Back to Journals » Journal of Multidisciplinary Healthcare » Volume 16
The Effect of Visual Impairment and Its Severity on Vision-Related and Health-Related Quality of Life in Jordan: A Comparative Cross-Sectional Study
Authors Jammal HM , Khader Y , Kanaan SF, Al-Dwairi R, Mohidat H, Al-Omari R , Alqudah N , Saleh OA, Alshorman H, Al Bdour M
Received 18 July 2023
Accepted for publication 5 October 2023
Published 18 October 2023 Volume 2023:16 Pages 3043—3056
DOI https://doi.org/10.2147/JMDH.S431159
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Hisham M Jammal,1 Yousef Khader,2 Saddam F Kanaan,3 Rami Al-Dwairi,1 Hasan Mohidat,1 Rami Al-Omari,4 Noor Alqudah,1 Omar A Saleh,1 Haneen Alshorman,1 Muawyah Al Bdour5
1Department of Ophthalmology, Faculty of Medicine, Jordan University of Science and Technology, Irbid, Jordan; 2Department of Public Health, Faculty of Medicine, Jordan University of Science and Technology, Irbid, Jordan; 3Department of Rehabilitation Science, College of Health Sciences, QU Health, Qatar University, Doha, Qatar; 4Department of Ophthalmology, Faculty of Medicine, Yarmouk University, Irbid, Jordan; 5Department of Ophthalmology, School of Medicine, The University of Jordan, Amman, Jordan
Correspondence: Hisham M Jammal, Department of Ophthalmology, Faculty of Medicine, Jordan University of Science and Technology, PO Box 3030, Irbid, 22110, Jordan, Tel +962-2-7201000, Fax + 962-2-7095123, Email [email protected]
Purpose: To assess the effect of visual impairment (VI), its severity, and ocular diseases on vision-related and health-related quality of life (QoL) in Jordan.
Patients and Methods: A comparative, cross-sectional, hospital-based study was conducted among a group of 278 patients with VI aged ≥ 18 years, and age and sex-matched control group of 278 individuals with no VI. An interviewer administered the National Eye Institute Visual Function Questionnaire (NEI VFQ-25) and the Medical Outcomes Study 12-Item Short Form Health Survey (SF-12) to all participants.
Results: All the mean VFQ-25 subscales scores, physical component scale (PCS) and the mental component scale (MCS) of the SF-12 were significantly lower in patients with VI compared to controls with no VI. The VFQ-25 subscales (except general health and ocular pain), PCS, and MCS scores significantly decreased with more severity of VI. In the adjusted multivariate analysis, lower level of education (p=0.013), male sex (p=0.016), and the presence of cerebrovascular disease (p=0.019) were significantly associated with lower VFQ-25 composite scores in visually impaired patients compared to controls. Ocular disease duration of > 5 years and progressive VI were significantly associated with lower VFQ-25 composite scores (p= 0.026 and p< 0.001) respectively, in patients with VI. Glaucoma had a significantly larger reduction in mean scores of all the VFQ-25 subscales, and the PCS of the SF-12 compared to all other ocular diseases.
Conclusion: Both VI and increasing severity of impairment were associated with reduced vision-related and health-related quality of life in adult Jordanians. Glaucoma patients and less educated people were particularly affected. Routine assessment of QoL in visually impaired patients and improving referral protocols to vision rehabilitation services is recommended to improve the QoL in those patients.
Keywords: quality of life, SF-12, VFQ-25, vision impairment, vision rehabilitation
Introduction
Vision plays a crucial role in daily life activities like reading, driving, and recognizing faces. It is also essential for social functioning and physical and emotional well-being. Visual impairment (VI) is defined as
Vision loss to such a degree as to qualify as an additional support need through a significant limitation of visual capability resulting from either disease, trauma, or congenital or degenerative conditions that cannot be corrected by conventional means, such as refractive correction, medication, or surgery.1
Globally, the age-standardized prevalence of blindness decreased by 28.5% while the prevalence of moderate and severe visual impairment increased slightly during the last 3 decades, with more than 90% of visually impaired individuals residing in developing countries, possibly attributed to higher poverty rates and limited access to health care services.2,3 In 2020 in Jordan, there were an estimated 6.8% of the population with vision loss including blindness, severe, moderate and mild VI.4 Rabiu reported rates of 1.3%, 1.8%, and 9.5% for blindness, severe VI, and moderate VI in the north of Jordan, respectively.5
Visual impairment has significant health and economic implications for affected individuals, their families, society, and the healthcare system. It impacts their physical functions and mental health and increases dependency on others. Elderly visually impaired people suffer from difficulties in daily life activities, frequent falls, social isolation, less life satisfaction, cognitive difficulties, mental health problems, increased need for nursing care and increased use of health services. Younger patients may experience anxiety due to the financial implications of their illness, such as loss of income or the fear of losing income.6 Therefore, knowledge of the impact of VI on vision-related quality of life (VR-QoL), early clinical intervention, and vision rehabilitation help improving patients’ functional and social life.
In the assessment of the impact of VI on affected patients and response to treatment, eye caregivers (ophthalmologists and optometrists) typically rely on objective measures such as visual acuity, often underestimating the functional, psychological and emotional consequences of VI that can occur with even mild vision loss.7 Several instruments were developed and tested over the past two decades to evaluate the subjective, patient-reported quality of life (QoL) in VI.8–14 These instruments have become essential when conducting trials on new treatments to assess their effectiveness in improving patients’ QoL.6,9,15
While generic, health-related QoL (HR-QoL) measures can determine the effects of various diseases on different aspects of QoL (including physical functioning, social functioning, role functioning, mental health, and general health perceptions) and can be used to compare different populations and different diseases, they may not be sensitive to changes in vision-related function or to the severity of impairment in ocular diseases. On the other hand, disease-specific, vision-related QoL (VR-QoL) measures can be more responsive to changes in visual and functional status, so they have better discrimination between the levels of severity of VI, but are incapable of comparisons between VI and other conditions.6 Therefore, it is advisable to include both health-related and vision-related measures when conducting research on the impact of VI on quality of life to facilitate comparison of the impact of vision disorders with other disorders and provide a broader assessment of clinical and health outcomes.8,15 However, limited studies have assessed the cross-sectional correlation between these two distinctly different QoL measures.16,17
Evaluating the QoL provides valuable insight into how patients perceive their illness and helps caregivers determine the most effective intervention by focusing on the most pronounced disabilities to improve the patient’s well-being. Additionally, QoL research can assist in determining how healthcare resources should be allocated across diverse conditions and populations.18
Currently, there is a lack of data and research on the impact of VI on the VRQoL and HRQoL in visually impaired people in Jordan. Therefore, the objectives of this study were to assess the impact of VI, its severity, and ocular morbidity on the VR-QoL and HR-QoL in adult Jordanians and to assess the association of sociodemographic and clinical factors with QoL scores.
Materials and Methods
Study Design and Population
This was a comparative cross-sectional, hospital-based, observational study involving 278 consecutive patients with VI aged 18 years and older, and 278 control participants, matched by age and sex, who attended the eye clinics at two tertiary referral university hospitals in north and central Jordan between December 2021 and June 2022. Control participants with normal vision were selected from those who accompanied all patients who attended the eye clinics. The study was approved by the institutional review board at Jordan University of Science and Technology (Approval No. 27/140/2021) and adhered to the tenets of the Declaration of Helsinki. All participants signed a written informed consent to participate in the study.
Patients with VI of at least 3 months and onset of the disease after the age of 18 years were included. Patients with a recent history of acute loss of vision and who were receiving active treatment, as well as those with cataract as the primary diagnosis and awaiting cataract surgery, were excluded. Participants with conditions that may impair their response to the questions (such as mental illness or cognitive impairment), cancer, or childhood onset of visual impairment were also excluded.
Data Collection and Ocular Examination
An interviewer collected sociodemographic data from the participants, including age, sex, marital status, whether living with family members or not, residence (urban, suburban, rural), level of education, medical insurance, employment, and monthly household income. Comorbid systemic conditions including cardiovascular disease, hypertension, diabetes mellitus, cerebrovascular disease, and arthritis were also recorded as reported by both control and patient groups. Patient-reported information on the duration, onset (acute/gradual), and progression of visual impairment (stable/progressive) were recorded. The ocular condition causing VI was extracted from the medical records.
All patients underwent ocular examination, including visual acuity (VA) measurement, intraocular pressure measurement, and slit-lamp examination of the anterior and posterior segments. Visual acuity was measured with Snellen’s chart, with the patient wearing habitual distant correction, or after subjective refraction. Control subjects had their VA measured to ensure they had no VI. The level of visual impairment was documented based on VA in the better-seeing eye and was defined according to the world health organization as follows: no VI (control subjects, ≥6/12), mild VI (<6/12 to 6/18), moderate VI (<6/ 18 to 6/60), severe VI (<6/60 to 3/60), and blindness (<3/60).19
Quality of Life Measures
For the VR-QoL, we implemented the National Eye Institute Visual Functioning Questionnaire (NEI VFQ-25), which specifically addresses visual function and has been used to assess visual impairment in different eye conditions.20 Its psychometric properties have been validated, and it covers the physical, functional, emotional and social aspects of visual disability, which are the main parts of the multidimensional concept of quality of life.10,16,21 Further advantages of using the VFQ-25 include the patient-based nature of the content, being observer administered (less missing items), and the ability to compare VI in different ocular conditions on the same scale. For the current study, an interviewer administered the Arabic version of the NEI VFQ-25, which is a reliable and valid measure for assessing visual functions of Arabic speaking patients.22 There are 12 subscales included in the VFQ-25: general health (1 item), general vision (1 item), ocular pain (2 items), near activities (3 items), distance activities (3 items), vision-specific social functioning (2 items), vision-specific mental health (4 items), vision-specific role difficulties (2 items), vision-specific dependency (3 items), driving (3 items), color vision (1 item), and peripheral vision (1 item). A scoring algorithm was used to calculate each subscale’s score, which was then transformed to a 0–100 scale with a higher score denoting better functioning. The average of 11 subscale scores was then calculated, removing the general health subscale’s single item, to produce an overall composite score.23 Subjects who have never driven or stopped driving due to “other reasons” or due to “eye sight and other reasons” were set to missing for all driving items, whereas subjects who stopped driving mainly due to their eyesight were entered as “0”.
Regarding HR-QoL assessment, an interviewer administered an Arabic translated version of the 12 items Medical Outcomes Study Short Form Health Survey (SF-12) version 1, which was previously validated and shown to have very good reliability.24,25 The SF-12, which is a briefer version of the 36 items health survey (SF-36), was demonstrated to be a valid measure of general health status for ophthalmic research,26 and benefits from the reduced administration time compared to the SF-36 form.27,28 Two summary component scores, the physical component score (PCS), and the mental component score (MCS) were calculated using a norm-based scoring algorithm utilizing data from a US general population survey, where the mean is 50, and standard deviation is 10.29
Statistical Analysis
Descriptive statistics were used to summarize data. The chi square test was used to analyze the differences in distribution of sociodemographic factors between patients with VI and control subjects. Student’s t-test and analysis of variance was used to compare the mean scores of the VFQ-25 scales and the SF-12 summary scales among the two study groups and groups of the level of visual impairment, respectively. Spearman correlation test was used to assess the correlation between the VFQ-25 composite score, and the SF-12 summary components. A general linear model procedure was used to test the differences in the QoL between patients and controls after adjusting for potential confounders and to analyze the differences in QoL scores among patients according to severity of VI. Also, a general linear model was used to analyze the effect of eye diseases on QoL scores in patients compared to controls. The general linear model multivariate procedure provides regression analysis and analysis of variance for multiple dependent variables by one or more factor variables or covariates. A p-value was considered significant if it was less than 0.05.
Results
Participants Sociodemographic and Clinical Characteristics
This study included 278 patients with visual impairment, and 278 controls with normal vision. Details of the sociodemographic and clinical characteristics and their differences between visually impaired patient and control subjects are shown in Table 1. Visually impaired people were significantly more likely to be single, have lower levels of education, be unemployed, or have lower income. No statistically significant differences were present regarding the other factors. The prevalence of diabetes mellitus among people with VI was significantly higher than controls (67.3% vs 36.7%), but the prevalence of other comorbidities was not significantly different between the two groups.
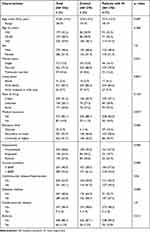 |
Table 1 Participants’ Sociodemographic and Clinical Characteristics |
Ocular Conditions and Visual Impairment
Table 2 shows the distribution and characteristics of the ocular conditions and the level of VI among visually impaired people. The majority of the 278 patients with visual impairment had moderate VI (51.8%), followed by mild VI (24.5%), blindness (15.1%), and severe VI (8.8%). Diabetic retinopathy was the most common ocular cause (58.6%), followed by corneal disease (16.9%). More than one ocular condition was present in 24 patients (8.6%). Most patients reported ocular diseases with gradual onset (82.0%), and the level of VI was felt to be progressing by 62.2%. Approximately half of the patients had their condition for more than 5 years, with a mean (SD) of 8.3 (7.0) years for all patients.
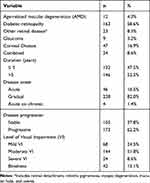 |
Table 2 Clinical Characteristics of Patients with Visual Impairment |
QoL for Patients with Visual Impairment Compared to Controls with Normal Vision
The mean VFQ-25 composite and subscale scores and the mean of SF-12 physical component (PCS), and the mental component (MCS) scores for patients with visual impairment and controls with normal vision are shown in Table 3. The mean composite score was significantly much lower in patients compared to controls (53.2 vs 98.0, p<0.001). Similarly, patients scored much significantly lower than controls in all VFQ-25 subscales, indicating a worse quality of life in patients across all domains. On average, the difference in subscales between the two groups (controls vs patients) ranged from 14.9 for ocular pain to 67 for driving. Moreover, patients had a significantly lower scores in PCS (40.9 vs 51.7, p<0.001) and MCS (36.4 vs 46.9, p<0.001) compared to controls, indicating poorer physical and mental health functioning in patients compared to controls.
 |
Table 3 The Mean Score for Vision-Related Quality of Life (VR-QoL) and Health-Related Quality of Life (HR-QoL) for Persons with Normal Vision and Patients with Visual Impairment |
When patients were categorized according to the severity of visual impairment (Table 4), they differed significantly in the mean PCS and MCS scores and the VFQ-25 composite and subscale scores except general health and ocular pain, with a tendency toward decreased score with an increasing the severity of visual impairment.
 |
Table 4 The Mean Score for Vision-Related Quality of Life (VR-QoL) and Health-Related Quality of Life (HR-QoL) for Patients with Visual Impairment According to the Severity of Visual Impairment |
Multivariate Analysis
Table 5 shows the multivariate analysis of the differences in VFQ-25 composite and subscale scores and the mean SF-12 components between patients and controls. After adjusting for important variables, patients scored lower than controls in the composite score by 43.4 points (95% CI: 40.6, 46.2), PCS by 9.0 points (95% CI: 7.5, 10.6), and MCS by 9.4 points (95% CI: 7.2, 11.6). Similarly, patients scored lower than controls in all VFQ-25 subscales, with a difference ranging from 23.1 for color vision to 65.9 for driving. Lower level of education (p=0.013), male sex (p=0.016), and the presence of cerebrovascular disease (p=0.019) were significantly associated with lower composite scores.
 |
Table 5 Multivariate Analysis of the Differences Between Patients and Controls in the Vision-Related Quality of Life (VR-QoL) and Health-Related Quality of Life (HR-QoL) |
Table 6 shows the multivariate analysis of the differences in VFQ-25 composite and subscale scores and the mean SF-12 components in patients according to severity of the VI. Patients with moderate VI scored lower than patients with mild VI in all subscales and domains except general health, ocular pain, and color vision. On the other hand, patients with severe VI scored lower than patients with mild VI in all subscales and domains except general health. Patients with blindness scored lower than patients with mild VI in the composite score and all domains except general health, ocular pain, and MCS. Among visually impaired subjects, ocular disease duration >5 years and progressive VI were significantly associated with lower VFQ-25 composite scores (p-value 0.026 and <0.001 respectively). The difference in scores between patients with mild VI and controls after adjustment was separately analyzed. Those with mild VI scored significantly less than controls in VFQ-25 composite score by 29.7 points 95% (CI: 26.7, 32.8), PCS by 5.8 points (95% CI: 3.8, 7.7), and MCS by 5.5 points (95% CI: 2.3, 8.7).
 |
Table 6 Multivariate Analysis of the Vision-Related Quality of Life (VR-QoL) and Health-Related Quality of Life (HR-QoL) Among Patients with Visual Impairment According to Impairment Severity* |
Table 7 shows the multivariate analysis of the impact of ocular conditions compared with controls on VFQ-25 and SF-12 components after adjustment for demographic and clinical variables. Patients with different ocular conditions had significantly lower scores compared to controls in all VFQ-25 subscales and the SF-12 components, except color vision in patients with Age-related macular degeneration (AMD) and corneal disease, and MSC in patients with corneal disease. Those with glaucoma had a larger reduction of the composite VFQ-25 score and all its subscales, and the PCS of the SF-12 compared to all other ocular conditions, whereas those with corneal disease scored better than other ocular diseases in both SF-12 components and in composite VFQ-25 score and all its subscales except ocular pain, mental health, peripheral vision.
 |
Table 7 Multivariate Analysis of the Impact of Ocular Conditions on VFQ-25 and SF-12 Components Compared with Controls† |
Among patients with VI, the VFQ-25 composite score had a moderate correlation with both the SF-12 PCS (r=0.579, p<0.001), and the MCS (r=0.409, p<0.001). Also, the vision related mental health subscale of the VFQ-25 had a moderate correlation with the MCS (r=0.666, p<0.001).30
Discussion
Our results showed that VI had a significant effect on all VFQ-25 subscales and SF-12 component scores compared to control subjects and was consistent with results from previous studies.16,31–34 This effect was significantly related to the severity of VI,12,14 however general health and ocular pain appeared to be unrelated to the severity of VI, indicating that as visual acuity becomes worse, the patients’ ability to perform other social and physical tasks is further compromised which further reflects on their dependency and emotional well-being. The lack of association between severity of VI and general health and ocular pain has also been reported in previous population-based studies.11–14
Although patients had significantly lower general health VFQ-25 scores than controls, no significant difference was found between the different levels of VI, possibly reflecting the lack of association between the various general comorbid medical conditions and the VR-QoL, except for cerebrovascular disease, which may contribute to other vision-related tasks through its central nervous system involvement.
In this study, subscales that rely directly or indirectly on visual acuity like driving, vision-specific mental health, social functioning, general vision, and near vision were the highest affected domains in the VFQ-25 respectively, similar to previously reported results.20,32,35
As in prior studies, the missing data rate for the driving subscale was high among patients (never driven or stopped driving due to other reasons),36,37 due to Jordan’s high car ownership costs. However, the impact of VI was greatest on the driving subscale, which could indirectly affect dependency and social functioning if patients eventually lose the ability to drive, especially in a country with a poor public transportation system that is not accessible to people with disabilities.
Scores of vision-specific mental health subscale and the SF-12 MCS were significantly lower in visually impaired subjects, confirming that VI is associated with various mental and psychological problems such as social isolation, lower life satisfaction, anxiety, and depression.12,28,38
As visual impairment progresses, patients become less independent, more socially isolated, and more physically restricted, and may rely on reading to maintain their quality of life, sense of engagement, and connection with the world. This may cause anxiety and depression due to fear of losing their near vision. They often ask if they can maintain their ability to read, or express their concern about losing near vision when general vision is deteriorating as this would leave a “destabilizing void in their lives”.39 Qutishat reported that near tasks were the main functional needs for patients with low vision (74.9%) in a vision rehabilitation center in Jordan.40
Ocular pain, color vision, and peripheral vision subscales were least influenced by VI, as reported previously.16,32 Ocular pain and color vision abnormalities are not usually associated with most ocular conditions that cause VI, while peripheral vision is affected in glaucoma and other diseases involving peripheral retina, as shown by low scores in this study.
The VFQ-25 was initially developed involving only patients with moderate to severe eye disease,16 thus it was unclear if it would be sensitive to milder VI. We found that all VFQ-25 subscales and SF-12 components were significantly lowered in patients with mild VI compared to control subjects, similar to subsequent studies.31,35,41 The mental health VFQ-25 subscale score was the most affected, emphasizing the psychological impacts of mild VI and the necessity to address this issue even in early vision loss patients.
Many population-based studies have assessed how visual impairment affects HR-QoL, mostly using SF-36 and SF-12.26 One study found no association between levels of VI and PCS or MCS,35 while VA levels were monotonically associated with PCS,33,34 and MCS before controlling for other variables.34 In our study, VI significantly affected both PCS and MCS, while the severity of VI was solely linked with PCS. VFQ-25 composite score had a large and medium correlation with SF-12 PCS and MCS scores, respectively. This shows that VI can affect dimensions covered by SF-12, and that SF-12 PCS may be sensitive to different levels of VI. More recently, other HR-QoL instruments such as EQ-5D, 15D, and EUROHIS-QOL8 have been assessed regarding the effect of distance VA on generic HR-QoL and were found to be highly sensitive to vision (EQ-5D, 15D). Their implementation in future studies may be needed to confirm their reproducibility in our population.14
As in earlier studies, higher education levels were associated with better vision-related QoL scores in this study.41,42 Higher-literacy patients may research their eye condition and VI to better understand the problem and implement strategies to improve their quality of life. Male sex was associated with lower composite VFQ-25 scores, however, many studies among different populations showed no association between sex and VR-QoL.7,31,32,35,43
Longer ocular disease or VI duration was linked to worse VR-QoL scores in some studies.43,44 We found similar results, however, this may indicate failure to adjust to VI over time or underutilization of visual rehabilitation services. A longitudinal study in patients with stable VI may clarify this issue. We also found that patient-reported progression of VI was associated with worse VR-QoL scores, emphasizing the importance of early clinical and rehabilitation management.
Ocular diseases assessed in this study were significantly associated with lower VFQ-25 scores, similar to other literature.37,45–48 However, few studies assessed the comparative effect of eye diseases on QoL using VFQ-25.31,32 Glaucoma had the highest impact on most VR-QoL scores, including ocular pain and color vision, and the impact was highest on dependency and peripheral vision. Glaucoma patients may report ocular pain due to side effects of glaucoma medications, intermittent angle closure, or exposure to various clinical interventions. In view of this, glaucoma patients need special attention to their QoL level, as the preservation of central vision until late in the disease may give a false impression of a relatively good QoL. On the other hand, patients with corneal disease scored the highest in most items.
Other ocular conditions had variable degrees of impact on the VFQ-25 scores. As expected, central visual loss in patients with AMD had a large impact on near and distance vision scores, which also affected dependency, social functioning, and mental health.37 Diabetic retinopathy is a major cause of blindness and visual impairment in Jordan,5 and in the present study was responsible alone for 58.6% of the total cases of VI, and was present in 75% of the cases with combined ocular disease. Although both groups (diabetic retinopathy, combined disease) were associated with moderate reductions of the VFQ-25 scores, post hoc multiple comparisons showed larger reductions of the scores (p-value <0.05) in the combined group in the ocular pain, distance activities, role difficulties, dependency, peripheral vision, and composite scales, suggesting that having an additional ocular morbidity further impacts the quality of life,22,31 but the additive or synergetic nature of this observation is not clear and may further be explored in a separate study.
Data on VR-QoL assessment using VFQ-25 in Middle eastern populations is sparse. In Egypt, Abdelfattah22 and El-Banna49 administered the Arabic VFQ-25 to visually impaired people with various ocular conditions. Our findings were different across different VFQ-25 subscales despite the close geographical, cultural, and language proximity. When comparing quality of life studies, the source of information, age distribution, hospital versus population-based samples, and study location (nursing homes, low vision rehabilitation groups) should be considered. Psychosocial support, culture, language, religion, habits, and lifestyles may change how VI is perceived in different populations.
This study is mainly limited by its cross-sectional nature and being hospital-based, hence causal relationship between the different variables and QoL measures could not be established, and selection bias may be present. However, we have included a control group, used a health-related QoL questionnaire (SF-12), and the collected data was from the two major university hospitals in the north and central regions of Jordan, making the results more generalizable for the rest of the population.
Conclusion
This study is the first study to evaluate the impact of visual impairment and various ocular diseases on vision-related and health-related quality of life in adults in Jordan. Both visual impairment and increasing severity of impairment were associated with significant negative impact on vision-related and health related QoL. Glaucoma patients and those with lower levels of literacy were particularly affected. Eye care givers are encouraged to routinely assess QoL in visually impaired patients and improve their referral protocols to vision rehabilitation services including patients with mild VI, where early therapeutic intervention should be made to delay the progression of VI, thus maintaining their quality of life.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
The study was approved by the institutional review board at Jordan University of Science and Technology (Approval No. 27/140/2021) and adhered to the tenets of the Declaration of Helsinki. All participants signed a written informed consent to participate in the study.
Funding
This work was funded by the deanship of research, Jordan University of Science and Technology, Grant No: [20210196]. The sponsor was not involved in any stage of this work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Visual impairment (Concept Id: C3665347) - MedGen - NCBI. Available from: https://www.ncbi.nlm.nih.gov/medgen/777085.
2. Bourne R, Steinmetz JD, Flaxman S, et al. Trends in prevalence of blindness and distance and near vision impairment over 30 years: an analysis for the global burden of disease study. Lancet Glob Health. 2021;9(2):e130–e143. doi:10.1016/S2214-109X(20)30425-3
3. Naipal S, Rampersad N. A review of visual impairment. Afr Vis Eye Health. 2018;77(1):4. doi:10.4102/aveh.v77i1.393
4. Steinmetz JD, Bourne RRA, Briant PS, et al. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the right to sight: an analysis for the global burden of disease study. Lancet Glob Health. 2021;9(2):e144–e160. doi:10.1016/S2214-109X(20)30489-7
5. Rabiu MM, Al Bdour MD, Abu Ameerh MA, Jadoon MZ. Prevalence of blindness and diabetic retinopathy in Northern Jordan. Eur J Ophthalmol. 2015;25(4):320–327. doi:10.5301/ejo.5000557
6. Wang CW, Chan CLW, Chi I. Overview of quality of life research in older people with visual impairment. Adv Ageing Res. 2014;2014. doi:10.4236/aar.2014.32014.
7. Panigrahi A, Nageswar Rao G, Kumari Konar A. Vision-related quality of life and its sociodemographic correlates among individuals with visual impairments. J Vis Impair Blind. 2021;115(4):319–328. doi:10.1177/0145482X211028938
8. Stelmack J. Quality of life of low-vision patients and outcomes of low-vision rehabilitation. Optom Vis Sci. 2001;78(5):335. doi:10.1097/00006324-200105000-00017
9. Margolis MK, Coyne K, Kennedy-Martin T, Baker T, Schein O, Revicki DA. Vision-specific instruments for the assessment of health-related quality of life and visual functioning. Pharmacoeconomics. 2002;20(12):791–812. doi:10.2165/00019053-200220120-00001
10. de Boer MR, Moll AC, de Vet HCW, Terwee CB, Volker-Dieben HJ, van Rens GH. Psychometric properties of vision-related quality of life questionnaires: a systematic review. Oph Phys Optics. 2004;24(4):257–273. doi:10.1111/j.1475-1313.2004.00187.x
11. Chia EM, Wang JJ, Rochtchina E, Smith W, Cumming RR, Mitchell P. Impact of bilateral visual impairment on health-related quality of life: the blue mountains eye study. Invest Ophthalmol Vis Sci. 2004;45(1):71–76. doi:10.1167/iovs.03-0661
12. Taipale J, Mikhailova A, Ojamo M, et al. Low vision status and declining vision decrease health-related quality of life: results from a nationwide 11-year follow-up study. Qual Life Res. 2019;28(12):3225–3236. doi:10.1007/s11136-019-02260-3
13. Wu J, Ji QQ, Lin CX, et al. Burden of visual impairment in mainland China: the Handan eye study and Beijing eye study. Graefes Arch Clin Exp Ophthalmol. 2021;259(11):3501–3509. doi:10.1007/s00417-021-05234-9
14. Purola PKM, Koskinen SVP, Uusitalo HMT. Comparison of three health-related quality of life instruments in relation to visual acuity: EQ-5D, 15D, and EUROHIS-QOL8. Qual Life Res. 2023;32(2):543–552. doi:10.1007/s11136-022-03293-x
15. Elliott DB, Pesudovs K, Mallinson T. Vision-related quality of life. Optom Vis Sci. 2007;84(8):656. doi:10.1097/OPX.0b013e31814db01e
16. Mangione CM, Lee PP, Pitts J, et al. Psychometric properties of the national eye institute visual function questionnaire (NEI-VFQ). Arch Ophthalmol. 1998;116(11):1496–1504. doi:10.1001/archopht.116.11.1496
17. Swamy BN, Chia EM, Wang JJ, Rochtchina E, Mitchell P. Correlation between vision- and health-related quality of life scores. Acta Ophthalmol. 2009;87(3):335–339. doi:10.1111/j.1755-3768.2008.01203.x
18. Patrick DL, Erickson P. Health Status and Health Policy: Quality of Life in Health Care Evaluation and Resource Allocation. New York: Oxford University Press; 1993.
19. World report on vision. Available from: https://www.who.int/publications-detail-redirect/9789241516570.
20. Mangione CM, Lee PP, Gutierrez PR, et al. Development of the 25-list-item national eye institute visual function questionnaire. Arch Ophthalmol. 2001;119(7):1050–1058. doi:10.1001/archopht.119.7.1050
21. Aaronson NK. Quality of life: what is it? How should it be measured? Oncology. 1988;2(5):69–76, 64.
22. Abdelfattah NS, Amgad M, Salama AA, et al. Development of an Arabic version of the national eye institute visual function questionnaire as a tool to study eye diseases patients in Egypt. Int J Ophthalmol. 2014;7(5):891–897. doi:10.3980/j.issn.2222-3959.2014.05.27
23. Mangione C. Version 2000 the national eye institute 25-item visual function questionnaire (VFQ-25). Available from: https://www.nei.nih.gov/sites/default/files/2019-06/manual_cm2000.pdf.
24. Al -shehri Amer H, Taha AZ, Bahnassy AA, Salah M. Health-related quality of life in type 2 diabetic patients. Ann Saudi Med. 2008;28(5):352–360. doi:10.5144/0256-4947.2008.352
25. Ware JE, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220. doi:10.1097/00005650-199603000-00003
26. Purola P, Koskinen S, Uusitalo H. Impact of vision on generic health-related quality of life – a systematic review. Acta Ophthalmol. 2023. doi:10.1111/aos.15676.
27. Globe DR, Levin S, Chang TS, Mackenzie PJ, Azen S. Validity of the SF-12 quality of life instrument in patients with retinal diseases. Ophthalmology. 2002;109(10):1793–1798. doi:10.1016/S0161-6420(02)01124-7
28. Pinquart M, Pfeiffer JP. Psychological well-being in visually impaired and unimpaired individuals: a meta-analysis. Br J Vis Impair. 2011;29(1):27–45. doi:10.1177/0264619610389572
29. Ware JE, Keller SD, Kosinski M. SF-12: How to Score the SF-12 Physical and Mental Health Summary Scales. Health Institute, New England Medical Center; 1995.
30. Schober P, Boer C, Schwarte LA. Correlation coefficients: appropriate use and interpretation. Anesth Analg. 2018;126(5):1763. doi:10.1213/ANE.0000000000002864
31. Broman AT, Munoz B, Rodriguez J, et al. The impact of visual impairment and eye disease on vision-related quality of life in a Mexican-American population: proyecto VER. Invest Ophthalmol Vis Sci. 2002;43(11):3393–3398.
32. Harutyunyan T, Giloyan A, Petrosyan V. Factors associated with vision-related quality of life among the adult population living in Nagorno Karabagh. Public Health. 2017;153:137–146. doi:10.1016/j.puhe.2017.09.004
33. Esteban JJN, Martínez MS, Navalón PG, et al. Visual impairment and quality of life: gender differences in the elderly in Cuenca, Spain. Qual Life Res. 2008;17(1):37–45. doi:10.1007/s11136-007-9280-7
34. Leung JCS, Kwok TCY, Chan DCC, et al. Visual functioning and quality of life among the older people in Hong Kong. Int J Geriatr Psychiatry. 2012;27(8):807–815. doi:10.1002/gps.2789
35. Varma R, Wu J, Chong K, Azen SP, Hays RD. Impact of severity and bilaterality of visual impairment on health-related quality of life. Ophthalmology. 2006;113(10):1846–1853. doi:10.1016/j.ophtha.2006.04.028
36. Lešin Gaćina D, Škegro B, Jandroković S, Škegro I, Bešlić I, Bukvić M. Psychometric properties of the Croatian version of the 25-item national eye institute visual function questionnaire (NEI VFQ-25). Int Ophthalmol. 2021;41(12):4025–4036. doi:10.1007/s10792-021-01975-y
37. Schippert AC, Jelin E, Moe MC, Heiberg T, Grov EK. The impact of age-related macular degeneration on quality of life and its association with demographic data: results from the NEI VFQ-25 questionnaire in a Norwegian population. Gerontol Geriatr Med. 2018;4:2333721418801601. doi:10.1177/2333721418801601
38. Augestad LB. Mental health among children and young adults with visual impairments: a systematic review. J Vis Impair Blind. 2017;111(5):411–425. doi:10.1177/0145482X1711100503
39. Creaser C, Spacey RE, Hicks D. Assessing the impact of reading for blind and partially sighted adults. Loughborough University; 2012. Available from: https://repository.lboro.ac.uk/articles/report/Assessing_the_impact_of_reading_for_blind_and_partially_sighted_adults/9414506/1.
40. Qutishat Y, Shublaq S, Masoud M, Alnuman N. Low vision profile in Jordan: a vision rehabilitation center-based study. Healthcare. 2021;9(1):20. doi:10.3390/healthcare9010020
41. Finger RP, Fenwick E, Marella M, et al. The impact of vision impairment on vision-specific quality of life in Germany. Invest Ophthalmol Vis Sci. 2011;52(6):3613–3619. doi:10.1167/iovs.10-7127
42. Adigun K, Oluleye TS, Ladipo MM, Olowookere SA. Quality of life in patients with visual impairment in Ibadan: a clinical study in primary care. JMDH. 2014;7:173–178. doi:10.2147/JMDH.S51359
43. Yibekal BT, Alemu DS, Anbesse DH, Alemayehu AM, Alimaw YA. Vision-related quality of life among adult patients with visual impairment at university of Gondar, Northwest Ethiopia. J Ophthalmol. 2020;2020:e9056097. doi:10.1155/2020/9056097
44. Briesen S, Roberts H, Finger RP. The impact of visual impairment on health-related quality of life in Rural Africa. Ophthalmic Epidemiol. 2014;21(5):297–306. doi:10.3109/09286586.2014.950281
45. Mitchell J, Bradley C. Quality of life in age-related macular degeneration: a review of the literature. Health Qual Life Outcomes. 2006;4(1):97. doi:10.1186/1477-7525-4-97
46. Parrish RK II, Gedde SJ, Scott IU, et al. Visual function and quality of life among patients with glaucoma. Arch Ophthalmol. 1997;115(11):1447–1455. doi:10.1001/archopht.1997.01100160617016
47. Fenwick EK, Xie J, Ratcliffe J, et al. The impact of diabetic retinopathy and diabetic macular edema on health-related quality of life in type 1 and type 2 diabetes. Invest Ophthalmol Vis Sci. 2012;53(2):677–684. doi:10.1167/iovs.11-8992
48. Kandel H, Nguyen V, Piermarocchi S, et al. Quality of life impact of eye diseases: a save sight registries study. Clin Experiment Ophthalmol. 2022;50(4):386–397. doi:10.1111/ceo.14050
49. El-banna MA, Ismail GM, Sharaa HM. Relationship between visual impairment of elderly and their quality of life. Saudi J Nurs Health Care. 2019;2(5):189–196. doi:10.21276/sjnhc.2019.2.5.4
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
