Back to Journals » Nutrition and Dietary Supplements » Volume 13
The Effect of Socioeconomic and Behavioral Factors on Childhood Stunting in Janamora District, Ethiopia
Authors Azmeraw Y, Akalu TY , Boke M , Gelaye K
Received 5 April 2021
Accepted for publication 1 June 2021
Published 14 June 2021 Volume 2021:13 Pages 91—101
DOI https://doi.org/10.2147/NDS.S314411
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Chandrika J Piyathilake
Yibrie Azmeraw,1 Temesgen Yihunie Akalu,2 Moges Boke,3 Kassahun Gelaye2
1Janamora District Health Office, North Gondar Zone, Ethiopia; 2Department of Epidemiology, Institute of Public Health, College of Medicine and Health Science, University of Gondar, Gondar, Ethiopia; 3Department of Reproductive Health, Institute of Public Health, College of Medicine and Health Science, University of Gondar, Gondar, Ethiopia
Correspondence: Moges Boke Tel +251 926114631
Email [email protected]
Purpose: Globally in 2016, 22.9% of under-five children were stunted. In Ethiopia, the current reduction rate was 2.8%, which was far from the global nutritional target of 2025. However, evidence on the prevalence of stunting and its associated factors in Janamora district was very limited. Therefore, this study aimed to determine the prevalence of stunting and to identify the risk factors among 6– 59 months children in Janamora district.
Methods: A community-based cross-sectional study was conducted in Janamora district from February to March 2020. A multistage cluster sampling technique was used to select 845 study participants. Data were collected using an interviewer-administered technique from caregivers, and anthropometric measurements were taken from the child. An adjusted odds ratio and a P-value < 0.05 were used to declare statistical significance.
Results: The prevalence of stunting in this study was 44.9% (95% CI: 41.5, 48.4). The following conditions were significantly affected stunting: age of child 6– 11 months (AOR=2.5 (1.18– 5.29)), 12– 24 months (AOR=3.38 (1.95– 5.88)), 24– 35 months (AOR=2.33 (1.35,4.01)), wealth status: poorest (AOR=2.79 (1.66,4.68)), poor (AOR=2.15 (1.29,3.57)), medium (AOR=2.09 (1.25,3.49)), good knowledge of mothers/caregivers on handwashing: (AOR = 0.64 (0.43,0.92)), presence of diarrhea: (AOR = 1.9 (1.23,2.95)), start complementary feeding at six months: (AOR=0.58 (0.39,0.88)), start complementary feeding before six months: (AOR=1.58 (1.07,2.34)), and large family size: (AOR=2.33 (1.70,3.21)).
Conclusion: In this study, the prevalence of stunting was high. Being a younger child, living with a large family size, low wealth status, starting complementary feeding before and at 6-months, and diarrhea were provoking factors for stunting. On the other hand, good knowledge of mothers on handwashing during the critical time was associated with a low risk of stunting. Therefore, the nutrition programs need to give focus activities that enhance caregiver’s handwashing knowledge at the critical time and to start complementary feeding at the appropriate age.
Keywords: prevalence, stunting, 6 to 59 months, Janamora district
Introduction
Malnutrition is a general term and comprises under-nutrition (stunting, wasting, underweight, and micronutrient deficiencies or insufficiencies) and over-nutrition (overweight and obesity).1 Stunting is measured by height for age anthropometric indices which indicate long-term chronic malnutrition, the presence of recurrent infection, and the failure of normal growth. Stunting is the most commonly prevalent malnutrition problem among under-five children.2,3
Stunting leads to cognitive and physical development deficits, which puts children at a disadvantage for the rest of their lives. They may develop with lower mental capacity, poor school performance, and in the long run, while they will become adults, they may less productive, earn less and face a higher risk of chronic disease due to overweight gain.4–7
Globally in 2016, 151 million under-five children were stunted; of which Africa and Asia account for 39.1% and 57.6%, respectively.8 In Sub-Saharan Africa, one in every three children was stunted.1,9 According to the four series of Ethiopian Demographic and health survey (EDHS) reports, the prevalence of stunting among under-five children was 58%, 49%, 41%, and 38% in 2000, 2005, 2011, and 2016, respectively. In the Amhara region, according to the recent EDHS report, nearly 46% of under-five children were stunted.10
Undernutrition in the first 1000 days post-conception influences a child’s early growth and development beginning in utero and contributes importantly to the risk of stunting. Maternal undernutrition during the pregnancy period accounts for 20% of childhood stunting by causing intrauterine growth restriction.1 The foundation for brain development and future growth is being formed during these critical days. In addition, the childhood period particularly the first five years is the special time for future optimal development, growth, and health of a child.11 Unfortunately, micronutrient deficiencies (iodine and iron) are prevalent during this period.1
About half of infant and child deaths in Ethiopia are associated with stunting and other forms of undernutrition.1 Ethiopia has the intention to contribute to the countrywide goal of dropping stunting among under-five children in selected regions, through provision intervention to 1.5 million stunted children and to reduce the occurrence of stunting by 40% between 2015 and 2025. According to the current progress report, the recent reduction rate was 2.8% and it needs an average annual reduction rate of 6% to achieve the 2025 targets.12 However, the occurrence of childhood stunting is still high and it requires massive efforts to achieve the 2025 target.13
In Ethiopia, promoting optimal breastfeeding practices for infants 0–6 months at community and facility level through individual and group counseling, appropriate feeding and dietary practices, prevent and control micronutrient deficiencies, detect and manage acute malnutrition and common childhood infections, conduct monthly growth monitoring and promotion, and build the capacity of service providers on complementary feeding initiatives that have been implemented for the last decades to end childhood undernutrition.14
Little information is known about the influence of altitude on stunting in Ethiopia, where most people live at highlands, and poor access to infrastructure (transportation), and there is no recent study done on this specific topic in the study area. In addition, a high number of people were being treated for severe acute malnutrition (SAM) and poor hygiene and sanitation coverage in this district. Subsequently, SAM was the leading problem in the health profile assessment in the district. Moreover, mother’s/caregiver’s knowledge of handwashing during the critical time was not investigated in previous similar studies. Therefore, this study was aimed at determining the prevalence of stunting and identifying risk factors of stunting among 6 to 59 months children in Janamora district.
Materials and Methods
Study Design and Period
A community-based cross-sectional study was conducted from February 3 to March, 30/2020 in Janamora district, North Gondar zone, northwest Ethiopia.
Study Area
The study was conducted in the Janamora district which was found in the North Gondar zone, Amhara Region. Janamora district is located 125 km from Debark and 230 km from Gondar. The district is located between 2400 meters and 4500 meters above sea level and the average annual rainfall amount ranges from 1200 millimeters (mm) to 1500 mm per year. Traditional agriculture (farming) is the main source of their economy and barley and wheat are the common cereal products in the district. The district has 37 rural and 1 urban kebeles and 2 supportive non-governmental organizations (NGOs). The district has a total population of 205,380, of which 27,808 were under-five children. The district has one hospital, six health centers, and 32 health posts.
Population
All children aged 6–59 months in Janamora district were the source population while all 6–59 months children who were present in the randomly selected kebeles were the study population.
Sample Size Estimation and Sampling Procedure
The sample size was calculated by Epi-Info version 7 statistical software using the following assumptions: 95% level of confidence, 80% power, 5% margin of error, and proportion (P) of stunting 49.4% studied in Libo-Kemkem District, Northwest Ethiopia.35 Besides, the sample for the 2nd objective was determined using the double proportion formula in Epi-info. Finally, the largest sample of 845 was used (Table 1). The multistage cluster sampling technique was used to identify study participants. The district has 38 kebeles. Of which, 12 Kebeles (32% of the study area) were selected using a simple random sampling technique (lottery method). Then, the study population was allocated to each selected kebeles by considering a conversion factor of 12.88%. The estimated sample size was proportionally allocated for each selected kebeles then households were visited to assess the presence of under-five children. Each household was visited until the proportioned sample size was reached; the remaining household that have children were excluded since the cluster sampling technique was used for selected households. A child was selected by lottery method if households have more than one eligible child.
 |
Table 1 Summary of Sample Size Calculation for the Specific Objective |
Data Collection Procedure
Data were collected using a structured interviewer-administered questionnaire from mothers/caregivers. The questionnaire was composed of socioeconomic, maternal and obstetric, child health, and hygiene and sanitation characteristics (Annex 1). Initially, the questionnaire was prepared in the English language. Then, it was translated to the local language (Amharic). Six experienced diploma clinical nurses were recruited as data collectors and supervised by two-degree nurses daily. During home visits, if the caregiver was absent, a second visit was made. Anthropometric measurements were collected using the procedure stipulated by the WHO for taking anthropometric measurements. Adherence to this procedure was ensured. The protocol used was UNICEF protocol.15
Age of child was determined using immunization card (if available) and a local-events calendar or from mothers’/caregivers report. Rural wealth index questions were used to collect household assets and classified them into five categories according to the World Food Program Wealth Index creation.16
Operational Definitions
Stunting: Children whose height-for-age Z-score is below minus two standard deviations (−2 SD) from the median of the WHO reference population are considered stunting.17 Presence of diarrhea: a child who has three (3) or more episodes of diarrhea begins within 24 hours determined as perceived by the mother.7,18
Recurrent diarrhea in the last two weeks prior to data collection: determined as perceived by mother or caretaker, or three or more loose or watery stools per day, or blood in stool was reported in the last two weeks prior to data collection.19
Repeated respiratory tract infection: Children who had greater than or equal to two times cough accompanied by short or rapid breathing two or more times in their life perceived by mothers or caretakers.20
Recurrent respiratory tract infection in the last two weeks: The child acquires greater than or equal to two times acute respiratory infection in the last two weeks before data collection.21
Pre-lacteal feeding: Children receive something other than breast milk during the first three days of life.7
Inappropriate complementary feeding: The child starts complementary food before six months of age.19,22
Unvaccinated: where data indicate that no single vaccine dose has been received.23
Fully vaccinated: children received completed primary schedule vaccine with or without a booster dose.10
Long-distance: Health facility greater than or equal to 3 hours to reach a health facility.
Good knowledge of handwashing during critical times: Among six (yes/no) handwashing during critical time questions, mothers/caregivers who scored ≥mean.10
Data Quality Assurance
During the development of the tool, all authors reviewed the data collection instruments and pretested on 5% of the total samples in Debark district before the actual data collection. The training was given for two days on survey objectives, survey methodology, measurement, and recording of anthropometric measurements, and basic nutrition index. Theoretical sessions and practical demonstrations on children’s height measurement, weight measurement and age certainty, and collection of other nutrition-related information were provided.
In addition, strong daily field supervision was carried out to monitor the performance of data collectors and to deliver on-the-spot correction/feedback on any mistakes noted.
Data management and analysis
The collected data was checked out for completeness, accuracy, and clarity by the principal investigator and supervisors before the data entry. Then, the data were entered into EPi-Info version 7 statistical software and exported into AntroPlus and statistical package for social science (SPSS) version 20.0 software for further analysis.
To define the outcome variable, anthropometric data were converted into Z-Score using WHO Anthro-plus software. Z-Score of the child was categorized based on the WHO NCHS reference standard. Then, stunting was defined and coded as 1= stunted, 0 = normal.
A descriptive statistical analysis was done and presented in the form of tables, texts, and graphs.
Furthermore, bivariate and multivariable logistic regression analyses were used to identify the influencing factors. All explanatory variables that were associated with outcome variables in bivariate analysis with a p-value of 0.2 or less were included in the final logistic regression model. Collinearity was checked using variance inflation factor (VIF). The Crude Odds Ratio (COR) and Adjusted Odds Ratios (AOR) together with their corresponding 95% confidence intervals were computed and interpreted. The variables which have a statistically significant association were identified on the basis of p-values < 0.05 and AOR with 95% confidence intervals in the multivariable logistic regression model. The model fitness was checked using Hosmer and Lemeshow’s test (p value=0.152).
Results
Socioeconomic Characteristics of Households
A total of 845 study participants were included in the study with a response rate of 99%. Nearly half (50.9%) of study participants were female. The mean age of children was 28.26 months (±14.33 SD). The educational status of participants showed that 546 (65.1%) of mothers and 541 (64.5%) fathers cannot read and write. Of the total, 759 (90.5%) mothers were married. Among respondents, 578 (68.9%) mothers/caregivers had <5 family members (Table 2).
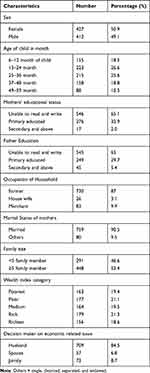 |
Table 2 Demographic and Socioeconomic Characteristics of Children, and Households at Janamora District, Northwest Ethiopia, 2020 (N=839) |
Maternal Health Service and Child Caring Practices Characteristics
Regarding maternal health service, 677 (80.7%) mothers had ANC visit at least two to three times. Among all children, 745 (88.8%) were fully vaccinated (BCG, OPV, Pentavalent, PCV, Rotarix, and measles vaccine were received). More than one-third, 324 (38.6%) of children started complementary feeding before six months of their age. About 61.4% of children had exclusive breastfeeding. Among the total participants, 284 (33.8%) had a history of pre-lacteal feeding, and 358 (42.7%) children had repeated diarrhea in the past two weeks prior to data collection (Table 3).
 |
Table 3 Maternal Health Service and Child-Caring Practices Janamora District, Northwest Ethiopia, 2020 (N=839) |
Hygiene and Sanitation Characteristics
About 631 (73.7%) of the households used protected sources of drinking water. Five hundred ninety households (70.3%) had a latrine. The majority, 663 (79%) of respondents had good knowledge of handwashing during the critical time (Table 4).
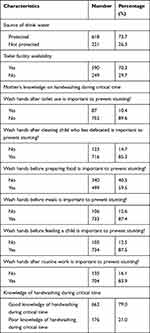 |
Table 4 Hygiene and Sanitation and Mother’s Knowledge of Handwashing During Critical Time Janamora District, Northwest Ethiopia, 2020 (N=839) |
Prevalence of Stunting Among 6–59 Months’ Children
The prevalence of stunting among children of 6–59 months was 44.9% with a 95% CI: (41.5, 48.4). The prevalence of stunting in age category; 8.3%, 14.2%, 11.4%, 7.4%, and 3.6% among age group 6–11, 12–23, 24–35, 36–47, and 48–59 months respectively.
Factors Associated with Stunting Among 6–59 Months’ Children
In the binary logistic regression analysis: sex of the child, household occupation, family size, wealth status of the household, age of the child, maternal age, vaccination status of the child, the household main source of drinking water, the child received pre-lacteal feeds, age complementary feeding started, types of food that children were taken, duration of breastfeeding, knowledge of handwashing during a critical time, toilet availability of in the house, recurrent diarrhea in the past two weeks before data collection were significantly associated with stunting at p-value < 0.2. However, age of the child, family size, wealth status of the household, age started complementary feeding, diarrhea morbidity in the past two weeks prior to data collection and knowledge of handwashing was significantly associated with stunting at P-value < 0.05 in the multivariable analysis.
The odds ratio of being stunted were 2.5 times 95% CI: (1.18–5.29), 3.38 times 95% CI: (1.95–5.88), and 2.33 times 95% CI: (2.33 (1.35,4.01)) higher among age groups 6–11 months,12–23 months, and 24–35 months as compared to children 48–59 months old, respectively.
The odds ratio of being stunted was 2.35 times 95% CI: (1.70–3.20) higher among children living with large family size (≥5) households than those living small family size (<5). In addition, the odds ratio of being stunted were 2.79 times 95% CI: (1.66–4.68) higher among poorest, about 2.15 times 95% CI; (1.29–3.57) higher among poor, and 2.09 times 95% CI: (1.25–3.45) higher among medium wealth status compared to richest households.
The odds ratio of having stunting was 1.58 times 95% CI: (1.07–2.33) higher among children who started complementary feeding before six months as compared to children who start after six months. When compared with children who start complementary feeding at six months, the odds ratio of being stunting was 42%; 95% CI: (0.39–0.88) less likely than they started after six months.
Furthermore, the odds ratio of being stunted was among children having recurrent diarrhea in the past two weeks prior to the data collection 1.9 times 95% CI: (1.25–2.95) higher than children who had no recurrent diarrhea in the past two weeks before data collection. The odds ratio of being stunted was 36.5%; 95% CI; (0.49–0.92) less likely among children were living who have mother/caregiver’s good knowledge of handwashing during critical times than those were living who have mothers/caregiver’s poor knowledge of handwashing during the critical time (Table 5).
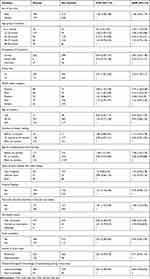 |
Table 5 Factors Associated with Stunting Among 6-to-59-Month Children in Janamora District, Northwest Ethiopia, 2020 (N=839) |
Discussion
In this study, about 44.9% (95% CI: 41.5, 48.4%) of under-five children had stunted. Age of child, family size, poor wealth status, early age starting complementary feeding, diarrhea, and knowledge regarding handwashing were factors affecting childhood chronic malnutrition.
This finding is in line with other studies done in Bahir Dar city (42.5%),24 Holeta town, Oromia regional state (48.2%),25 in Bule-Hora district (47.6%),26 Harmaya (45.8%),27 East Badawacho district (45.6%),28 the high land area of Ethiopia (47%),29 and Nigeria (47.6%).4 However, this finding is higher than mini-EDHS 2019 (37%),10 Amhara region report (41.3%),29 Sodo Zuria district (24.9%),30 Hosanna town (35.4%),7 in Shey bench district, Southwest Ethiopia (33.3%),22 and Korahay Zone, Somalia regional state (31.9%),22 Sudan (24.9%),31 and Bangladesh (41%).5 The reason for this discrepancy might be differences in altitude. Sodo Zuria, Hossana town, Shey bench studies were conducted in a lowland setting, while the current study was conducted in a highland setting. As evidence showed high stunting rate in highlands might be due to the hypoxic environment, which may alone or interact with other highland-specific socio-cultural, ecological, and genetic characteristics. In highland populations, specific genes and polymorphisms have been discovered.32,33
On the other hand, the result was lower than the study conducted in Dabat district (64.5%),34 Libo-Kemkem (49.4%),35 Gurage zone (52.5%),34 and Lasta (49.7%).36 The difference might be attributed to the difference in the educational status of caregivers. Caregivers’ knowledge will determine the level of engagement in growth monitoring, feeding, hygiene, and sanitation practice.
The odds ratio of having stunting was reduced as age increased. This finding is consistent with studies conducted in the Shire Endesilasie and Gurage zone.37 However, this finding is in contrast with studies done in Hosanna town,7 Libo-Kemkem district,35 Sodo-Zuria District,30 and Lasta district.36 The possible reason for reduction of stunting as age increment might be, the risk of diarrheal disease incidence. Diarrheal disease is the immediate cause of malnutrition. As evidence suggested that the risk of acquiring the diarrheal disease is high in under two years of children due to multiple effects; lack of active immunity in the infant and decreasing levels of antibodies acquired from the mother. Moreover, children age between 12 and 23 months are either crawling or walking, as result, they can easily pick dirt or other contaminated materials.38
The odds ratio of stunting was linearly associated with large family size. This finding is in line with studies done in Libo-Kemkem,35 Sodo-Zuria,30 Lasta district,36 Albuko district,5 and Shekel district, and western Ethiopia.39 This might be due to a shortage of food, food competition with the family, and lack of care. The other possible reason could be high fertility in the households would result in a high number of families, low socioeconomic status, and poor child caring practice.
In this study, the poor and middle wealth status were associated with a high odds ratio of stunting. This finding is consistent with the study done in Dabat district,34 and Lasta district.36 This might be poverty related to insufficient intake of nutrients, less acquisition of food, as well as it is also associated with less education and more difficult access to health services, clean water and sanitation.
Children who started complementary foods before six months of age had a higher odds ratio of stunting than children who started at the age of six months. This finding is consistent with studies done in northwest Ethiopia,40 and Shiny district, Southwest Ethiopia,22 urban slums of Bahir Dar city,24 and Nepal.8 This might be due to the inappropriate timing of introducing complementary foods may affect the child’s nutritional status negatively. Early introduction of complementary foods, particularly under unsanitary conditions, predisposed to illness and malnutrition due to the immaturity of digestive and immune systems. In addition, early initiation of complementary feeding might be a negative impact on breastfeeding frequency and duration.
The presence of diarrhea has increased the odds ratio of stunting among under-five children. This is consistent with studies conducted in Sodo Zuria district,30 west Gojjam zone,41 Wollega,42 and Bure-hora southwest Ethiopia.26 This might be diarrhea causes loss of appetite, poor absorption of nutrients, and increased nutrient demand.
In addition, mothers’ knowledge of handwashing at critical times showed a remarkable difference with childhood stunting. This result is in line with the studies done in Wondo Genet district,43 and rural Ethiopia.44 This might be, mothers’ awareness about handwashing prevents the incidence of diarrhea or soil-transmitted helminth infections.
The study has the following limitations. The study may introduce recall bias to certain variables such as initiation of breastfeeding within one hour, age of the child at complementary feeding started, prelacteal feeding, and age of the child as the study was conducted in a rural area. Moreover, it is difficult to establish a cause–effect relationship because of the cross-sectional nature of the study. Even though standard measures were used to measure height and weight, measurement errors were inevitable between assessors.
Conclusion
The prevalence of stunting among children aged 6–59 months in Janamora district was high. Being a younger child, living with large family size, poorest, poor and medium wealth status household, age of complementary feeding starting before six months, and diarrhea morbidity in the last two weeks prior to the data collection was positively associated with stunting. Start complementary feeding at six months and mothers have good knowledge of handwashing practice were negatively associated with stunting. Therefore, water, sanitation, and hygiene (WASH) programs need to give attention that enhances caregiver’s handwashing knowledge at critical times and nutrition programs on timely initiation of complementary feeding. Moreover, income-generating programs need to give priority to low quartile wealth index households.
Data Sharing Statement
Data will be available upon reasonable request from the corresponding author.
Ethical Clearance and Participants Consent
Letter of permission was obtained from the University of Gondar College of Medicine and Health Sciences Research Ethical Review Board (IRB). A support letter was obtained from the Janamora district health office. Informed verbal consent was approved from the University of Gondar IRB, and verbal consent was taken from mothers/caregivers. For the sake of confidentiality, the name of participants was not recorded on the questionnaire. All mothers/caregivers of study participants or were informed that participation in this research project has no incentives or direct benefit. The mothers/caregivers involved in the study were voluntary, and children participated through voluntary, consultation, child initiation and child direction. Participants who were unwilling to participate in the study and those who wish to quit their participation at any stage were informed to do so without any restriction. The parents/caretakers also provided consent on behalf of the children. Finally, the stunted children were linked to nearby health posts for malnutrition treatment. The whole procedure was done according to Helsinki declaration.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The Amhara Regional Health Bureau provides minimal financial support. The funder has no role in the conception, design, analysis, interpretation, and decision on publication.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Solomon D, Kebede Z, Bogale F, et al. Reducing stunting in Ethiopia:—from promise to impact‖: an evidence informed policy brief. Addis Ababa: Ethiopian Public Health Institute; 2019. Availble from: https://ephi.gov.et/wp-content/uploads/2014/04/B-Stunting-policy-brief-full-report-05-23-19.pdf. Accessed June 04, 2021.
2. World Health Organization. Reducing stunting in children: equity considerations for achieving the global nutrition targets 2025; 2018.
3. De Onis M, Borghi E, Arimond M, et al. Prevalence thresholds for wasting, overweight and stunting in children under 5 years. Public Health Nutr. 2018. doi:10.1017/S1368980018002434
4. Akombi BJ, Agho KE, Hall JJ, et al. Stunting and severe stunting among children under-5 years in Nigeria: a multilevel analysis. BMC Pediatr. 2017;17(1):15. doi:10.1186/s12887-016-0770-z
5. Sarma H, Khan JR, Asaduzzaman M, et al. Factors influencing the prevalence of stunting among children aged below five years in Bangladesh. Food Nutr Bull. 2017;38(3):291–301. doi:10.1177/0379572117710103
6. Yalew BM. Prevalence of malnutrition and associated factors among children age 6–59 months at Lalibela town administration, North WolloZone, Anrs, Northern Ethiopia. J Nutr Disorders Ther. 2014;4(132):2161–2509. doi:10.4172/2161-0509.1000147
7. Moges B, Feleke A, Meseret S, et al. Magnitude of stunting and associated factors among 6–59 months old children in Hossana Town, Southern Ethiopia. J Clin Res Bioeth. 2015;6(1):1.
8. Paudel R, Pradhan B, Wagle R, et al. Risk factors for stunting among children: a community based case control study in Nepal. Kathmandu Univ Med J. 2012;10(3):18–24. doi:10.3126/kumj.v10i3.8012
9. World Health Organization. Reducing Stunting in Children; 2018. ISBN 978-92-4-151364-7.
10. Edhs E. Mini demographic and health survey 2014: key indicators report. The DHS Program ICF. 2014;363:364.
11. Emmanuel A, Nwachukwu JO, Adetunji OE, et al. Malnutrition and associated factors among underfive in a Nigeria local government area; 2016.
12. De Onis M, Dewey KG, Borghi E, et al. The W orld H ealth O rganization’s global target for reducing childhood stunting by 2025: rationale and proposed actions. Matern Child Nutr. 2013;9:6–26.
13. Gebru KF, Haileselassie WM, Temesgen AH, et al. Determinants of stunting among under-five children in Ethiopia: a multilevel mixed-effects analysis of 2016 Ethiopian demographic and health survey data. BMC Pediatr. 2019;19(1):176. doi:10.1186/s12887-019-1545-0
14. Federal Democratic Republic of Ethiopia. Government of Ethiopia National Nutrition Program 2016–2020. Ethiopia; 2020.
15. World Health Organization. UNICEF-WHO-the World Bank: joint child malnutrition estimates. In: Levels and Trends in Child Malnutrition. (Updated September 2014). NY; 2014. Available from: https://www.who.int/nutgrowthdb/estimates/en/. Accessed June 04, 2021.
16. Hjelm L, Dm AM, Wadhwa A World food program Creation of a Wealth Index. VAM guidance paper; 2017.
17. Institute EPH, ICF. Ethiopia Mini Demographic and Health Survey 2019: Key Indicators. USA: EPHI and ICF Rockville; 2019.
18. Sisay Shine FT, Shiferaw Z, Mideksa L, Seifu W. Prevalence and associated factors of stunting among 6–59 months children in pastoral community of Korahay Zone, Somali Regional State, Ethiopia. J Nutr. 2017;7(1):1000208.
19. Wasihun AG, Dejene TA, Teferi M, et al. Risk factors for diarrhoea and malnutrition among children under the age of 5 years in the Tigray Region of Northern Ethiopia. PLoS One. 2018;13(11):11. doi:10.1371/journal.pone.0207743
20. Yurochko F. Recurrent respiratory infections in children. CHILDS HEALTH. 2012;2(37):79–83.
21. Saaka M. Relationship between mothers’ nutritional knowledge in childcare practices and the growth of children living in impoverished rural communities. J Health Popul Nutr. 2014.
22. Teferi M, Hassen H, Kebede A, Adugnaw E, Gebrekrstos G, Guesh M. Prevalence of stunting and associated factors among children aged 06–59 months in Southwest Ethiopia: a cross-sectional study. JNHFS. 2016.
23. World Health Organization. WHO_SurveillanceVaccinePreventable_16_Pertussis; 2015.
24. Demilew YM, Abie DD. Undernutrition and associated factors among 24–36-month-old children in slum areas of Bahir Dar city, Ethiopia. Int J Gen Med. 2017;10:79. doi:10.2147/IJGM.S126241
25. Tesfamariam K, Yilma D. Prevalence of stunting and its associated factors among children under 5 age in Holeta town, West Shoa zone, Oromia region, Ethiopia, 2017. EC Nutr. 2017;12(2):90–98.
26. Mandefro A, Mekitie W, Mohammed T, Lamessa DJBPH. Prevalence of undernutrition and associated factors among children aged between six to fifty nine months in Bule Hora district, South Ethiopia. BMC Public health. 2015;15(1):41.
27. Yisak H, Gobena T, Mesfin F. Prevalence and risk factors for under nutrition among children under five at Haramaya district, Eastern Ethiopia. BMC Pediatr. 2015;15(1):212. doi:10.1186/s12887-015-0535-0
28. Betebo B, Ejajo T, Alemseged F, et al. Household food insecurity and its association with nutritional status of children 6–59 months of age in east Badawacho District, south Ethiopia. J Environ Public Health. 2017;2017:1–17. doi:10.1155/2017/6373595
29. Mohammed SH, Habtewold TD, Abdi DD, et al. The relationship between residential altitude and stunting: evidence from> 26,000 children living in highlands and lowlands of Ethiopia. Br J Nutr. 2020;1–21.
30. Samson Kastro Dake FBS, Bobe TM, Tekle HA, Eg T. Predictors of stunting among children 6– 59 months of age in Sodo Zuria District, South Ethiopia. BMC Nutr. 2019.
31. Musa TH, Musa HH, Ali EA, Musa NE. Prevalence of malnutrition among children under five years old in Khartoum State, Sudan. Polish Ann Med. 2014;21(1):1–7. doi:10.1016/j.poamed.2014.01.001
32. Beall CM, Blangero J, Williams-Blangero S, et al. Major gene for percent of oxygen saturation of arterial hemoglobin in Tibetan highlanders. Am J Phys Anthropol. 1994;95(3):271–276. doi:10.1002/ajpa.1330950303
33. Beall CM. Andean, Tibetan, and Ethiopian patterns of adaptation to high-altitude hypoxia. Integr Comp Biol. 2006;46(1):18–24. doi:10.1093/icb/icj004
34. Zeritu Dewana TF, Facha W, Mekonnen N. Prevalence and predictors of stunting among children of age between 24 to 59 months in Butajira Town and Surrounding District, Gurage Zone, Southern Ethiopia. Health Sci J. 2017.
35. Selamawit Bekele Geberselassie, Solomon Mekonnen Abebe, Melsew YA, et al. Prevalence of stunting and its associated factors among children 6–59 months of age in Libo-Kemekem district, Northwest Ethiopia. PLoS One. 2018.
36. Birhanu A, Mekonen S, Atenafu A, Abebaw DC. Stunting and associated factors among children aged 6–59 months in Lasta woreda, north east Ethiopia, 2015: a community based cross sectional study design. J Fam Med. 2017;4(3):8.
37. Brhane G, Regassa N. Nutritional status of children under five years of age in Shire Indaselassie, North Ethiopia: examining the prevalence and risk factors. Kontakt. 2014;16(3):e161–e70. doi:10.1016/j.kontakt.2014.06.003
38. Melese B, Paulos W, Astawesegn FH, et al. Prevalence of diarrheal diseases and associated factors among under-five children in Dale District, Sidama zone, Southern Ethiopia: a cross-sectional study. BMC Public Health. 2019;19(1):1–10. doi:10.1186/s12889-019-7579-2
39. Mulu E, Mengistie B. Household food insecurity and its association with nutritional status of under five children in Sekela District, Western Ethiopia: a comparative cross-sectional study. BMC Nutr. 2017;3(1):35. doi:10.1186/s40795-017-0149-z
40. Beyene M, Worku AG, Wassie MM. Dietary diversity, meal frequency and associated factors among infant and young children in Northwest Ethiopia: a cross- sectional study. BMC Public Health. 2015;15(1):1007. doi:10.1186/s12889-015-2333-x
41. Teshome B, Kogi-Makau W, Getahun Z, Taye G. Magnitude and determinants of stunting in children underfive years of age in food surplus region of Ethiopia: the case of West Gojam Zone. Ethiop J Health Dev. 2009;23(2).
42. Wondemagegn A, Cheme M, Gerbi E. Predictors of chronic undernutrition (stunting) among under five children in Rural East Wollega, Oromiya Region, West Ethiopia: a community based unmatched case-control study. J Nutr Health Food Eng. 2017;7(2):00233.
43. Demssie A, Daniel D, Tefera A, Kindu H, Abebe S, Sanbata H. Knowledge, attitude and practice (KAP) of hand washing among mothers of under five children in Gotu Kebele Wondogenet Woreda Oromia, Ethiopia. Int J Environ Sci. 2017;6(4):146–153.
44. Kwami CS, Godfrey S, Gavilan H, Lakhanpaul M, Parikh P. Water, sanitation, and hygiene: linkages with stunting in Rural Ethiopia. Int J Environ Res Public Health. 2019;16(20):3793. doi:10.3390/ijerph16203793
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
