Back to Journals » Nutrition and Dietary Supplements » Volume 9
The effect of micronutrient supplementation on active TB incidence early in HIV infection in Botswana
Authors Campa A, Baum MK, Bussmann H, Martinez SS, Farahani M, van Widenfelt E, Moyo S, Makhema J, Essex M, Marlink R
Received 30 September 2016
Accepted for publication 12 May 2017
Published 14 July 2017 Volume 2017:9 Pages 37—45
DOI https://doi.org/10.2147/NDS.S123545
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Chandrika J Piyathilake
Adriana Campa,1 Marianna K Baum,1 Hermann Bussmann,2 Sabrina Sales Martinez,1 Mansour Farahani,3 Erik van Widenfelt,2 Sikhulile Moyo,2,3 Joseph Makhema,2 Max Essex,2,3 Richard Marlink3
1Department of Dietetics and Nutrition, Robert Stempel College of Public Health and Social Work, Florida International University, Miami, FL, USA; 2Botswana Harvard AIDS Institute Partnership, Gaborone, Botswana; 3The Harvard T.H. Chan School of Public Health, Boston, MA, USA
Background: Coinfection with active tuberculosis (TB) is one of the leading causes of death in people living with HIV (PLWH) in Africa. This investigation explores the role of micronutrient supplementation in preventing active TB in PLWH.
Methods: A randomized trial of nutritional supplementation was conducted among antiretroviral-
naïve (without previous antiretroviral treatment [ART]) HIV-infected people in Botswana between 2004 and 2009. The study had a factorial design with four arms: the selenium (Se) alone arm, the multivitamins (MVT) alone arm that contained vitamin B complex and vitamins C and E, the combined Se+MVT group and the placebo group. Those participants with prior or current active TB were excluded, as were participants with advanced HIV disease (CD4 <250 cells/µL) or who had already qualified for ART. HIV-positive adults (N=878) were followed monthly for study pill dispensation, every 3 months for CD4 cell count and every 6 months for viral load during 24 months or until they were started on ART.
Results: The participants’ characteristics were not significantly different among the four groups at baseline. Supplementation with Se alone (hazard ratio =0.20, 95% confidence interval: 0.04, 0.95, P=0.043) and the two combined SE groups (Se and Se+MVT) had significantly lower risk of developing incident TB disease compared with placebo in multivariate adjusted models (hazard ratio=0.32, 95% confidence interval: 0.11, 0.93, P=0.036). Multivitamins alone did not affect the incidence of TB. Isoniazid preventive therapy was received by 12.2% of participants, a rate that was not significantly different among the four study arms (P=0.122) and the newly diagnosed cases.
Conclusion: Se supplementation, alone and with MVT, decreased the incidence of TB disease in PLWH who were ART-naïve. Supplementation with these micronutrients should be considered in HIV infection, prior to ART, in areas where TB and malnutrition are endemic.
Keywords: selenium, multivitamin, TB and HIV
Introduction
Human immunodeficiency virus (HIV) has become one of the main infectious causes of death among adults in the world, and closely behind HIV in global number of deaths is tuberculosis (TB).1 In Africa, where most of the new cases of HIV and TB are reported, 31% of TB cases are in people living with HIV (PLWH) and the rate surpasses 50% in the southern part of the continent, where HIV/TB coinfection is the main cause of death, including in those receiving antiretroviral treatment (ART).2 The global incidence of HIV/TB coinfections was 1.2 million (11% of all new TB cases) in 2015, of which the majority were reported in Africa.1,3
As with HIV, the majority of TB cases (>95%) are in countries with limited resources, in which malnutrition is also highly prevalent.3 TB is caused by Mycobacterium tuberculosis. TB infection remains in latency while the immune system controls the mycobacteria.4 The lifetime risk of conversion to active TB is 5%–10% in otherwise healthy populations,5 but this risk increases approximately 19 times in HIV infection.2,3,6
With adequate treatment, TB is curable; but without treatment, and in combination with HIV, it is highly fatal.2 The World Health Organization recommends the provision of ART to those who are HIV/TB coinfected, irrespective of their CD4 cell count.1,6,7 The recent increase in access to ART in sub-Saharan Africa has contributed to the decline of TB case fatality in the region,8 with 78% of HIV/TB coinfected people receiving ART.
Botswana reports one of the highest HIV prevalence rates (24.1% rate of HIV infection in the 15–49-year-old age group), and has a TB incidence of 503 per 100,000 inhabitants and 65% of HIV/TB coinfection.9,10 Despite the magnitude of the HIV epidemic in Botswana and the high cost of ART,11 45% of all known HIV patients with active TB coinfection were started on ART.9 During the time of this study, a plan to increase access to preventive isoniazid (INH) for 6 months against TB was implemented in PLWH in Botswana, without obtaining a tuberculin skin test (TST).12 During participation in this clinical trial, 12.5% of participants received INH preventive therapy (IPT). Observational studies showed low serum levels of micronutrients,13 including selenium,14 iron,15 zinc16 and vitamins A, C, E17 and D18 in patients with active TB.19 Micronutrient supplementation in HIV/TB coinfected patients improved both HIV and TB outcomes.20–22 All of these previous studies, however, were conducted in participants who were in the late stages of HIV/TB coinfection. In contrast, our study differed from these HIV or TB-related studies in that multivitamin (MVT) and selenium (Se) supplementation alone or in combination was started in participants who were diagnosed with HIV infection early and who did not have a prior diagnosis of TB. Patients with a prior medical history of TB disease were excluded. Different from the parent study,23 this research considered newly diagnosed TB as its main endpoint. The analyses were conducted to provide evidence to prospectively determine the efficacy of Se and MVT supplementation, individually or combined, on the prevention of newly diagnosed active TB.
Methods
Study design
The study was a randomized, double-blind, placebo-controlled trial of nutritional supplementation conducted in Gaborone, Botswana.23 Before the study was started, it was registered at ClinicalTrials.gov, identifier: NCT00149656. Participants were enrolled and followed from 2004 to 2009 when the database was censored. This is the first report of a defined secondary endpoint for this trial, which considered newly diagnosed TB as an acquired immune deficiency syndrome (AIDS)-defining condition. The original primary endpoint for this registered trial was CD4 cell count <200 cells/µL.23 The phases of the analysis are described in Figure 1. The Harvard Institutional Review Board, the Florida International University Institutional Review Board, the Botswana Health Research Unit of the Botswana Ministry of Health, and the Independent Data and Safety Monitoring Board consisting of two members from Botswana (statistician and HIV physician) and three members from the US (two infectious disease physicians from Tufts and Harvard Universities and one epidemiologist from Florida International University), approved the protocols before initiation and monitored the study’s performance. The study adhered to the Guidelines for the Protection of Human Subjects of Research of the US National Research Act, the Commission for the Protection of Human Subjects of Biomedical and Behavioral Research that were created by the Act, and the Belmont Report. Each participant in this investigation provided a signed consent form before any study activity was performed.
There were two initial visits to assess compliance with scheduled visits, which included screening and randomization of asymptomatic adults who were recently diagnosed with HIV disease. At these visits, eligibility for the trial was assessed and demographic information collected. Participants needed to be ART-naïve, early in the HIV disease process (CD4 cell count >350 cells/μL), with a body mass index (BMI) more than 18 kg/m2 for women and 18.5 kg/m2 for men, of age 18 years or older and have no medical history of AIDS-defining conditions, including TB disease. Pregnancy was an exclusion criterion, because it requires prenatal micronutrient supplementation. Using a factorial design, the participants were randomized into four arms and followed monthly for 24 months for supplement dispensation and follow-up.
During the baseline visit and every 3 months for 2 years, blood was drawn to measure CD4 cell counts and the study nurse or study physician completed physical examinations and collected the participants’ medical history and prescriptions with an emphasis on TB status. Time and duration of IPT was abstracted from medical charts. At the baseline visit and every 6 months, blood was also drawn to assess HIV viral load, plasma micronutrient levels (in 10% random subsample), high-sensitive C-reactive protein (hsCRP), and complete blood counts and blood chemistry.
For the treatment protocol (Table 1), participants were assigned randomly to MVTs alone, selenium alone, MVTs plus selenium, or placebo. The content of each of the supplements is shown in Table 1. The acceptability and adherence to the supplement, as well as the adverse effects and morbidity were documented monthly. Adherence was measured with monthly pill counts and a five-question questionnaire about the frequency of missed doses and the reasons for missing them. To maintain blinding, all the pills were coated with an opaque vanilla flavor to look and taste similarly. Only the statistician and the pharmacist were not blinded to the assignments, and they would communicate with the investigators in case unblinding or stopping the study were necessary. Both investigators and participants were blinded.
  | Table 1 Detailed formula composition by study group |
Once an HIV-positive participant was diagnosed as a TB case, according to Botswana’s health care standards, he/she qualified for ART because TB was considered an AIDS-defining illness. At this point, nutrient supplementation was discontinued and the participant was referred to receive ART and TB treatment at the Princess Marina Hospital, Gaborone, Botswana. All participants received their routine medical care at the Botswana–Harvard Partnership/Florida International University Research Clinic, and any abnormal laboratory values found in the process of research were referred to their physician. Contacts authorized by the participants provided details of the participants’ death. Death certificates were issued by the Botswana Ministry of Health Department of Vital Statistics.
Biochemical assays
The following biochemical assessments were performed.
Every 3 months
Differential counts and phenotyping lymphocytes were determined by a Coulter MaxM and a four-color panel of monoclonal antibodies.
Every 6 months
Pulmonary TB and lymph node (glandular) TB were confirmed using Ziehl–Neelsen staining of sputum or fine-needle aspirate to detect acid-fast bacilli. Extrapulmonary new TB cases other than glandular TB were diagnosed by typical imaging findings (X-rays, ultrasound) or cerebrospinal fluid. HIV viral load was measured by the Amplicor reagents and protocol (Roche Diagnostics, Branchburg, NJ, USA). The high-sensitivity C-reactive protein (hsCRP) was assessed using the CRP Ultra-Range Reagent Kit (Equal Diagnostics, Exton, PA, USA), and plasma minerals, selenium and zinc, were measured by atomic absorption spectrophotometry.
The laboratory staff was blinded to a 5% duplicate testing that was conducted for quality assurance.
Sample size
To test the hypothesis that taking MVT and selenium alone or in combination was associated with a significantly longer time to TB diagnosis than taking placebo pills and to estimate the sample size,24 we assumed that the probability of having an event was 30% during the 24 months of follow-up; therefore, the total sample size needed was 704 participants. The power to test the hypothesis with this sample size for this endpoint was 0.97. We also assumed that the attrition rate was 15% during the follow-up period, which was based on the attrition rates from other US National Institutes of Health funded studies conducted by Harvard University in Botswana. It was estimated that 828 study participants were needed to have more than adequate power. Thus, 207 were assigned to each of the four groups. Toward the end of the study, we requested permission from the regulatory boards to increase the recruitment by 50 participants to make up for those who were lost between the randomization visit and the first visit. The final sample size was 878 participants.
Statistical analyses
The endpoint for these analyses was an active TB diagnosis. Intent-to-treat analyses were conducted. The Kaplan–Meier method tested by a two-sided log-rank test was used to estimate the time to TB diagnosis curves. Cox’s proportional hazards models generated hazard ratios (HRs) and 95% confidence intervals. The models included age, gender, supplementation arms, hsCRP, BMI and CD4 counts, and HIV viral loads at baseline and over time. Statistical significance was set as P<0.05. After the last scheduled follow-up visit took place on July 24, 2009, the database was censored. A test for interaction between the Se and MVT arms using the SAS F-test general linear model procedure (PROC GLM) was conducted to assess the independent effect of each intervention.25,26 The Peto stopping rule was set for potential early discontinuation of the research at a nominal P=0.001 for efficacy and P=0.05 for safety endpoints.27
Results
This study randomized 878 participants, and 24 newly diagnosed TB cases were confirmed during the follow-up period. The research trial took place between December 2004 and July 2009; the 25th to 75th percentiles for the time of follow-up were 15–24 months. Nonparametric Wilcoxon test showed no significant differences in the parameters of HIV disease progression and demographic characteristics between the four groups at baseline (P>0.05), which implies successful randomization. The median CD4 cell count at baseline was 420 (interquartile range, 336–550) cells/μL, with 33% of the participants (286/872) having a CD4 count ≥500 cells/μL. The median HIV viral load was 4.14 (interquartile range, 3.49–4.78) log10 copies/mL and the mean BMI was 24.29± standard deviation (SD) 5.36 kg/m2 (Table 2). In Figure 1, the Consolidated Standards of Reporting Trials flowchart describes the disposition of participants throughout the clinical trial. Among the 878 participants, 6 were lost to follow-up between the randomization and baseline visits, 4 in the Se arm and 2 from the placebo group, and were not included in the intent-to treat analyses. The average loss to follow-up was 3.5% per year, for a total of 17.5% over 5 years. The losses to follow-up between the groups were not significantly different (df=3, chi-square value =1.75, P=0.63; Figure 1). Adherence to the micronutrient supplement was high (96%), and attendance to the research visits was 89.84%. When compared with the placebo group, the comparison of adverse events indicated that the supplements were safe.23
  | Table 2 Population statistics of the overall cohort (878) and tuberculosis cases (n=24) Abbreviations: BMI, body mass index; IQR, interquartile range; SD, standard deviation. |
IPT was used in 12.2% (107/878) of the cases during or before participation. There were no significant differences in the use of INH preventively among the four study groups (P=0.122; Figure 1). The rate of preventive INH among the incident cases of TB was 12.5% and not different from the total rate, that is, 107/878 (12.2%). Only one case of pulmonary TB was diagnosed by smear positivity; the rest of the pulmonary TB cases (n=16) were diagnosed by chest X-rays. Out of the 24 cases, 7 (29%) were extrapulmonary TB. Of these seven cases, four cases were TB lymphadenitis, two were diagnosed with pleural effusion and one was a case of TB meningitis. The TB incidence rate in our study was 1.4% per year, which is higher (1.26% per year)28 or similar (1.6% per year)28 to other reports of the incidence rates of active TB in HIV-positive patients receiving IPT for 6 months in Botswana. This rate is 2.7 times higher than that in the general population (503 per 100,000 people/year).9 Mortality was low (0.34%), probably because the participants were early in the disease at the beginning of the follow-up period.
The test of interaction between Se and MVT supplementation was not significant (P=0.82). Comparing the groups that received selenium methionine supplementation (Se alone and Se+MVT) with those that were not supplemented with selenium (the MVT and placebo groups) also tended to show reduced risk for selenium supplementation, although it did not reach significance (multivariate adjusted HR=0.41, 95% confidence interval [CI]: 0.15, 1.07, P=0.067; Table 3). Figures 2 and 3 and Table 4 show that supplementation with selenium alone and in combination with MVTs prolonged the time to a TB diagnosis. Those who were supplemented with selenium alone (Figure 2; Table 4) had a significantly lower risk of being diagnosed with TB disease (3/219, 1.37%), compared to placebo (10/217, 4.6%; multivariable adjusted HR=0.20, 95% CI: 0.04, 0.95, P=0.043). When the two groups that received Se, the Se and Se+MVT groups, were compared to placebo (Figure 3; Table 4), the risk of developing TB disease was also decreased (9/439, 2.05%, multivariable adjusted HR=0.32, 95% CI: 0.11, 0.93, P=0.036). MVT supplementation alone (5/219, 2.28%) had no significant effect on the number of cases diagnosed with TB (Table 4). All analyses were controlled for CD4 cell count at baseline and over time, viral load at baseline and over time, age and hsCRP at baseline and over time as the continuous variables and gender as a dichotomous variable.
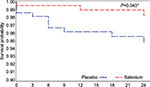  | Figure 2 Kaplan–Meier survival curve comparing supplementation with Se only vs placebo. Note: *Significant. |
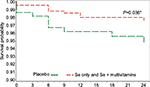  | Figure 3 Kaplan–Meier survival curve comparing the two groups receiving selenium (Se only + combination of Se and multivitamins) vs placebo. Note: *Significant. |
Plasma levels of selenium were measured in a subset of participants (n=133). Across the four randomized groups, there was a high frequency (126/133, 95%) of deficient Se levels (<0.085 μg/mL) at baseline. The frequency of selenium deficiency was not significantly different among the four randomized arms. Blood was drawn during the trial for Se levels and analyzed at the end of the trial to avoid unblinding. Those supplemented with Se (Se only and Se+MVT) had an increase in their plasma Se levels from a mean of 0.067 mg/L ±0.018 SD to a mean of 0.163 mg/L ±0.119 SD (P<0.001), while the plasma Se levels of participants who were not supplemented with selenium (placebo and MVT only) remained stable (0.07 mg/L ±0.02 SD vs 0.072 mg/L ±0.013 SD, P=0.44).
Discussion
The design of the study ensured that randomization successfully created four groups that were similar in parameters of HIV disease stage and demographic characteristics at baseline. The results demonstrated that supplementation with micronutrients may be a cost-effective prophylactic and therapeutic intervention for PLWH, who are at high risk for TB disease in low-resource countries. Both HIV29 and TB30 were associated with decreased levels of serum micronutrients, and supplementation with combined MVT and selenium reduced the risk of the recurrence of TB22 and reduced the TB-related mortality late in HIV/TB coinfected participants.21
Our randomized clinical trial of MVT and selenium supplementation for 24 months significantly decreased the risk of a new TB diagnosis in PLWH, who were asymptomatic, early in the disease process and who had not received prior ART (ART-naïve). The analyses of both groups containing selenium (selenium alone and Se+MVT) showed a lower risk of a case of newly diagnosed TB. In addition, we have shown previously that supplementation with MVT (vitamin B complex and vitamins C and E) and selenium delayed HIV disease progression and was safe in this population.23 The rate of extrapulmonary TB was unusually high in comparison with the rate of cases of pulmonary TB, but this is consistent with other studies which found that in HIV/TB coinfection, the cases of extrapulmonary TB are almost double.31,32
Other studies of nutritional supplementation among TB patients and HIV/TB coinfection have supported the beneficial effect of selenium supplementation on cases of active TB. Range et al21 supplemented MVTs, Se and copper with or without zinc in a factorial design to 499 TB patients, of whom 213 (43%) were HIV/TB coinfected and were undergoing TB treatment for 8 months. Compared to placebo, supplementation reduced mortality by ~70% in the HIV/TB coinfected group at the end of their TB treatment. In Tanzania, Villamor et al22 provided a formula with MVTs and selenium to 887 patients diagnosed with TB disease, who were initiating their TB treatment; of these participants, 471 were coinfected with HIV. In this study, MVTs with selenium significantly decreased the risk of TB recurrence by 45% overall and by 63% in the HIV/TB coinfected patients. Supplementation also increased the CD3+ and CD4+ cell counts and decreased the incidence of extrapulmonary TB and genital ulcers in patients who were HIV negative. In contrast, in a research trial in Malawi, Semba et al33 did not find significant differences on survival in patients with TB regardless of their HIV status, when supplementation of an MVT–mineral formula for 24 months was compared with placebo pills.
In HIV/TB coinfection, the combined infections have a depressive synergistic effect on immunity and nutritional status and accelerate HIV disease progression.34 Treatment with micronutrients has an immune-stimulatory effect,21,22 especially in areas where micronutrient deficiencies are common.35 The short-term supplementation with micronutrients, however, seems to be less effective in changing disease outcomes than long-term supplementation,21,22,36 which seems to be effective in supporting nutritional status and reversing the metabolic abnormalities produced by the combined infections.37 While micronutrient supplementation, including MVT and selenium, has improved both HIV and TB outcomes in patients who were either already diagnosed with TB or were HIV/TB coinfected,21,33 this is the first clinical trial that showed a significant effect of micronutrients early in HIV disease, prior to treatment, in delaying HIV disease progression23 and preventing the development of new cases of TB in an ART-naïve, HIV-infected adult population.9
Several mechanisms of action may be involved in the relationship between nutrients and these infections. Both HIV and TB disease may produce micronutrient deficiencies and rapid weight loss by increasing nutrient demands, altering metabolic mechanisms or by inducing anorexia.38,39 In turn, low BMI and deficiencies in nutrients needed to mount adequate immune responses, such as selenium, may lower cell-mediated immunity, an important host defense against both HIV and TB, as well as increase the susceptibility to TB activation and delay or prevent the response to anti-TB treatment.30,40,41
Our studies, as well as those of other investigators, have shown that deficiencies of several micronutrients, such as B vitamins, vitamins C and E, and selenium, are associated with HIV-1–related prognosis and mortality in HIV-infected people.42,43,44 The evidence from this study supports the public health recommendation of providing low-cost supplementation with selenium and MVT to PLWH in the asymptomatic stages of HIV infection to slow the disease progression and decrease the incidence of TB. Adequate nutritional status is critical at all stages of infection, because malnutrition, as a comorbid condition in infections, weakens the immune response, allowing infections to fester and become fatal.45
In agreement with other studies of effectiveness of IPT in this region, our findings suggested that the earlier strategy of 6-month IPT without TST did not provide additional protection to PLWH, once the therapy ended.46 New research by Smith et al47 conducted in Botswana suggests that to increase the benefits and cost-effectiveness of IPT in PLWH, it needs to be targeted on TST-positive, HIV-infected people with CD4 cell counts <250 cell/µL and provided for at least 36 months.47
Limitations
The study targets a secondary outcome of the original study on the effect of nutritional supplementation on HIV disease progression, including AIDS-defining conditions, of which TB is one of the most devastating. The parent clinical trial study23 had a strong design, high internal validity, adequate power and adherence to supplementation, and very modest drop-out rates. Randomization of the cohort ensured that the exposure characteristics of the four groups were balanced, including exposure to IPT, TB and other opportunistic infections. Our findings are preliminary and demonstrate the need for a well-designed study on the effect of supplementation on the manifestation of TB disease in PLWH before and after ART. Our results might be generalizable only to other HIV-infected cohorts in resource-limited settings, where TB disease and inadequate nutritional status are endemic, and the initiation of ART is withheld depending on the levels and rate of decline of CD4 cell counts, unless the treatment is clinically indicated.
The effect of nutrient supplementation on morbidity and mortality in HIV infection might have been understated, as some participants might have discontinued from the study because of developing other endpoints that might have qualified them for ART before developing TB disease. In addition, participants with the potential for developing active TB might have been lost to follow-up, have died or have become severely ill with other conditions during the time of this research. However, there were no differences in the percentage of participants lost to follow-up in the four arms of the study (Figure 1). Initiation of ART is recommended after the initiation of TB treatment in cases with a diagnosis of TB disease,1,7 and supplementation with micronutrients is not a substitute for early ART; however, it is one more tool for supporting the immune system that it is under the double attack of HIV and TB.
Conclusion
This randomized clinical trial confirmed that micronutrient supplementation of ART-naïve PLWH, who were early in the disease processes, was safe and reduced TB-related morbidity in Botswana. Considering our earlier finding from this clinical trial that MVTs plus selenium supplementation significantly delayed HIV disease progression,23 this combination of micronutrients should also be considered for the prevention of TB in this at-risk population.
Summary
A randomized, double-blind nutritional trial of supplementation was conducted. Participants received selenium alone, multivitamins alone and combined selenium and multivitamin supplementation and were compared to placebo in a factorial design. Participants were people living with HIV, who had not been previously exposed or received antiretrovirals (ART-naïve), in Botswana, Africa.
Acknowledgments
The authors are grateful to people living with HIV in Botswana who participated in this research. Without their contributions, the findings from this study would have never been possible. The investigators are also grateful for the continuous support and cooperation of the Government of Botswana and the Botswana–Harvard Partnership. The authors are also grateful for the advice and leadership provided by Dr Jag Khalsa, Chief, Division of Pharmacotherapies and Medical Consequences of Drug Abuse at the National Institutes on Drug Abuse, NIH, USA. Very special acknowledgments to our Program Manager at the Harvard School of Public Health, Patricia J Burns, to the Study Coordinator at the Botswana Harvard AIDS Institute Partnership, Lesedi Tsalaile, and to our Data Manager at Florida International University, Yinghui Li, without whose efforts and dedication to this research, it could not have been completed.
Dr Marianna K Baum, Principal Investigator, Adriana Campa, Co-Principal Investigator, and Richard Marlink, Principal Investigator of the Harvard subcontract for the NIDA grant, and coauthors of this report had full access to the database and take responsibility for the integrity and accuracy of data analysis.
This trial was funded by the National Institute on Drug Abuse (R01 DA016551). The agency declares no role in study design, data collection, analysis, interpretation, writing or submission of this manuscript for publication.
Disclosure
The authors report no conflicts of interest in this work.
References
World Health Organization. Global Tuberculosis Report. Available from: http://www.who.int/tb/publications/global_report/gtbr2016_executive_summary.pdf?ua=1. Accessed May 2, 2017. | ||
World Health Organization. Tuberculosis (TB). TB and HIV. Available from: http://www.who.int/tb/areas-of-work/tb-hiv/en/. Accessed May 2, 2017. | ||
World Health Organization. HIV-Associate Tuberculosis. Fact sheet. http://www.who.int/tb/areas-of-work/tb-hiv/tbhiv_factsheet_2016_web.pdf?ua=1. Accessed May 2, 2017. | ||
Barry CE, Boshoff HI, Dartois V, Dick T, Ehrt S, Flynn J, et al. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat Rev Microbiol. 2009;7(12):845–855. | ||
Harries AD, Dye C. Tuberculosis. Ann Tropical Med Parasitol. 2006;100(5–6):415–431. | ||
WHO. HIV/AIDS. Tuberculosis and HIV. Available from: http://www.who.int/hiv/topics/tb/about_tb/en/. Accessed May 2, 2017. | ||
World Health Organization. Interim Policy on Collaborative TB/HIV Activities; WHO/HTM/TB/2004.330 WHO/HTM/HIV/2004.1. Available from: http://citeseerx.ist.psu.edu/viewdoc/summary?doi=10.1.1.384.7644. Accessed June 14, 2017. | ||
UNAIDS. Getting to zero. 2011–2015 strategy. Geneva, Joint United Nations Programme on HIV/AIDS. Available from http://www.unodc.org/documents/southeastasiaandpacific/Publications/2011/JC2034_UNAIDS_Strategy_en.pdf. Accessed June 14, 2017. | ||
World Health Organization. Botswana Tuberculosis profile. Available from: https://extranet.who.int/sree/Reports?op=Replet&name=%2FWHO_HQ_Reports%2FG2%2FPROD%2FEXT%2FTBCountryProfile&ISO2=BW&outtype=html. Accessed June 12, 2012. | ||
Wester W, Bussman H, Avalos A, et al. Establishment of public antiretroviral treatment clinic for adults in urban Botswana: Lessons learned. Clin Iinfect Dis. 2005:40(7):1041–1044. | ||
Bussmann H, Wester CW, Thomas A, et al. Response to zidovudine/didanosine-containing combination antiretroviral therapy among HIV-1 subtype C-infected adults in Botswana: two-year outcomes from a randomized clinical trial. J Acquir Immune Defic Syndr. 2009;51(1):37–46. | ||
Sibanda T, Tedla Z, Nyirenda S, et al. Anti-tuberculosis treatment outcomes in HIV-infected adults exposed to isoniazid preventive therapy in Botswana. Int J Tuberc Lung Dis. 2013;17(2):178–185. | ||
Sinclair D, Abba K, Grobler L, Sudarsanam TD. Nutritional supplements for people being treated for active tuberculosis. Cochrane Database Syst Rev. 2011;(11):CD006086. | ||
Ramakrishnan K, Shenbagarathai R, Kavitha K, Thirumalaikolundusubramanian P, Rathinasabapati R. Selenium levels in persons with HIV/tuberculosis in India, Madurai City. Clin Lab. 2012;58(1–2):165–168. | ||
Isanaka S, Aboud S, Mugusi F, et al. Iron status predicts treatment failure and mortality in tuberculosis patients: a prospective cohort study from Dar es Salaam, Tanzania. PLoS One. 2012;7(5):e37350. | ||
Afridi HI, Kazi TG, Kazi N, et al. Evaluation of zinc, copper and iron in biological samples (scalp hair, blood and urine) of tuberculosis and diarrhea male human immunodeficiency virus patients. Clin Lab. 2011;57(9–10):677–688. | ||
Plit ML, Theron AJ, Fickl H, van Rensburg CE, Pendel S, Anderson R. Influence of antimicrobial chemotherapy and smoking status on the plasma concentrations of vitamin C, vitamin E, beta-carotene, acute phase reactants, iron and lipid peroxides in patients with pulmonary tuberculosis. Int J Tuberc Lung Dis. 1998;2(7):590–596. | ||
Nnoaham KE, Clarke A. Low serum vitamin D levels and tuberculosis: a systematic review and meta-analysis. Int J Epidemiol. 2008;37(1):113–119. | ||
Louw JA, Werbeck A, Louw MEJ, Kotze TJvW, Cooper R, Labadarios D. Blood vitamin concentrations during the acute phase response. Crit Care Med. 1992;20(7):934–941. | ||
Mehta S, Mugusi FM, Bosch RJ, et al. A randomized trial of multivitamin supplementation in children with tuberculosis in Tanzania. Nutr J. 2011;10:120. | ||
Range N, Changalucha J, Krarup H, Magnussen P, Andersen AB, Friis H. The effect of multi-vitamin/mineral supplementation on mortality during treatment of pulmonary tuberculosis: a randomised two-by-two factorial trial in Mwanza, Tanzania. Br J Nutr. 2006;95(4):762–770. | ||
Villamor E, Mugusi F, Urassa W, et al. A trial of the effect of micronutrient supplementation on treatment outcome, T cell counts, morbidity, and mortality in adults with pulmonary tuberculosis. J Infect Dis. 2008;197(11):1499–1505. | ||
Baum MK, Campa A, Lai S, et al. Effect of micronutrient supplementation on disease progression in asymptomatic, antiretroviral-naïve, HIV-infected adults in Botswana. JAMA. 2013;310(20):2154–2163. | ||
Goggins WB, Finkelstein DM, Schoenfeld DA, Zaslavsky AM. A Markov chain Monte Carlo EM algorithm for analyzing interval-censored data under the Cox proportional hazards model. Biometrics. 1998;54(4):1498–1507. | ||
McAllister FA, Straus SE, Sackett DL, Altman DG. Analysis and reporting of factorial trials: systematic review. JAMA. 2003, 289(19):2545–2553. | ||
Gaugler T, Akritas MG. Testing for interaction in two-way random and mixed effects models: the fully nonparametric approach. Biometrics. 2011;67(4):1314–1320. | ||
Hughes MD, Pocock SJ. Interim monitoring of clinical trials. In: Finkelstein DM, Schoenfeld DA, editors. AIDS Clinical Trials. Hoboken, NJ: Wiley & Sons; 1995:177–196. | ||
Samandari T, Agizew TB, Nyirenda S, et al. 6-month versus 36-month isoniazid preventive treatment for tuberculosis in adults with HIV infection in Botswana: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;377(9777):1588–1598. | ||
Campa A, Baum MK. Micronutrients and HIV-Infection. HIV Therapy. 2010;4(4):437–469. | ||
30Cegielski JP, McMurray DN. The relationship between malnutrition and tuberculosis: evidence from studies in humans and experimental animals. Int J Tuberc Lung Dis. 2004;8(3):286–298. | ||
World Health Organization.2015. TB/HIV facts 2015. Available from: http://www.who.int/hiv/topics/tb/tbhiv_facts_2015/en/. Accessed May 3, 2017. | ||
Gopi A, Madhavan SM, Sharma SK, Sahn SA. Diagnosis and treatment of tuberculous pleural effusion in 2006. Chest. 2007;131(3):880–889. | ||
Semba RD, Kumwenda J, Zijlstra E, et al. Micronutrient supplements and mortality of HIV-infected adults with pulmonary TB: a controlled clinical trial. Int J Tuberc Lung Dis. 2007;11(8):854–859. | ||
Beisel WR. AIDS. Chapter 32. In: Gershwin ME, German JB, Keen CL, editors. Nutrition and Immunology: Principles and Practices. Totowa, NJ: Humana Press; 2000:389–403. | ||
Taylor EW, Nadimpalli RG, Ramanathan CS. Genomic structure of viral agents in relation to the biosynthesis of seleno proteins. In: Scharauzer GN, Montagnier L, editors. Proceedings of the First International Symposium on “Human Viral Diseases: Selenium, Antioxidants and other Emerging Strategies of Therapy and Prevention,” April 1996, Nonwiler, Germany: Biol Trace Elem Res; 1997;56:63–91. | ||
Ndeezi G, Tylleskär T, Ndugwa CM, Tumwine JK. Effect of multiple micronutrient supplementation on survival of HIV-infected children in Uganda: a randomized, controlled trial. J Int AIDS Soc. 2010;13:18. | ||
Ndeezi G, Tumwine JK, Ndugwa CM, Bolann BJ, Tylleskär T. Multiple micronutrient supplementation improves vitamin B12 and folate concentrations of HIV infected children in Uganda: a randomized controlled trial. Nutr J. 2011;10:56. | ||
Macallan DC. Malnutrition in tuberculosis. Diagn Microbiol Infect Dis. 1999;34(2):153–157. | ||
Kotler DP. Nutritional alterations associated with HIV infection. J Acquir Immune Defic Syndr. 2000;25(Suppl 1):S81–S87. | ||
Chandra RK. Nutrient supplementation as adjunct therapy in pulmonary tuberculosis. Int J Vitam Nutr Res. 2004;74(2):144–146. | ||
Zachariah R, Spielmann MP, Harries AD, Salaniponi FM. Moderate to severe malnutrition in patients with tuberculosis is a risk factor associated with early death. Trans R Soc Trop Med Hyg. 2002;96(3):291–294. | ||
Tang AM, Graham NM, Saah AJ. Effects of micronutrient intake on survival in human immunodeficiency virus type 1 infection. Am J Epidemiol. 1996;143(12):1244–1256. | ||
Baum MK, Shor-Posner G, Lai S, et al. High risk of HIV-related mortality is associated with selenium deficiency. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;15(5):370–374. | ||
Baum MK, Shor-Posner G. Micronutrient status in relationship to mortality in HIV-1 disease. Nutr Rev. 1998;56(1 Pt 2):S135–S139. | ||
De Maayer T, Saloojee H. Clinical outcomes of severe malnutrition in a high tuberculosis and HIV setting. Arch Dis Child. 2011;96(6):560–564. | ||
Briggs MA, Emerson C, Modi S, Taylor NK, Date A. Use of isoniazid preventive therapy for tuberculosis prophylaxis among people living with HIV/AIDS: a review of the literature. J Acquir Immune Defic Syndr. 2015;68(Suppl 3):S297–S305. | ||
Smith T, Samandari T, Abimbola T, Marston B, Sangrujee N. Implementation and operational research: cost-effectiveness of antiretroviral therapy and isoniazid prophylaxis to reduce tuberculosis and death in people living with HIV in Botswana. J Acquir Immune Defic Syndr. 2015;70(3):e84–e93. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



