Back to Journals » Journal of Pain Research » Volume 15
The Effect of Single-Shot Erector Spinae Plane Block (ESPB) on Opioid Consumption for Various Surgeries: A Meta-Analysis of Randomized Controlled Trials
Authors Cui Y, Wang Y, Yang J, Ran L, Zhang Q, Huang Q, Gong T, Cao R, Yang X
Received 29 October 2021
Accepted for publication 27 February 2022
Published 6 March 2022 Volume 2022:15 Pages 683—699
DOI https://doi.org/10.2147/JPR.S346809
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Timothy Atkinson
Yu Cui,1,* Yu Wang,2,* Jing Yang,1,* Longqing Ran,1,* Qianqian Zhang,1 Qinghua Huang,1 Tianqing Gong,1 Rong Cao,1 Xiao Yang3
1Department of Anesthesiology, The Affiliated Hospital, School of Medicine, UESTC Chengdu Women’s & Children’s Central Hospital, Chengdu, People’s Republic of China; 2Department of Anesthesiology, No.363 Hospital, Chengdu, People’s Republic of China; 3Department of Hospital Management, The Affiliated Hospital, School of Medicine, UESTC Chengdu Women’s & Children’s Central Hospital, Chengdu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiao Yang, Tel/Fax +86 13882288881, Email [email protected]
Study Objective: Pain management plays a pivotal role in enhanced recovery after surgery (ERAS). Erector spinae plane block (ESPB) is widely used in many regions to treat perioperative pain, but its benefits are still somewhat controversial. We, therefore, intent to systematically review the available literature on ESPB, to elucidate its effects on opioid-sparing analgesia, and summarize its potential complications.
Design: Systematic review of randomized controlled trials (RCTs) with meta-analysis.
Setting: Postoperative opioid consumption for various surgeries.
Patients: Patients undergoing various surgeries.
Intervention: We searched relevant studies in PubMed, EMBASE, Medline, and the Cochrane Library up to May 16, 2021. All prospective and RCTs that compared ESPB and sham block or no block were enrolled.
Measurements: The primary outcomes were postoperative opioid consumption during the first 24 hours. The secondary outcomes were the requirement of rescue analgesia, time to first rescue analgesic and ESPB-related adverse events.
Results: We included 52 trials that reported postoperative opioid consumption during the first 24 hours. The results presented that compared to control group (ie, no intervention or a sham block), ESPB reduced the accumulated opioid consumption during the first 24 h after surgery [mean difference (MD) of − 12.83 (95% CI: − 17.29 to − 8.38; p < 0.001) mg; I2 = 100%]. Besides, ESPB could prolong time to first rescue analgesia after surgery [SMD = 5.31; 95% CI 4.01– 6.61; p < 0.001; I2 = 97%]. The number of patients who received rescue analgesia after surgery in the ESPB group was less than that in the control group (OR 0.13; 95% CI 0.09, 0.21; p < 0.001; I2 = 54%), and the incidence of PONV was lower in the ESPB group (OR 0.51; 95% CI 0.43, 0.62; p < 0.001; I2 = 19%).
Conclusion: ESPB is an effective technique on pain management with few complications.
Keywords: erector spinae plane block, ESPB, opioid consumption, postoperative nausea and vomiting, PONV
A Letter to the Editor has been published for this article.
Introduction
In recent years, multimodal approaches related to Enhanced Recovery After Surgery (ERAS), including shortening fasting time before surgery, combined with regional blocks, reducing opiate usage, early feeding after surgery, early mobilization, and optimal pain control to avoid stress, have been proposed to reduce complications and decrease hospital costs.1,2 Among those, pain management plays a pivotal role as it ensures patients’ satisfaction and early rehabilitation, and further improves outcomes.
Traditionally, opioids have been considered as an important component for pain management not only intraoperatively but also postoperatively due to their perfect analgesic efficacy. However, their relevant complications (ie, respiratory depression, nausea and vomiting, constipation, pruritis and opioid dependence) have been realized by providers. A large-scale retrospective study of 319,898 surgical procedures showed 12.2% of patients experienced opioid-related adverse events. Patients suffering from the opioid-related adverse events have a longer hospital stays, a greater overall hospital costs,3 and even a higher rate of mortality.4 More importantly, morphine may associate with tumor progression in animal model.5 The evidence indicates that opioid-related adverse events have become a major issue in deteriorating patient outcomes. Thus, the concept of opioid sparing or opioid free anesthesia has been proposed, which can be achieved by multimodal analgesia,6 such as nonopioid analgesics, regional techniques, and neuraxial anesthesia.7
Erector spinae plane block (ESPB), one of novel regional techniques, was described in 2016 by Forero et al8 and it had been performed for breast surgery,9 lumbar spine surgery,10,11 thoracoscopic surgery,12 cholecystectomy,13 and cardiac surgery.14 Despite ESPB is widely used in many regions to treat perioperative pain, its benefits are still somewhat controversial. Several meta-analyses have shown that ESPB can provide sufficient analgesic effects and reduce postoperative opioid consumption; however, the results are not convincing enough due to the small number of cases included and significant heterogeneity among studies.15,16 Besides, the mechanism of ESPB is still indeterminate. In the cadaveric study, no spreading of the dye into the paravertebral space was observed to involve the origin of the ventral and dorsal branches of the thoracic vertebral nerve,17 indicating the extent of blockage was not as wide as that observed in the initial clinical finding.8 Besides, ESPB was performed in six male volunteers, and the authors found that cutaneous sensory loss varied greatly between individuals.18
We, therefore, intent to systematically review the available literature on ESPB in various surgeries, to elucidate its effects on opioid-sparing analgesia, and summarize its potential complications.
Methods
Literature Review and Search Strategy
This review was conducted in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and assessing the methodological quality of systematic reviews (AMSTAR). The scope of this review included randomized controlled trials (RCT) reporting the ESPB in human subjects. PubMed, Embase, Ovid Medline, and Cochrane Central Register of Controlled Trial (CENTRAL) were searched without language restriction up to May 16, 2021. We considered for inclusion all prospective and randomized controlled trials which compared ESPB and sham block or no block. All search terms were listed in Supplementary Table 1. This meta-analysis was registered at PROSPERO with No. CRD42021265173.
Criteria for This Review
Inclusion Criteria
Types of patients: Adults (aged≥18 years)
Types of interventions: The intervention group was defined patients received ESPB, and the control group was defined the patients received a sham block (block with normal saline) or no intervention.
Outcomes: Outcomes include the postoperative opioid consumption.
Types of studies: Only RCTs were included in the current study.
Exclusion Criteria
Studies were excluded if the patients received a continuous infusion of local anesthetic or different ESPB methods were compared (ie, deep vs superficial ESP block, bilateral vs unilateral ESP block). Conference abstracts, letters, and study protocols, that do not contain full-text context, were also excluded.
Type of Outcome Measures
The primary outcomes were postoperative opioid consumption during the first 24 hours. A standardized conversion calculator was used to estimate the consumption of opioids, and all data was converted to intravenous morphine equivalents.19
The secondary outcomes were the requirement of rescue analgesia, time to the first rescue analgesic and ESPB-related adverse events.
Data Retraction
Three co-authors (YC, YW, JY) extracted the data according to the aforementioned inclusion and exclusion criteria independently. Disagreements over eligibility between the three reviewers were resolved by discussion. If necessary, we would take a vote to make a judgement. The data was collected as follows: the first author, the year of publication, sample size, number of patients in each group, type of surgery, ESPB group (type and dosage of local anesthetics), control group (a sham block or no block) and outcomes. The first reviewer (YC) input the data, and the data accuracy was double-checked by co-authors.
Quality Assessment
The quality of studies was evaluated by three authors (YC, YW, LR) using GRADEpro (McMaster University, Hamilton, ON, Canada, 2014) and Review Manager ® Version 5.3 for Windows (RevMan, The Cochrane Collaboration, Oxford, UK) independently, including random sequence generation, allocation concealment, performance bias, detection bias, attribution bias, reporting bias, and others. The risk of bias was judged at three levels (low risk, unclear risk and high risk).
Statistical Analysis
The continuous variables were expressed as means ± standard deviations (SD), and the dichotomous variables were presented as numbers. For dichotomous outcomes, the odds ratio (OR) or risk ratio (RR) with 95% confidence interval (95% CI) were calculated, and mean difference (MD) with 95% CI for continuous outcomes. If medians (IQR) or median (min, max) was reported, the means ± SD would be calculated according to the method described in the previous study.20 Statistical heterogeneity was estimated by I2 statistic. If a value of I2 > 50% which indicated the evidence of significant heterogeneity, the random-effect model would be used, otherwise we would use a fixed effect model. Moreover, in our study, a further subgroup analysis was conducted to identify the source of heterogeneity. We did subgroup analysis according to different type of surgery, the definition of the control group and different type of postoperative analgesics. Both the funnel plot and Egger’s test were used to identify potential publication bias, and Review Manager software (RevMan, version 5.3) was used to performed data analysis. P value< 0.05 was considered statistically significant.
Results
Search Results
A total of 1872 potentially relevant studies (PubMed 370, Embase 368, Ovid Medline 610, CENTRAL 524) were identified based on our criteria. Of these, 715 duplicated articles and 1002 studies (animal studies, editorials, pediatric surgery, protocols, retrospective studies, reviews, case reports and irrelevant studies) were excluded. The remaining 155 articles were fully reviewed. Finally, 52 RCTs with 3000 patients were included in the current review,10–12,14,21–68 each reporting the preplanned primary outcomes. Interestingly, all studies are published between 2018 and 2021, showing that ESPB is a novel technique. The process of literature selection was listed in Figure 1.
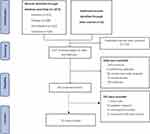 |
Figure 1 Flow chart of search strategy to identify the eligible randomized controlled trials. |
Out of the 52 RCTs, 11 were about breast surgeries,24–26,31,36,38,39,47,48,54,64 15 were about orthopedic surgeries,10,11,21–23,27,28,32,33,50,52,53,56,57,60 5 were about thoracoscopic surgeries,12,25,34,58,59 6 were about cholecystectomy,29,30,41,45,63,65 6 were about nephrolithotomy,37,44,49,61,62,68 2 were about cardiac surgeries,14,42 and 7 were others.40,43,46,51,55,66,67 The characteristics of enrolled 52 studies were listed in the Table 1.
 |  |  |
Table 1 Summary of Details About the Enrolled Trials |
Quality Assessment
The risk of bias is presented in Figure 2. All enrolled trials presented a low risk of random sequence generation, and 33 of 52 showed a low risk of allocation concealment by describing the randomized method in detail. This risk of performance and detection bias was considered as “unclear” or “high” in 30 and 13 out of 52, respectively. The funnel plot for postoperative opioid consumption showed symmetry. (Supplementary Figure 1).
 |
Figure 2 A summary of bias for each included study. |
Primary Outcomes
Postoperative Opioid Consumption During the First 24 Hours
The pooled effect of 52 RCTs examining the effect of ESPB on postoperative opioid consumption during the first 24 h after surgery revealed a significant beneficial effect compared to control group [mean difference (MD) of − 12.83 (95% CI: − 17.29 to − 8.38; p < 0.001) mg], but with extremely high heterogeneity (I2 = 100%). (Figure 3) We hypothesized that the possible reason was the different types of surgery included in the study or the difference in the definitions of the control group (ie, no intervention or sham block). Thus, the subgroup analysis was performed as follows.
 |
Figure 3 Forest plot for subgroup analysis of the effect of ESPB on postoperative opioid consumption during the first 24 h after surgery, according to the different types of surgeries. |
① Subgroup Analysis According to Surgical Types
Breast Surgery
As aforementioned, there were 11 studies (680 subjects) discussing the use of ESPB in breast surgery.24–26,31,36,38,39,47,48,54,64 The results showed that patients received ESPB were associated with a significant reduction of postoperative morphine consumption during the first 24 h after surgery (−7.01 mg, 95% CI −9.16 to −4.85; p<0.001) (Figure 3), but with a high heterogeneity (I2 = 96%).
Orthopedic Surgery
There were 15 RCTs (875 patients) evaluated the effect of ESPB in orthopedic surgery,10,11,21–23,27,28,32,33,50,52,53,56,57,60 which reported opioid consumption in postoperative 24 h. Meta-analysis demonstrated that compared to the non-block groups or the sham block, ESPB significantly reduced 24-hour opioid consumption (−9.97 mg; 95% CI: −12.58 to −7.37; p < 0.001; I2 = 98%) (Figure 3).
Thoracic Surgery or Cardiac Surgery
In the current study, we found that there were 5 RCTs studied the application of ESPB in thoracic surgery12,25,34,58,59 whereas 2 RCTs in cardiac surgery.14,42 In patients undergoing thoracic surgery or cardiac surgery, we also found that ESPB significantly reduced 24-hour opioid consumption (Thoracic surgery: −27.84.3 mg; 95% CI: −40.36 to −15.32; p < 0.001; I2 = 99% and Cardiac surgery: −56.13 mg; 95% CI: −85.03 to −27.23; p < 0.001; I2 = 97%) (Figure 3).
Nephrolithotomy or Cholecystectomy or Other Type of Surgery
Totally, there were 19 studies researching nephrolithotomy (6 studies),37,44,49,61,62,68 cholecystectomy (6 studies)29,30,41,45,63,65 and other type of surgeries (7 studies).40,43,46,51,55,66,67 By subgroup analyses, the findings were consistent with patients undergoing nephrolithotomy (MD: −13.29 mg; 95% CI: −19.59 to −6.99; p < 0.001; I2 = 97%), cholecystectomy (MD: −4.80 mg; 95% CI: −6.16 to −3.45; p < 0.001; I2 = 59%) and other types of surgery (−6.99 mg; 95% CI: −9.91 to −4.07; p < 0.001; I2 = 92%) (Figure 3).
② Subgroup Analysis According to the Definition of the Control Group
Seventeen RCTs compared ESPB with a sham block (block with normal saline),10,11,14,22,24,25,27,34,40,44–46,52,55,56,63,66 whereas 35 compared ESPB with no intervention.12,21,23,26,28–33,35–39,41–43,47–51,53,54,57–62,64,65,67,68 Although subgroup analysis was conducted, no significant reduction of heterogeneity was detected (Figure 4). Despite sensitivity analysis was performed by purging individual studies, the source of heterogeneity was still not found.
 |
Figure 4 Forest plot for subgroup analysis of the effect of ESPB on postoperative opioid consumption during the first 24 h after surgery, according to the definition of the control group. |
③ Subgroup Analysis According to Different Type of Postoperative Analgesics
For postoperative analgesics, 20 studies used morphine,11,21,27,31,32,35,36,40,43–45,47,48,50,54,55,60,64,65,68 11 studies used fentanyl,14,38,41,42,46,51,56–59,62 9 studies used tramadol,23,28–30,37,39,49,53,63 5 studies used sufentanil,12,22,24–26 4 studies used pethidine,33,34,66,67 2 studies used oxycodone10,52 and 1 study used nalbuphine.61 However, the heterogeneity did not reduce by the subgroup analysis (Figure 5).
 |
Figure 5 Forest plot for subgroup analysis of the effect of ESPB on postoperative opioid consumption during the first 24 h after surgery, different type of postoperative analgesics. |
Secondary Outcomes
Time to First Rescue Analgesia
Eighteen trials presented first analgesic demand time after surgery.11,14,21,23,27,32,33,37,40,44,45,49,50,53,54,66–68 The inverse-variance method and random effects were used to conduct analysis. Compared to control group, ESPB significantly prolonged the time to first rescue analgesia after surgery (SMD = 5.31; 95% CI 4.01–6.67; minutes; p < 0.001), but the heterogeneity was extremely high (p for heterogeneity < 0.001, I2 = 97%,) (Supplementary Figure 2).
Rescue Analgesia Requirement
Twenty-four studies, including 1287 patients, reported the number of patients who had a requirement of postoperative rescue analgesia.11,12,23,28–32,36–39,53–59,63–67 The pooled data demonstrated that ESPB significantly reduced the incidence of rescue analgesia (OR 0.13; 95% CI 0.09, 0.21; p < 0.001; I2 = 52%). Notably, sensitivity analysis by removing one study [38] decreased the heterogeneity dramatically (OR 0.12, 95% CI 0.09, 0.18; p<0.01; p for heterogeneity = 0.10, I2 = 28%) (Supplementary Figure 3).
Adverse Events Associated with ESPB
Among them, 41 studies reported the incidence of PONV after surgery.10–12,21–27,31,32,34,35,37–41,43–48,50,51,53,55–59,61–67 The results showed that compared to control group, ESPB significantly reduced the incidence of PONV (OR 0.51; 95% CI 0.43, 0.62; p<0.01; p for heterogeneity = 0.15, I2 = 19%). (Supplementary Figure 4) Because of low heterogeneity, it was unnecessary to conduct sensitivity analysis.
There were 44 RCTs mentioned the complications related to ESPB,10–12,14,21–27,31,32,34–47,50–59,61,63–68 such as local anesthetic toxicity, bleeding related to the block procedures, infection, pneumothorax, respiratory depression, and hematoma. Among them, only one study reported that 1 patient in control group experienced respiratory depression.51
Discussion
In the current meta-analysis, we included 52 trials that reported postoperative opioid consumption during the first 24 hours. The results from our study found that compared to control group (ie, no intervention or a sham block), ESPB reduced the accumulated opioid consumption during the first 24 h after surgery, but with considerable heterogeneity. Subgroup analysis, based on surgical procedures and the definition of control group and types of postoperative opioids, had little impact on reducing heterogeneity. Besides, ESPB could prolong time to first rescue analgesia after surgery. The number of patients who received rescue analgesia after surgery in the ESPB group was less than that in the control group. The incidence of PONV in the ESPB group was lower, as compared to the control group.
A study that assessed the incidence of post-surgical pain found that despite standardized pain therapy and pain management were utilized based on the national guidelines, more than half of patients still experienced moderate to severe pain after surgery.69,70 Severe pain was associated with higher resource utilization, reluctance to engage in early mobilization, psychological distress, unsatisfactory medical services, delayed early rehabilitation and prolonged hospital stay. Much effort had been put on perioperative pain management. Opioid was considered as classic drugs to treat pain after surgery for a long time. However, this view has been questioned in recent years. More and more literature results supported with sparing or even free opioid anesthesia since opioid overuse may associated with respiratory depression, the high incidence of PONV and constipation, and hyperalgesia. These adverse effects may not only lead to prolonged hospitalization, but also includes the unplanned hospital readmission, addiction, and development of chronic pain as well.7 To achieve desired pain control, regional techniques are becoming more popular because it can provide sufficient analgesia without opioid consumption. As already stated, since Forero et al described ESPB in 2016,8 lots of studies had discussed its mechanism of action both in clinical practice and in cadavers. Although the mechanism of action in ESPB was still under debate, most clinical and cadaver studies that were investigated with the use of radiological instrument showed that the spread of the contrast agent reached the neural foramina or the paravertebral/epidural space, which confirmed the effectiveness of ESPB.71 In our study, we summarized all the published RCTs on ESPB and demonstrated that ESPB was a good choice for pain relief after surgery, not only in breast and thoracic surgeries, but in orthopedics and abdominal procedures, which was consistent with the results of multiple meta-analyses.72,73
One of the strengths of our study was a large number of trials included. Most previous meta-analyses recruited only several RCTs, each involving only a few dozen patients. We enrolled 52 high-quality RCTs evaluating 3000 patients across multiple procedures, and subgroup analysis was conducted to confirm our findings. A recent study by Kendall et al reported that the patients receiving ESPB had lower postoperative pain scores during 7 surgical procedures as compared to control group, but only 13 trials with 679 patients were included.74 This implied that there were fewer RCTs for each surgical procedure, which reduced the reliable of the results. In our meta-analysis, 11 are about breast surgeries, 15 are about orthopedic surgeries, 5 are about thoracoscopic surgeries, 6 are about cholecystectomy, 6 are about nephrolithotomy, 2 are about cardiac surgeries, and 7 are others. However, the considerable heterogeneity limits our study to be generalized. The possible factors contributing to heterogeneity may be as follows. First, it is noted that the intensity of postoperative pain varied greatly among different surgical procedures. For example, patients undergoing breast surgery or orthopedics do not have visceral pain, whereas patients undergoing thoracic or abdominal surgery suffered both somatic and visceral pain. Thus, subgroup analysis according to surgical procedures was necessary to reduce heterogeneity. Disappointedly, the heterogeneity did not reduce significantly. Next, considering potential operator bias, the control group referring to a sham block might be provided in some studies, which helped reduce bias by blinding outcome assessors and participates. However, the potential negative impacts from the “sham injection” seems to be in contravention of the Declaration of Helsinki. Clearly, an invasive sham injection brought real risk such as infection or bleeding without the possibility of any clinical benefits. Hence, among the enrolled studies, 17 trials were designed with sham blocks, while 35 did not for ethical concerns. Although subgroup analysis according to the definition of control group was carried out, heterogeneity did not been significantly reduced. Last, an additional heterogeneity may be added due to the use of different formulations of opioids and rescue analgesics. One way to represent opioid utilization is by consulting an equianalgesic table, which is called “equianalgesic dose”. It is defined as the respective dose of various opioids when they provide approximately the same analgesic effect. However, in the literature, various published tables have different equivalence ratios. Shaheen et al and his colleagues reported that major variability of equianalgesic ratios recommended for both opioid rotation and conversion for commonly used opioids.75 Ratios between transdermal fentanyl and parenteral morphine were varied greatly, from 100ug:40mg to 100ug:10mg. We assumed that the utilization of different postoperative opioids might lead to the high heterogeneity, and subgroup analysis was performed but useless. However, the high-quality evidence in our study, which only included the well-designed RCTs, demonstrated ESPB is effective.
Recently, opioid-sparing or opioid-free anesthesia have been proposed based on the purpose of avoiding the adverse events related to perioperative opioid consumption. PONV is the most common side effect associated with opioid consumption. In this study, 41 trials reported the incidence of PONV, and ESPB reduced the incidence of PONV significantly. The logical explanation is that ESPB decreased the postoperative opioid consumption, and further reduced the incidence of PONV. Opioids increased the risk of PONV in a dose-dependent manner had been supported.76 Among 3000 subjects, 1497 patients received ESPB, and no patient experienced local anesthetic toxicity, bleeding, infection, pneumothorax, and hematoma, indicating ESPB is an easy and safe procedure.
Limitations
Finally, several potential limitations in our research should not be ignored when interpreting the results. First, the protocols for postoperative opioid administration are not standardized. For example, in Zhang’s study, patients-controlled intravenous analgesia was provided after surgery, meaning that patients could self-administer opioids as long as they felt pain, while the other authors designed to administer opioids if patients’ NRS≥4. Second, ESPB was performed after general anesthesia, showing that the authors did not know whether the block was successful owning to the level of dermatomal sensory loss could not be tested. Last, the advantages of ESPB in chronic pain are not investigated. In the future, some high-quality RCTs focus on the effects of ESPB in chronic pain after surgery should be performed. One third of patients suffered surgery-related chronic pain after thoracotomy.77 Unsatisfactory acute pain management may be a predisposition to develop chronic pain.78 Alleviating acute pain by regional techniques such as ESPB after surgery remains momentous area of investigation.
Conclusion
In conclusion, our meta-analysis demonstrated that compared to control group, ESPB reduced the accumulated opioid consumption during the first 24 h after surgery. Besides, ESPB could prolong time to first rescue analgesia after surgery. The number of patients who received rescue analgesia after surgery in the ESPB group was less than that in the control group. The incidence of PONV in the ESPB group was lower, as compared to the control group. All the above evidence indicates that ESPB is an effective technique on pain management with few complications.
Acknowledgment
We wish to thank Mr. Liming Luan, for providing language help and writing assistance.
Funding
This work is supported by Chengdu Municipal Health Commission (No.2020095).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ljungqvist O, Scott M, Fearon KC. Enhanced recovery after surgery: a review. JAMA Surg. 2017;152:292–298.
2. Medbery RL, Fernandez FG, Khullar OV. ERAS and patient reported outcomes in thoracic surgery: a review of current data. J Thorac Dis. 2019;11:S976–S986.
3. Oderda GM, Gan TJ, Johnson BH, et al. Effect of opioid-related adverse events on outcomes in selected surgical patients. J Pain Palliat Care Pharmacother. 2013;27:62–70.
4. Elzey MJ, Barden SM, Edwards ES. Patient characteristics and outcomes in unintentional, non-fatal prescription opioid overdoses: a systematic review. Pain Physician. 2016;19:215–228.
5. Nguyen J, Luk K, Vang D, et al. Morphine stimulates cancer progression and mast cell activation and impairs survival in transgenic mice with breast cancer. Br J Anaesth. 2014;113:i4–i13.
6. Shanthanna H, Ladha KS, Kehlet H, et al. Perioperative opioid administration. Anesthesiology. 2021;134:645–659.
7. Chia PA, Cannesson M, Bui CCM. Opioid free anesthesia: feasible? Curr Opin Anaesthesiol. 2020;33:512–517.
8. Forero M, Adhikary SD, Lopez H, et al. The erector spinae plane block: a novel analgesic technique in thoracic neuropathic pain. Reg Anesth PainMed. 2016;41:621–627.
9. Sotome S, Sawada A, Wada A, et al. Erector spinae plane block versus retrolaminar block for postoperative analgesia after breast surgery: a randomized controlled trial. J Anesth. 2021;35:27–34.
10. Zhu L, Wang M, Wang X, et al. Changes of Opioid Consumption After Lumbar Fusion Using Ultrasound-Guided Lumbar Erector Spinae Plane Block: a Randomized Controlled Trial. Pain Physician. 2021;24:E161–E168.
11. Yeşiltaş S, Abdallah A, Uysal Ö, et al. The Efficacy of Intraoperative Freehand Erector Spinae Plane Block in Lumbar Spondylolisthesis: a Randomized Controlled Study. Spine. 2021;46:E902–E910.
12. Liu L, Ni XX, Zhang LW, et al. Effects of ultrasound-guided erector spinae plane block on postoperative analgesia and plasma cytokine levels after uniportal VATS: a prospective randomized controlled trial. J Anesth. 2021;35:3–9.
13. Cesur S, Yörükoğlu HU, Aksu C, et al. Bilateral versus unilateral erector spinae plane block for postoperative analgesia in laparoscopic cholecystectomy: a randomized controlled study. Braz J Anesthesiol. 2021.
14. Athar M, Parveen S, Yadav M, et al. A Randomized Double-Blind Controlled Trial to Assess the Efficacy of Ultrasound-Guided Erector Spinae Plane Block in Cardiac Surgery. J Cardiothorac Vasc Anesth. 2021;S1053-0770(21):211.
15. Leong RW, Tan ESJ, Wong SN, et al. Efficacy of erector spinae plane block for analgesia in breast surgery: a systematic review and meta-analysis. Anaesthesia. 2021;76:404–413.
16. Ma J, Bi Y, Zhang Y, et al. Erector spinae plane block for postoperative analgesia in spine surgery: a systematic review and meta-analysis. Eur Spine J. 2021;30:3137–3149.
17. Ivanusic J, Konishi Y, Barrington MJ. A Cadaveric Study Investigating the Mechanism of Action of Erector Spinae Blockade. Reg Anesth Pain Med. 2018;43:567–571.
18. Byrne K, Smith C. Human volunteer study examining the sensory changes of the thorax after an erector spinae plane block. Reg Anesth Pain Med. 2020;45:761–762.
19. Svendsen K, Borchgrevink P, Fredheim O, et al. Choosing the unit of measurement counts: the use of oral morphine equivalents in studies of opioid consumption is a useful addition to defined daily doses. Palliat Med. 2011;25:725–732.
20. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13.
21. Zhang TJ, Zhang JJ, Qu ZY, et al. Bilateral Erector Spinae Plane Blocks for Open Posterior Lumbar Surgery. J Pain Res. 2020;13:709–717.
22. Zhang Q, Wu Y, Ren F, et al. Bilateral ultrasound-guided erector spinae plane block in patients undergoing lumbar spinal fusion: a randomized controlled trial. J Clin Anesth. 2021;68:110090.
23. Yayik AM, Cesur S, Ozturk F, et al. Postoperative Analgesic Efficacy of the Ultrasound-Guided Erector Spinae Plane Block in Patients Undergoing Lumbar Spinal Decompression Surgery: a Randomized Controlled Study. World Neurosurg. 2019;126:e779–e785.
24. Yao Y, Li H, He Q, et al. Efficacy of ultrasound-guided erector spinae plane block on postoperative quality of recovery and analgesia after modified radical mastectomy: randomized controlled trial. Reg Anesth Pain Med. 2019;5:
25. Yao Y, Fu S, Dai S, et al. Impact of ultrasound-guided erector spinae plane block on postoperative quality of recovery in video-assisted thoracic surgery: a prospective, randomized, controlled trial. J Clin Anesth. 2020;63:109783.
26. Wang HJ, Liu Y, Ge WW, et al. Comparison of ultrasound-guided serratus anterior plane block and erector spinae plane blockperioperatively in radical mastectomy. Zhonghua Yi Xue Za Zhi. 2019;99:1809–1813.
27. Wahdan AS, Radwan TA, Mohammed MM, et al. Effect of bilateral ultrasound-guided erector spinae blocks on postoperative pain and opioid use after lumbar spine surgery: a prospective randomized controlled trial. Egyptian J Anaesthesia. 2021;37(1):100–106.
28. Tulgar S, Kose HC, Selvi O, et al. Comparison of Ultrasound-Guided Lumbar Erector Spinae Plane Block and Transmuscular Quadratus Lumborum Block for Postoperative Analgesia in Hip and Proximal Femur Surgery: a Prospective Randomized Feasibility Study. Anesth Essays Res. 2018;12:825–831.
29. Tulgar S, Kapakli MS, Senturk O, et al. Evaluation of ultrasound-guided erector spinae plane block for postoperative analgesia in laparoscopic cholecystectomy: a prospective, randomized, controlled clinical trial. J Clin Anesth. 2018;49:101–106.
30. Tulgar S, Kapakli MS, Kose HC, et al. Evaluation of Ultrasound-Guided Erector Spinae Plane Block and Oblique Subcostal Transversus Abdominis Plane Block in Laparoscopic Cholecystectomy: randomized, Controlled, Prospective Study. Anesth Essays Res. 2019;13:50–56.
31. Singh S, Kumar G. Ultrasound-guided erector spinae plane block for postoperative analgesia in modified radical mastectomy: a randomised control study. Indian J Anaesth. 2019;63:200–204.
32. Singh S, Choudhary NK, Lalin D, et al. Bilateral Ultrasound-guided Erector Spinae Plane Block for Postoperative Analgesia in Lumbar Spine Surgery: a Randomized Control Trial. J Neurosurg Anesthesiol. 2020;32:330–334.
33. Siam EM, Abo Aliaa DM, Elmedany S, et al. Erector spinae plane block combined with general anaesthesia versus conventional general anaesthesia in lumbar spine surgery. Egyptian J Anaesthesia. 2020;36(1):201–226.
34. Shim JG, Ryu KH, Kim PO, et al. Evaluation of ultrasound-guided erector spinae plane block for postoperative management of video-assisted thoracoscopic surgery: a prospective, randomized, controlled clinical trial. J Thorac Dis. 2020;12:4174–4182.
35. Sharma S, Arora S, Jafra A, et al. Efficacy of erector spinae plane block for postoperative analgesia in total mastectomy and axillary clearance: a randomized controlled trial. Saudi J Anaesth. 2020;14:186–191.
36. Seelam S, Nair AS, Christopher A, et al. Efficacy of single-shot ultrasound-guided erector spinae plane block for postoperative analgesia after mastectomy: a randomized controlled study. Saudi J Anaesth. 2020;14:22–27.
37. Prasad MK, Varshney RK, Jain P, et al. Postoperative analgesic efficacy of fluoroscopy-guided erector spinae plane block after percutaneous nephrolithotomy (PCNL): a randomized controlled study. Saudi J Anaesth. 2020;14:480–486.
38. Park S, Park J, Choi JW, et al. The efficacy of ultrasound-guided erector spinae plane block after mastectomy and immediate breast reconstruction with a tissue expander: a randomized clinical trial. Korean J Pain. 2021;34:106–113.
39. Oksuz G, Bilgen F, Arslan M, et al. Ultrasound-Guided Bilateral Erector Spinae Block Versus Tumescent Anesthesia for Postoperative Analgesia in Patients Undergoing Reduction Mammoplasty: a Randomized Controlled Study. Aesthetic Plast Surg. 2019;43:291–296.
40. Mostafa SF, Abdelghany MS, Abu Elyazed MM. Ultrasound-Guided Erector Spinae Plane Block in Patients Undergoing Laparoscopic Bariatric Surgery: a Prospective Randomized Controlled Trial. Pain Pract. 2021;21:445–453.
41. Kwon HM, Kim DH, Jeong SM, et al. Does Erector Spinae Plane Block Have a Visceral Analgesic Effect?: a Randomized Controlled Trial. Sci Rep. 2020;10:8389.
42. Krishna SN, Chauhan S, Bhoi D, et al. Bilateral Erector Spinae Plane Block for Acute Post-Surgical Pain in Adult Cardiac Surgical Patients: a Randomized Controlled Trial. J Cardiothorac Vasc Anesth. 2019;33:368–375.
43. Kim D, Kim JM, Choi GS, et al. Ultrasound-guided erector spinae plane block for postoperative analgesia in laparoscopic liver resection: a prospective, randomised controlled, patient and observer-blinded study. Eur J Anaesthesiol. 2021;38:S106–S112.
44. Ibrahim M, Elnabtity AM. Analgesic efficacy of erector spinae plane block in percutaneous nephrolithotomy: a randomized controlled trial. Anaesthesist. 2019;68:755–761.
45. Ibrahim M. Erector Spinae Plane Block in Laparoscopic Cholecystectomy, Is There a Difference? A Randomized Controlled Trial. Anesth Essays Res. 2020;14:119–126.
46. Hamed MA, Goda AS, Basiony MM, et al. Erector spinae plane block for postoperative analgesia in patients undergoing total abdominal hysterectomy: a randomized controlled study original study. J Pain Res. 2019;12:1393–1398.
47. Gürkan Y, Aksu C, Kuş A, et al. Ultrasound guided erector spinae plane block reduces postoperative opioid consumption following breast surgery: a randomized controlled study. J Clin Anesth. 2018;50:65–68.
48. Gürkan Y, Aksu C, Kuş A, et al. Erector spinae plane block and thoracic paravertebral block for breast surgery compared to IV-morphine: a randomized controlled trial. J Clin Anesth. 2020;59:84–88.
49. Gultekin MH, Erdogan A, Akyol F. Evaluation of the Efficacy of the Erector Spinae Plane Block for Postoperative Pain in Patients Undergoing Percutaneous Nephrolithotomy: a Randomized Controlled Trial. J Endourol. 2020;34:267–272.
50. GhamryMR E, Elgebaly AS, Anwar AG, et al. Ultrasound-guided erector spinae plane block for acute pain management in patients undergoing posterior lumbar interbody fusion under general anaesthesia. South Afr J Anaesth Analg. 2019;25:26–31.
51. Fu J, Zhang G, Qiu Y. Erector spinae plane block for postoperative pain and recovery in hepatectomy: a randomized controlled trial. Medicine. 2020;99:e22251.
52. Finnerty D, Ní Eochagáin A, Ahmed M, et al. A randomised trial of bilateral erector spinae plane block vs. no block for thoracolumbar decompressive spinal surgery. Anaesthesia. 2021;76:1499–1503.
53. Eskin MB, Ceylan A, Özhan MÖ, et al. Ultrasound-guided erector spinae block versus mid-transverse process to pleura block for postoperative analgesia in lumbar spinal surgery. Anaesthesist. 2020;69:742–750.
54. Elsabeeny WY, Shehab NN, Wadod MA, et al. Perioperative Analgesic Modalities for Breast Cancer Surgeries: a Prospective Randomized Controlled Trial. J Pain Res. 2020;13:2885–2894.
55. Dost B, Kaya C, Ozdemir E, et al. Ultrasound-guided erector spinae plane block for postoperative analgesia in patients undergoing open radical prostatectomy: a randomized, placebo-controlled trial. J Clin Anesth. 2021;72:110277.
56. Ciftci B, Ekinci M, Gölboyu BE, et al. High Thoracic Erector Spinae Plane Block for Arthroscopic Shoulder Surgery: a Randomized Prospective Double-Blind Study. Pain Med. 2021;22:776–783.
57. Ciftci B, Ekinci M, Celik EC, et al. Ultrasound-Guided Erector Spinae Plane Block versus Modified-Thoracolumbar Interfascial Plane Block for Lumbar Discectomy Surgery: a Randomized, Controlled Study. World Neurosurg. 2020;144:e849–e855.
58. Ciftci B, Ekinci M, Celik EC, et al. Ultrasound-Guided Erector Spinae Plane Block and Thoracic Paravertebral Block for Postoperative Analgesia Management following Video-Assisted Thoracic Surgery: a Prospective, Randomized, Controlled Study. Anestezi Dergisi. 2020;28:170–178.
59. Ciftci B, Ekinci M, Celik EC, et al. Efficacy of an Ultrasound-Guided Erector Spinae Plane Block for Postoperative Analgesia Management After Video-Assisted Thoracic Surgery: a Prospective Randomized Study. J Cardiothorac Vasc Anesth. 2020;34:444–449.
60. Calia R, La Brocca L, Ventimiglia M, et al. The erector spinae plane block as an analgesic regional technique in acute post-surgical pain control in lumbar surgery. preliminary findings of a randomized trial. Reg Anesth Pain Med. 2019;44:A237.
61. Bryniarski P, Bialka S, Kepinski M, et al. Erector Spinae Plane Block for Perioperative Analgesia after Percutaneous Nephrolithotomy. Int J Environ Res Public Health. 2021;18:3625.
62. Anushree RT, Vasudevan D, Simha J, et al. To study the efficacy of bilateral ultrasound guided erector spinae plane block for postoperative analgesia in donor laparoscopic nephrectomy surgery. Indian J Anaesth. 2020;1:ISAP226.
63. Altiparmak B, Toker MK, Uysal Aİ, Kuşçu Y, Demirbilek SG. Efficacy of ultrasound-guided erector spinae plane block for analgesia after laparoscopic cholecystectomy: a randomized controlled trial. Braz J Anesthesiol. 2019;69:561–568.
64. Aksu C, Kuş A, Yörükoğlu HU, et al. Analgesic effect of the bi-level injection erector spinae plane block after breast surgery: a randomized controlled trial. Agri. 2019;31:132–137.
65. Aksu C, Kus A, Yörükoglu HU, et al. The effect of erector spinae plane block on postoperative pain following laparoscopic cholecystectomy: a randomized controlled study. JARSS. 2019;27:9–14.
66. Abu Elyazed MM, Mostafa SF, Abdelghany MS, et al. Ultrasound-Guided Erector Spinae Plane Block in Patients Undergoing Open Epigastric Hernia Repair: a Prospective Randomized Controlled Study. Anesth Analg. 2019;129:235–240.
67. Abdelhamid BM, Khaled D, Mansour MA, et al. Comparison between the ultrasound-guided erector spinae block and the subcostal approach to the transversus abdominis plane block in obese patients undergoing sleeve gastrectomy: a randomized controlled trial. Minerva Anestesiol. 2020;86:816–826.
68. Abd Ellatif SE, Sara M. Ultrasound guided erector spinae plane block versus quadratus lumborum block for postoperative analgesia in patient undergoing open nephrectomy: a randomized controlled study. Egyptian J Anaesthesia. 2021;37:123–134.
69. Simanski CJ, Althaus A, Hoederath S, et al. Incidence of chronic postsurgical pain (CPSP) after general surgery. Pain Med. 2014;15:1222–1229.
70. Edgley C, Hogg M, De Silva A, et al. Severe acute pain and persistent post-surgical pain in orthopaedic trauma patients: a cohort study. Br J Anaesth. 2019;123:350–359.
71. De Cassai A, Bonvicini D, Correale C, et al. Erector spinae plane block: a systematic qualitative review. Minerva Anestesiol. 2019;85:308–319.
72. Zhang Y, Liu T, Zhou Y, et al. Analgesic efficacy and safety of erector spinae plane block in breast cancer surgery: a systematic review and meta-analysis. BMC Anesthesiol. 2021;21:59.
73. Huang W, Wang W, Xie W, et al. Erector spinae plane block for postoperative analgesia in breast and thoracic surgery: a systematic review and meta-analysis. J Clin Anesth. 2020;66:109900.
74. Kendall MC, Alves L, Traill LL, et al. The effect of ultrasound-guided erector spinae plane block on postsurgical pain: a meta-analysis of randomized controlled trials. BMC Anesthesiol. 2020;20:99.
75. Shaheen PE, Walsh D, Lasheen W, et al. Opioid equianalgesic tables: are they all equally dangerous? J Pain Symptom Manage. 2009;38:409–417.
76. Hozumi J, Egi M, Sugita S, et al. Dose of intraoperative remifentanil administration is independently associated with increase in the risk of postoperative nausea and vomiting in elective mastectomy under general anesthesia. J Clin Anesth. 2016;34:227–231.
77. Bayman EO, Parekh KR, Keech J, et al. A Prospective Study of Chronic Pain after Thoracic Surgery. Anesthesiology. 2017;126:938–951.
78. Glare P, Aubrey KR, Myles PS. Transition from acute to chronic pain after surgery. Lancet. 2019;393:1537–1546.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
