ramírez-Maestre C, Esteve R, Ruiz-Párraga G, Gómez-Pérez L, López-Martínez AE. The key role of pain catastrophizing in the disability of patients with acute back pain. Int J Behav Med. 2017;24(2):239–248. doi:10.1007/s12529-016-9600-9
28. Hudcova J, McNicol E, Quah C, Lau J, Carr DB. Patient controlled opioid analgesia versus conventional opioid analgesia for postoperative pain. Cochrane Database Syst Rev. 2006 Oct 18;(4):CD003348. doi:10.1002/14651858.CD003348.pub2. Update in: Cochrane Database Syst Rev. 2015;6:CD003348.
29. Agency for Healthcare Research and Quality U.S. Comparative effectiveness of analgesics to reduce acute pain in the prehospital setting. Department of Health and Human Services, AHRQ Publication No. 19-EHC021-EF, Number 220; 2019.
30. Nadler SF, Steiner DJ, Petty SR, Erasala GN, Hengehold DA, Weingand KW. Overnight use of continuous low-level heat wrap therapy for relief of low back pain. Arch Phys Med Rehabil. 2003;84(3):335–342. doi:10.1053/apmr.2003.501035
31. Michlovitz S, Hun L, Erasala GN, Hengehold DA, Weingand KW. Continuous low-level heat wrap therapy is effective for treating wrist pain. Arch Phys Med Rehabil. 2004;85(9):1409–1416. doi:10.1016/j.apmr.2003.10.016
32. Sánchez-Moreno A, Guevara-Hernández E, Contreras- Cervera R, et al. Irreversible temperature gating in trpv1 sheds light on channel activation. Elife. 2018;7:36372. doi:10.7554/eLife.36372
33. Alawi K, Keeble J. The paradoxical role of the transient receptor potential vanilloid 1 receptor in inflammation. Pharmacol Ther. 2010;125(2):181–195. doi:10.1016/j.pharmthera.2009.10.005
34. Pingle SC, Matta JA, Ahern GP. Capsaicin receptor: TRPV1 a promiscuous TRP channel. Handb Exp Pharmacol. 2007;179:155–171. doi:10.1007/978-3-540-34891-7_9
35. Leonard G, Goffaux P, Marchand S. Deciphering the role of endogenous opioids in high-frequency TENS using low and high doses of naloxone. Pain. 2010;151(1):215–219. doi:10.1016/j.pain.2010.07.012
36. Nadler SF, Steiner DJ, Erasala GN, et al. Continuous low-level heat wrap therapy provides more efficacy than Ibuprofen and acetamino- phen for acute low back pain. Spine. 2002;27(10):1012–1017. doi:10.1097/00007632-200205150-00003
37. Mayer JM, Ralph L, Look M, et al. Treating acute low back pain with continuous low-level heat wrap therapy and/or exercise: a randomized controlled trial. Spine J. 2005;5(4):395–403. doi:10.1016/j.spinee.2005.03.009
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.