Back to Journals » Drug Design, Development and Therapy » Volume 17
The Effect of Low-Dose Esketamine on Postoperative Neurocognitive Dysfunction in Elderly Patients Undergoing General Anesthesia for Gastrointestinal Tumors: A Randomized Controlled Trial
Authors Ma J , Wang F, Wang J, Wang P, Dou X, Yao S, Lin Y
Received 15 February 2023
Accepted for publication 7 June 2023
Published 29 June 2023 Volume 2023:17 Pages 1945—1957
DOI https://doi.org/10.2147/DDDT.S406568
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Jiamin Ma,1– 3,* Fuquan Wang,1,2,* Jingxu Wang,1,2,* Pengcheng Wang,4 Xiaoke Dou,1,2 Shanglong Yao,1,2 Yun Lin1,2
1Department of Anesthesiology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430022, People’s Republic of China; 2Institute of Anesthesia and Critical Care Medicine, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, 430022, People’s Republic of China; 3Department of Anesthesiology, Shanghai General Hospital, Shanghai Jiao Tong University, Shanghai, 201620, People’s Republic of China; 4Department of Anesthesiology, Zhumadian Central Hospital, Zhumadian, 463000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yun Lin, Department of Anesthesiology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, No. 1277, Jiefang Avenue, Wuhan, Hubei, 430022, People’s Republic of China, Tel/Fax +86 02785351606, Email [email protected]
Purpose: This study aims to evaluate the effects of the intraoperative application of low-dose esketamine on postoperative neurocognitive dysfunction (PND) in elderly patients undergoing general anesthesia for gastrointestinal tumors.
Methods: Sixty-eight elderly patients were randomly allocated to two groups: the esketamine group (group Es) (0.25 mg/kg loading, 0.125mg/kg/h infusion) and the control group (group C) (received normal saline). The primary outcome was the incidence of delayed neurocognitive recovery (DNR). The secondary outcomes were intraoperative blood loss, the total amount of fluid given during surgery, propofol and remifentanil consumption, cardiovascular adverse events, use of vasoactive drugs, operating and anesthesia time, the number of cases of sufentanil remedial analgesia, the incidence of postoperative delirium (POD), the intraoperative hemodynamics, bispectral index (BIS) value at 0, 1, 2 h after operation and numeric rating scale (NRS) pain scores within 3 d after surgery.
Results: The incidence of DNR in group Es (16.13%) was lower than in group C (38.71%) (P < 0.05). The intraoperative remifentanil dosage and the number of cases of dopamine used in group Es were lower than in group C (P < 0.05). Compared with group C, DBP was higher at 3 min after intubation, and MAP was lower at 30 min after extubation in group Es (P< 0.05). The incidence of hypotension and tachycardia in group Es was lower than in group C (P < 0.05). The NRS pain score at 3 d after surgery in group Es was lower than in group C (P < 0.05).
Conclusion: Low-dose esketamine infusion reduced to some extent the incidence of DNR in elderly patients undergoing general anesthesia for gastrointestinal tumors, improved intraoperative hemodynamics and BIS value, decreased the incidence of cardiovascular adverse events and the intraoperative consumption of opioids, and relieved postoperative pain.
Keywords: esketamine, postoperative neurocognitive dysfunction, elderly patients, gastrointestinal surgery
A Letter to the Editor has been published for this article.
Introduction
After surgical anesthesia, postoperative cognitive dysfunction (POCD) is a common central nervous system complication, with the predominant clinical symptom being a reduction in cognitive function (including learning, memory, mood, emotion, and judgment) in patients.1 POCD often occurs in patients over the age of about 65 yr and has recently been recommended as perioperative neurocognitive disorders (PND).2,3 According to the time of onset, PND includes pre-existing neurocognitive disorders, postoperative delirium (POD) occurring hours to days after surgery, delayed neurocognitive recovery (DNR) occurring within 30 days after surgery, and postoperative neurocognitive disorder (POND) occurring weeks to months after surgery.3 The definition of POCD has changed over the years, and various cognitive assessment tests have been employed in different research, making it difficult to pinpoint the exact incidence of PND.4,5 As the population ages, the proportion of elderly patients undergoing surgery increases accordingly.
Gastrointestinal tumors mainly include gastric cancer (GC) and colorectal cancer (CRC). GC is the fourth most common malignancy and the second most deadly of all malignancies worldwide.6,7 CRC is the world’s fourth most deadly cancer with nearly 900,000 deaths yearly.8 The incidence of gastrointestinal tumors is highest in developed countries and has significantly burdened global healthcare finances.9–11 In senior patients, aging causes increasing levels of pro-inflammatory factors, poorer inflammation inhibition, and a higher prevalence of PND. During gastrointestinal surgery, the trauma and extreme stress cause severe hemodynamic fluctuations and a massive release of inflammatory mediators that affect the central nervous system (CNS), which can result in some postoperative neurological complications (such as cognitive dysfunction and delirium).12 Therefore, finding practical solutions to avoid PND in elderly patients after gastrointestinal surgery is essential for their prognosis.
Cognitive dysfunction is present in various psychoneurological disorders, such as depressive disorders, Alzheimer’s disease (AD), schizophrenia, Parkinson’s disease, and others. Many psychiatric disorders may be associated with over-activating the N-methyl-D-aspartic acid receptor (NMDAR).13 Esketamine is a derivative of ketamine used as a nasal spray for treating depression. Both ketamine and esketamine work by blocking the NMDAR in the brain, however, esketamine has been found to have fewer cognitive side effects compared to ketamine.14–16 It has been shown that in depressed patients receiving low-dose esketamine, most patients showed a rapid and sustained improvement in their depressive symptoms.17 Tu et al showed that the induction and maintenance of intraoperative anesthesia with esketamine for the spinal operation had good safety and reliability, facilitated postoperative cognitive recovery, and had relatively mild adverse effects.18
There is still a lack of research on esketamine used for the induction and maintenance of anesthesia in elderly patients, especially with gastrointestinal tumors. In conclusion, this study aims to investigate the effect of low-dose esketamine on PND in elderly patients undergoing general anesthesia for gastrointestinal tumors, and to provide a reference for the selection and rational use of clinical anesthetic drugs.
Methods
Study Design
The current study was approved by the Ethics Committee at Union Hospital Tongji Medical College of Huazhong University of Science and Technology (No. UHCT21683) and prospectively registered at the Chinese Clinical Trial Registry (ChiCTR2200059003, date of registration: April 22, 2022). This study is a single-center, double-blind, prospective, randomized controlled clinical trial in Union Hospital Tongji Medical College of Huazhong University of Science and Technology. Patients who would like to participate in this study were selected from May 2022 to August 2022 at Union Hospital Tongji Medical College of Huazhong University of Science and Technology. Written informed consent was obtained from all patients.
Inclusion Criteria
Participants must meet the following inclusive criteria: (1) age ≥65 years; (2) American Society of Anesthesiology (ASA) physical status I–III; (3) body mass index (BMI) 18–30kg/m2 (4) elective major abdominal surgery under general anesthesia for gastrointestinal tumors; (5) operation time ≥2 hours.
Exclusion Criteria
The exclusion criteria are as follows: (1) preoperative cognitive dysfunction as determined by Mini-Mental State Examination (MMSE) (less than 17 for illiteracy, 20 for primary school education, and 24 for high school education or above); (2) unable to communicate because of aphasia, severely impaired hearing or other difficulties; (3) use of psychotropic drugs (such as clozapine, risperidone, olanzapine, haloperidol, and chlorpromazine); (4) patients with a history of alcohol abuse or acetylcholine drug use; (5) use of sevoflurane, dexmedetomidine, scopolamine, penehyclidine hydrochloride during the study period; (6) severe circulatory disease (such as severe arrhythmia, hypertension, coronary artery disease, and cardiac insufficiency) and history of cardiac surgery; (7) severe respiratory disease (respiratory failure, severe chronic obstructive pulmonary disease); (8) the history of cerebral hemorrhage; (9) coagulation abnormalities; (10) patients with contraindications to the use of esketamine; (11) patients who experienced severe subcutaneous emphysema, serious adverse events (such as intraoperative hemorrhage and severe hemodynamic fluctuations); (12) emergency surgery; (13) admitted to ICU after surgery; (14) decided to withdraw from the study during the study period; (15) had a second operation within three days after the operation.
Sample Size Calculation
The study was a randomized controlled trial with the intervention group as group Es and the control group as group C. The primary outcome indicator of the study was the incidence of DNR based on the MMSE score on the third day after the operation. According to the literature review and pre-experimental results, the average MMSE score in group C on the third day after the operation was 26.88±1.91. The score on the third day after operation in group Es is estimated to be increased by 1.5, assuming α is 0.05 (bilateral) and the degree of assurance (test efficiency) is 0.9. PASS 15 software was used to calculate that the sample size of group C is 28 and that of group Es is 28. The lost follow-up rate was considered to be 20%, and at least 34 patients were enrolled in each group. Therefore, a total of at least 68 patients are included.
Randomization and Blindness
A double-blind design was used in this study, and patients were divided into group Es and group C using the random number table method. The anesthesia physician was not allowed to observe and record intraoperative patient data during the study; another anesthesiologist recorded intraoperative data. The MMSE scale was used to assess patients’ cognitive function 1 day before and 3 days after surgery by a professionally trained anesthetist. POD was assessed using the Richmond Agitation-Sedation Scale (RASS) and the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) 1 to 3 d after surgery. The incidence of DNR and POD in the 3 d after surgery was recorded. After data collection, the random number table envelope was opened, and analysis was completed to determine the difference between group Es and group C. An overview of this study is presented in Figure 1.
 |
Figure 1 Flowchart of the study design. |
Anesthesia Management
Based on the same other anesthetics in the two groups, esketamine was given at 0.25 mg/kg for induction and 0.125 mg/kg/h for maintenance in group Es, while in group C, 0.9% saline was given in equal volume. Induction of anesthesia after pre-oxygenation with mask ventilation in both groups: intravenous injection of sufentanil (SFDA Approval No.: 11A10331, Yichang Renfu Pharmaceutical Co., Ltd.) 0.25 to 0.5 ug/kg, a bolus injection of etomidate (SFDA Approval No.: YT211016, Jiangsu Enhua Pharmaceutical Co., Ltd.) 0.15 to 0.3 mg/kg and a bolus injection of cisatracurium besilate (SFDA Approval No.: 220227BL, Jiangsu Hengrui Pharmaceutical Co., Ltd.) 0.15 to 0.2 mg/kg after the disappearance of consciousness. Group Es was given esketamine (SFDA Approval No: 211019BL, Jiangsu Hengrui Pharmaceutical Co., Ltd.) 0.25mg/kg, and group C was given an equal volume of 0.9% sodium chloride injection. Five minutes following the start of assisted ventilation, the patient underwent intubation, which was followed by connection to an anesthetic machine and mechanical ventilation. Respiratory parameters were set as follows: tidal volume 6–8 mL/kg, ventilation frequency 12–15 times/min, inspiration-expiration ratio 1:1.5–2, airway pressure <30mmHg, and postapneic end-tidal carbon dioxide pressure (PETCO2) maintained at 35–45mmHg (1 mmHg = 0.133 kPa, considering the effect of pneumoperitoneum, the standard was relaxed appropriately). Anesthesia was maintained by intravenous infusion of propofol (SFDA Approval No.: H20043272, Guangdong Jia Bo Pharmaceutical Co.) 6 to 10 mg/kg/h, remifentanil (SFDA Approval No.: 20A02051, Yichang Renfu Pharmaceutical Co.) 0.2 to 0.3 ug/kg/min and cisatracurium besilate 1 to 2 ug/kg/min. Group Es was given a continuous infusion of esketamine 0.125 mg/kg/h until it was stopped 20 minutes before the end of the operation, and the patients in group C were given an equal volume of 0.9% saline. ECG, HR, SpO2, blood pressure, and BIS were monitored. The BIS depth monitor was used to monitor the depth of sedation, and the pump speed was adjusted to keep the BIS value between 40 and 60 during operation. Under local anesthetic, puncture catheters measured arterial blood pressure through the radial artery. Adapt breathing parameters in accordance with intraoperative arterial blood gas measurement to preserve water-electrolyte and acid-base balance. During the operation, hemodynamic stability was maintained. The responsible anesthesiologist injected urapidil 12–25 mg intravenously if hypertension occurred (SBP increased by more than 30% compared to the baseline value or DBP was above 100 mmHg), and continuously nitroglycerin (5 μg/min) if it recurred more than three times. The responsible anesthesiologist administered dopamine 2 mg intravenously and continuously 2–10 μg/kg/min if hypotension frequently occurred more than three times (SBP decreased by more than 30% compared with the baseline value or the absolute value <80 mmHg). The responsible anesthesiologist should administer intravenous atropine 0.5 mg or esmolol 10–20 mg if bradycardia (HR 50<beats/min), tachycardia (HR >100 beats/min), or elevations and drops exceeding 30% of the patient’s baseline value for 10 minutes occur. Sufentanil 5 mg, nalbuphine (SFDA Approval No.: 11J12071, Yichang Renfu Pharmaceutical Co., Ltd.) 10mg and tropisetron hydrochloride (SFDA Approval No: 210804 Southwest Pharmaceutical Co., Ltd.) 5mg intravenously 30 minutes prior to the end of the operation. After the operation, the patient was sent to the PACU for observation. All patients were given nalbuphine for patient-controlled intravenous analgesia (PCIA). PCIA configuration includes the following: nalbuphine 50 mg and sufentanil 50 μg diluted to 100 mL, 2 mL/h, bolus 2 mL, lock time 15 min. Once it was determined that the patient was responding promptly to vocal commands and that his or her muscular strength had fully recovered, the tracheal tube was taken out. If the patient is still in pain after awakening, administer sufentanil 5ug for remedial analgesia. After confirming that the patient’s reflexes, consciousness, and muscle strength had fully recovered and no serious adverse effects occurred, he was returned to the ward.
Measurement
Data Collection
Patients completed preoperative cognitive status testing using the MMSE scale one day before surgery. Preoperative data collected included patient age, sex, ASA, BMI, education level, co-morbidities, and operative procedure. Intraoperative data were obtained from intraoperative blood pressure, mean arterial pressure (MAP) and heart rate (HR), cardiovascular adverse events, operating time, anesthesia time, the total amount of crystalloids and colloids given during surgery, intraoperative blood loss, propofol and remifentanil consumption, BIS value, number of cases of dopamine use and sufentanil remedial analgesia. Postoperative information includes the MMSE scale, the frequency of DNR at 3 days after surgery, the incidence of POD from the first to the third day following the operation, and the numerical rating scale (NRS) score three days following the operation.
Assessment of Delayed Neurocognitive Recovery
As most patients in this study had a low level of education, the MMSE scale was chosen to evaluate cognition. The MMSE scale is a reliable clinical test, which is easy to complete and commonly used to measure orientation, memory, attention, calculation, recall, and language skills, with a maximum score of 30.19 Due to cultural and language differences, we used the Chinese version of the MMSE scale.20 According to the educational level, the MMSE scale scores of illiterateness < 17, primary school < 20, middle school and above < 24 were defined as cognitive impairment. Patients were assessed for DNR using the MMSE scale when POD was excluded three days after surgery. The score decreased by 2 points after the operation compared with the preoperative score, which is considered DNR.
Assessment of Postoperative Delirium
After the surgery, the patients were observed and engaged in conversation to detect mental status. It is crucial to employ both sedation and agitation methods, such as RASS and a delirium screening tool, because POD is relevant in the postoperative context.21 CAM-ICU was applied by experienced researchers who were trained in neuropsychological assessment during each 8-h shift, and the patient’s mental status was evaluated in terms of the four CAM-ICU characteristics (1) acute changes or fluctuations in mental state, (2) inattentiveness, (3) altered level of consciousness, and (4) disorganized and incoherent thinking.22 POD was diagnosed if characteristics 1–3, 4 along with 1–2, or 1–4, were present. RASS should first assess the depth of sedation. Patients who are profoundly sedated or unarousable (RASS score ≤ −4) should not be assessed for delirium; if they are arousable (RASS score ≥ −3), they may proceed to assessment by CAM-ICU.
Statistical Analysis
This study is a randomized controlled trial. The primary outcome was the incidence of DNR as determined by MMSE score. The secondary measures were the preoperative, intraoperative, and postoperative variables. Patients were divided into two groups based on a random number table. The experimental group is group Es, and the control group is group C.
All research data were analyzed by IBM SPSS software 26.0. Research data of normal distribution were described as the mean±SD (x ± s), and measures that did not conform to a normal distribution were expressed as the median (interquartile). The two groups were compared by independent sample t-test (normal distribution) or non-parametric test (non-normal distribution). ANOVA with repeated measures design was used for comparison within groups. The chi-squared test performed the comparison of categorical data. P <0.05 was considered to be statistically significant.
Results
Sixty-eight patients were enrolled in this study; six patients (8.8%) dropped out, two had incomplete information due to poor compliance, three withdrew from the trial, and one had a second operation within three days after surgery. The remaining 62 patients completed perioperative information collection, and 31 patients in each group completed statistical analysis (Figure 1).
General Data
There was no significant difference in age, sex ratio, ASA, BMI, education level, preoperative complications (hypertension, diabetes, and coronary heart disease), and operative procedure between the two groups (P >0.05) (Table 1).
 |
Table 1 Patient Demographic and Clinical Parameters |
Intraoperative Conditions and Drug Use
There was no significant difference in the duration of surgery and anesthesia, the volume of infusion, blood loss, intraoperative propofol consumption, and the cases of postoperative PACU sufentanil remedial analgesia between the two groups (P >0.05). However, compared with group C, the intraoperative remifentanil consumption, the cases of dopamine use and sufentanil remedial analgesia in group Es were significantly lower (P <0.05). And the BIS value in group Es was significantly higher than that in group C (P <0.001) (Table 2).
 |
Table 2 Intraoperative Data |
Hemodynamic Indexes
Compared with T0, SBP and MAP at T1, T3–T5, DBP at T1, T5 in the two groups, HR at T2, T4–T5 in group C, and HR at T2–T5 in group Es were significantly decreased (P <0.05). Compared with group C, DBP at T1 in group Es was significantly higher (P <0.05), and MAP at T6 in group Es was significantly lower (P <0.05) (Figure 2). This demonstrated that the anesthetic induction and maintenance of esketamine were more conducive to hemodynamic stability in elderly patients.
Intraoperative Cardiovascular Adverse Events
Compared with group C, the incidence of hypotension and tachycardia in group Es was significantly lower (P <0.05). There were no significant differences in the incidence of hypertension and bradycardia between the two groups (P >0.05) (Table 3).
 |
Table 3 Intraoperative Cardiovascular Adverse Events |
MMSE Scores and the Incidence of DNR
The two groups had no significant difference in MMSE scores at D0 (P >0.05). Three days after surgery (D3), the MMSE scores decreased in the two groups. Compared with D0, the MMSE scores at D3 in group C were significantly lower (P <0.05); there was no significant difference in the MMSE scores at D3 in group Es (P >0.05). And there was no significant difference in MMSE scores at D3 between the two groups (P >0.05) (Figure 3). Comparing all patients in the two groups, the incidence of DNR was significantly lower among patients who used esketamine (12 of 31 patients in group C versus 5 of 31 patients in group Es, P = 0.046) (Figure 4). This suggested that the anesthetic induction and maintenance of esketamine were more conducive to promoting the recovery of cognitive function in elderly patients.
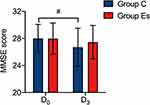 |
Figure 3 Comparison of MMSE scores between the two groups. Abbreviations: MMSE, Mini-Mental State Examination; D0,1 day before surgery; D3, 3 days after surgery; Group C, control group; Group Es, esketamine group. Notes: Compared with T0 in the same group, #P <0.05. An unauthorized version of the Chinese MMSE was used by the study team without permission. The MMSE is a copyrighted instrument and may not be used or reproduced in whole or in part, in any form or language, or by any means without written permission of PAR (www.parinc.com). |
NRS Scores and the Incidence of POD
In group C, the incidence of POD was 12.90% (D1), 9.68% (D2), and 6.45% (D3), whereas, in group Es, it was 9.68%, 3.23%, and 0.00%. Although the incidence of POD three days after the operation was lower in group Es than in group C, there was no significant difference between the two groups (P >0.05) (Table 4). Patients were checked on at the same time in the afternoon at D1, D2, and D3 to measure postoperative pain using the NRS scale. The results showed that the NRS scores were higher in group C than in group Es at D1 and D2, and there was no significant difference between the two groups (P >0.05). Compared with group C, the NRS scores at D3 in group Es was significantly lower (P <0.05) (Table 4).
 |
Table 4 NRS Scores and the Incidence of POD |
Discussion
In this study, we preliminary explored the effect of low-dose esketamine on PND in elderly patients undergoing general anesthesia for gastrointestinal tumors. We referred to the literature when determining the dose of esketamine for anesthesia induction and maintenance.23 The anesthesia induction dose in the instructions is 0.5mg/kg, but overdose reactions may occur at this dose in elderly patients with gastrointestinal tumors. In our study, we chose 0.25mg/kg esketamine intravenous injection and 0.125 mg/kg/h esketamine continuous infusion; and showed stable hemodynamics in group Es.
As the population ages, the proportion of elderly patients undergoing surgery increases accordingly. POCD is a common central nervous system complication following surgical anesthesia and has lately been recommended as PND.2,3 PND describes cognitive impairment identified in the preoperative or postoperative period. Depending on the time of onset, PND includes pre-existing neurocognitive disorders before surgery, POD, DNR, and POND. Previously the concept of POCD was distinguished from POD from each other, so this study will follow the POCD concept when discussing PND in addition to POD. POCD often occurs in elderly patients over 65, mainly manifested as memory impairment, mental retardation, language barrier, information processing ability decline, and anxiety.24 The mechanism of POCD is complex and there are many influencing factors. Previous studies have identified age, low education level, inflammation, postoperative pain, serum 25-OH-D level, and preoperative cognitive impairment as potential risk factors for POCD.25–29 A prospective longitudinal study of the incidence of POCD in patients of different ages showed that the incidence of POCD at three months after surgery was significantly higher in elderly patients than in young and middle-aged patients and that increasing age, lower education level, previous history of cerebrovascular accident and early POCD were independent risk factors for POCD at three months after surgery.30 The type and duration of surgery both have an impact on postoperative cognitive function. Due to the high degree of stress, operation time, complex procedure, and postoperative problems, major surgery (such as heart surgery, hip replacement, and gastrointestinal surgery) has varying degrees of influence on patients’ cognitive function. A randomized controlled study showed a significantly higher incidence of PND in patients undergoing coronary artery bypass surgery than in non-cardiac surgery.31
POD is a form of delirium that occurs in patients after anesthesia and surgery, usually with a high incidence in the first 1–3 days after surgery. Its clinical manifestations are divided into excited delirium, characterized by restlessness, irritability, hallucinations, and delusions, and indifferent delirium, characterized by decreased exercise, sparse speech, and slow reaction. Moreover, mixed delirium in which excited delirium and indifferent delirium coexist. Although most POD patients can recover independently within a few days, severe postoperative accidents (removal of the drainage tube and wound dehiscence caused by emotional excitement) will lead to delayed recovery, prolonged hospitalization, and even life-threatening. European Society of Anesthesiology put forward that POD risk factors include preoperative factors (cardiovascular-related complications, preoperative fasting, dehydration, and hyponatremia or hypernatremia), intraoperative factors (abdominal surgery, cardiothoracic surgery, intraoperative bleeding), and postoperative pain.21 Additionally, emergency surgery and postoperative complications can increase the incidence POD and the risk of long-term cognitive impairment.32,33
Radical operation of gastrointestinal tumor causes trauma and severe stress reaction during the operation, which leads to severe fluctuation of hemodynamics and release of inflammatory mediators thus involving CNS and causing postoperative neurological complications (such as cognitive dysfunction and delirium). According to the latest research results of Yongli, the incidence of early postoperative POCD for gastrointestinal tumors aged 65 and above is 41.0%.34 In this study, the incidence of POCD in the control group was 38.71% three days after the operation, consistent with the above results. A meta-analysis of risk factors of POD in gastrointestinal surgery showed that the incidence of POD ranged from 8.2% to 54.4%.35 In this study, the incidence of POD in the control group was 12.9%, 9.68%, and 6.45%, respectively, consistent with the above results.
Esketamine is a chiral cyclohexanone derivative with analgesic and anesthetic effects. It is combined with a sedative anesthetic (propofol) for anesthesia induction and general anesthesia or as a supplement to local anesthesia.36 Esketamine has high-fat solubility, its plasma concentration reaches its peak within 5 minutes after intramuscular injection, and it is easy to pass through the blood-brain barrier (BBB). Its product is converted into norketamine in vivo. The anesthetic effect of norketamine is 1/5–1/3 as potent as esketamine, and its elimination half-life is long, which can explain why esketamine still has a specific analgesic effect after the patient wakes up from anesthesia.37 It was found that norketamine mediated the acute and transient cardiovascular, respiratory, and sympathomimetic effects of esketamine by antagonizing the α7 nicotinic acetylcholine receptor.38,39 Intraoperative hypotension (IOH) hurts tissue perfusion and blood flow.40 A randomized controlled study performed by Siepe et al showed that patients undergoing elective or urgent on-pump coronary artery bypass grafting (CABG) had a significantly higher drop in postoperative MMSE scores and developed POD in patients with low MAP (60–70) compared with high MAP (80–90).41 Therefore, IOH may be related to postoperative neurocognitive impairment due to cerebral hypoperfusion. Tu et al showed that esketamine induction of anesthesia has good safety and reliability, which can improve hemodynamics, reduce surgical stress and inflammatory reaction, shorten the duration of anaesthesia, promote postoperative cognitive recovery, and the adverse effects are relatively mild.18 Our study showed that intraoperative administration of esketamine reduced the incidence of hypotension and tachycardia and prevented anesthesia-induced blood pressure dips. This phenomenon may be related to the sympathomimetic effect of esketamine and its strong analgesic effect.
Memory is divided into explicit and invisible memory, with explicit memory (declarative memory) involving the hippocampal-middle temporal lobe system and implicit memory (non-declarative memory) including essential associative learning and memory in the cerebellum, amygdala, and other systems.42 Most cognitive tests in PND relate to postoperative declarative memory, which is formed through lived experience and involves the acquisition of input information and consolidation of memory and output information. Therefore, the relatively clear structures of PND and the brain are hippocampi and related cortical structures, such as the limbic area, parahippocampal area, and hippocampal regions. Long-term potentiation and long-term inhibition, as synaptic plasticity mechanisms, can affect learning and memory.43 Studies of synaptic pathways associated with long-duration enhancement have found that Schaffer-collateral CA1 synapses in the hippocampus use glutamate to activate NMDA receptors and alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, which remove Mg2+ and then allow Ca2+ inward flow to bind to post-emergence neurons, thereby affecting learning and memory.
Studies on synaptic pathways related to long-term potentiation found that the CA1 synapse of Schaffer-collateral in the hippocampus using glutamate to activate the NMDA receptor and AMPA receptor to remove Mg2+, after that, Ca2+ inflow is allowed to bind to postsynaptic neurons, thus affecting learning and memory. However, a further increase in cellular Ca2+ levels will increase mitochondrial Ca2+, releasing cytochrome c and promoting apoptosis.44 Most studies suggest that the mechanism of PND after anesthetic surgery is related to the inflammatory response and central Ca2+ dysregulation.45,46 Gill et al showed that visual learning memory improved after intravenous ketamine and that intravenous subanesthetic doses of ketamine did not deteriorate cognitive function.47 Our study showed that the intraoperative application of esketamine improved the postoperative cognitive function of patients, with a significantly lower incidence of DNR compared to the control group. However, the difference in effect on POD was not significant.
Pain is an essential factor affecting perioperative inflammation. Preoperative pain can cause the release of inflammatory factors, and surgical stimulation further exacerbates the inflammatory response, potentially leading to CNS inflammation and dysfunction. Preoperative pain may also lead to changes in central neurotransmitters such as dopamine, acetylcholine, and 5-hydroxytryptamine, which may be associated with the development of PND. The use of opioid analgesics can relieve intraoperative and postoperative pain. However, excessive or inappropriate use may result in problems such as respiratory depression, pain allergy, drug tolerance, and related side effects (nausea, vomiting, constipation, and dizziness). American Society for Enhanced Recovery and Perioperative Quality Initiative Joint Consensus Statement on minimizing perioperative opioid use, including NMDA receptor antagonists in analgesic options for multimodal analgesia to reduce perioperative pain levels and opioid use.48 A meta-analysis showed that ketamine can reduce the side effects of opioids during operation, but it was not found to be beneficial to POD.49 A randomized controlled trial by Bornemann-Cimenti et al revealed that perioperative esketamine application decreased opioid use in major laparotomy, but there was no significant difference in postoperative pain levels between the groups. In addition, compared with minimal-dose esketamine (a 0.015 mg/kg/h infusion following a saline bolus) and placebo, there was a significant increase in the low-dose group (a 0.25 mg/kg bolus and 0.125 mg/kg/h infusion for 48 hours) in Intensive Care Delirium Screening Checklist (ICDSC) score (a score of more than three is considered delirium).23 This study demonstrated that the intraoperative application of low-dose esketamine was significantly associated with a lower incidence of DNR in the three days following surgery compared to the control group, and the intraoperative opioid dose was significantly reduced, which is consistent with the related research results in recent years.23,37 However, the incidence of POD in the esketamine group was lower than that in the control group three days after the operation, which was inconsistent with the above research results. The different selection of delirium assessment scales and the small sample size may have influenced the study results.
Clinical studies have been conducted to discuss whether using intraoperative electroencephalography (EEG) to control the depth of anesthesia affects PND. Bispectral index (BIS) is a monitoring parameter of EEG, and BIS monitoring is considered one of the crucial tools for potentially preventing PND.50–54 Farag et al found that according to BIS monitoring, a higher BIS regimen (mean BIS, 50.7) was superior to a lower BIS regimen (median BIS, 38.9) in terms of cognitive recovery (especially in terms of ability to process information) 4–6 weeks after surgery.55 Studies have shown that ketamine affects EEG activity, increasing the relative power of slow (θ) and fast (γ) waves, thus increasing the BIS value and therefore applying BIS does not objectively reflect the depth of anesthesia.56,57 However, other studies have found that the effect of low-dose ketamine on BIS values is transient, lasting only a short time after induction of anesthesia, and does not affect the intraoperative BIS-guided monitoring of depth of sedation.58 In this study, under the condition of keeping blood pressure, HR and MAP stable, BIS monitoring showed that the BIS value of esketamine group (53 ±3.61) was higher than that of the control group (45 ±2.92). Although higher BIS is a pharmacological effect of esketamine, it can also reduce the usage of propofol. A study revealed a greater prevalence of neurological deficits was linked to deep anesthesia with high-dose propofol.59 The results showed that intraoperative administration of esketamine increased BIS monitoring value, and a higher BIS regimen positively affected postoperative cognitive recovery.
Shortcomings of this study: 1. 68 patients were expected to be included, but 6 patients (8.8%) dropped out due to exclusion criteria. The sample size is relatively insufficient and is a single-center study, so the results are subject to error bias the experimental results. Therefore, conducting a multi-center, large sample study is necessary to explore further the effect of low-dose esketamine on PND in elderly patients; 2. The follow-up period of this study is relatively short, only 3 d postoperative cognitive function assessment was performed, which is not sufficient to explain the effect of esketamine on the long-term cognitive function, and the follow-up period of 7 d, 30 d, and 1 year postoperative should be improved to investigate the long-term effect of esketamine on the cognitive recovery of elderly patients; 3. This study did not collect blood specimens from patients for laboratory analysis. It lacked research on the mechanism of esketamine’s effect on patients’ cognitive recovery, which requires further improvement of related experiments in follow-up.
Conclusions
Low-dose esketamine infusion reduced to some extent the incidence of DNR in elderly patients undergoing general anesthesia for gastrointestinal tumors, improved intraoperative hemodynamics and BIS value, decreased the incidence of cardiovascular adverse events and the intraoperative consumption of opioids, and relieved postoperative pain.
Data Sharing Statement
An unauthorized version of the Chinese MMSE was used by the study team without permission. The MMSE is a copyrighted instrument and may not be used or reproduced in whole or in part, in any form or language, or by any means without written permission of PAR (www.parinc.com). All data generated or analyzed during this study were included in the published article. Further inquiries about the datasets can be directed to the corresponding author on reasonable request.
Ethics Approval
This study design was approved by the Ethics Committee at Union Hospital Tongji Medical College of Huazhong University of Science and Technology (No. UHCT21683).
Acknowledgments
This work was supported by the Hubei Technological Innovation Special Fund (No.2019ACA167) and the Wu Jieping Medical Foundation (No.320.6750.2020-21-12).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Steinmetz J, Christensen KB, Lund T, et al. Long-term consequences of postoperative cognitive dysfunction. Anesthesiology. 2009;110(3):548–555. doi:10.1097/ALN.0b013e318195b569
2. Eckenhoff RG, Maze M, Xie Z, et al. Perioperative neurocognitive disorder: state of the preclinical science. Anesthesiology. 2020;132(1):55–68. doi:10.1097/aln.0000000000002956
3. Evered L, Silbert B, Knopman DS, et al. Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery-2018. Br J Anaesth. 2018;121(5):1005–1012. doi:10.1016/j.bja.2017.11.087
4. Daiello LA, Racine AM, Yun Gou R, et al. Postoperative delirium and postoperative cognitive dysfunction: overlap and divergence. Anesthesiology. 2019;131(3):477–491. doi:10.1097/aln.0000000000002729
5. Snyder B, Simone SM, Giovannetti T, et al. Cerebral hypoxia: its role in age-related chronic and acute cognitive dysfunction. Anesth Analg. 2021;132(6):1502–1513. doi:10.1213/ane.0000000000005525
6. Sitarz R, Skierucha M, Mielko J, et al. Gastric cancer: epidemiology, prevention, classification, and treatment. Cancer Manag Res. 2018;10:239–248. doi:10.2147/cmar.S149619
7. Guggenheim DE, Shah MA. Gastric cancer epidemiology and risk factors. J Surg Oncol. 2013;107(3):230–236. doi:10.1002/jso.23262
8. Dekker E, Tanis PJ, Vleugels JLA, et al. Colorectal cancer. Lancet. 2019;394(10207):1467–1480. doi:10.1016/s0140-6736(19)32319-0
9. Thrift AP, El-Serag HB. Burden of gastric cancer. Clin Gastroenterol Hepatol. 2020;18(3):534–542. doi:10.1016/j.cgh.2019.07.045
10. Keum N, Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol. 2019;16(12):713–732. doi:10.1038/s41575-019-0189-8
11. Peery AF, Crockett SD, Murphy CC, et al. Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: update 2018. Gastroenterology. 2019;156(1):254–272.e11. doi:10.1053/j.gastro.2018.08.063
12. Liu X, Xie G, Zhang K, et al. Dexmedetomidine vs propofol sedation reduces delirium in patients after cardiac surgery: a meta-analysis with trial sequential analysis of randomized controlled trials. J Crit Care. 2017;38:190–196. doi:10.1016/j.jcrc.2016.10.026
13. Ohgi Y, Futamura T, Hashimoto K. Glutamate signaling in synaptogenesis and NMDA receptors as potential therapeutic targets for psychiatric disorders. Curr Mol Med. 2015;15(3):206–221. doi:10.2174/1566524015666150330143008
14. Wang J, Huang J, Yang S, et al. Pharmacokinetics and safety of esketamine in Chinese patients undergoing painless gastroscopy in comparison with ketamine: a randomized, open-label clinical study. Drug Des Devel Ther. 2019;13:4135–4144. doi:10.2147/dddt.S224553
15. Andrade C. Ketamine for depression, 3: does chirality matter? J Clin Psychiatry. 2017;78(6):e674–e677. doi:10.4088/JCP.17f11681
16. Muller J, Pentyala S, Dilger J, et al. Ketamine enantiomers in the rapid and sustained antidepressant effects. Ther Adv Psychopharmacol. 2016;6(3):185–192. doi:10.1177/2045125316631267
17. Singh JB, Fedgchin M, Daly E, et al. Intravenous esketamine in adult treatment-resistant depression: a double-blind, double-randomization, placebo-controlled study. Biol Psychiatry. 2016;80(6):424–431. doi:10.1016/j.biopsych.2015.10.018
18. Karki R, Sharma BR, Tuladhar S, et al. Synergism of TNF-α and IFN-γ triggers inflammatory cell death, tissue damage, and mortality in SARS-CoV-2 Infection and cytokine shock syndromes. Cell. 2021;184(1):149–168.e17. doi:10.1016/j.cell.2020.11.025
19. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi:10.1016/0022-3956(75)90026-6
20. Katzman R, Zhang MY, Ouang Ya Q, et al. A Chinese version of the mini-mental state examination; impact of illiteracy in a Shanghai dementia survey. J Clin Epidemiol. 1988;41(10):971–978. doi:10.1016/0895-4356(88)90034-0
21. Aldecoa C, Bettelli G, Bilotta F, et al. European society of anaesthesiology evidence-based and consensus-based guideline on postoperative delirium. Eur J Anaesthesiol. 2017;34(4):192–214. doi:10.1097/eja.0000000000000594
22. Evered LA, Chan MTV, Han R, et al. Anaesthetic depth and delirium after major surgery: a randomised clinical trial. Br J Anaesth. 2021;127(5):704–712. doi:10.1016/j.bja.2021.07.021
23. Bornemann-Cimenti H, Wejbora M, Michaeli K, et al. The effects of minimal-dose versus low-dose S-ketamine on opioid consumption, hyperalgesia, and postoperative delirium: a triple-blinded, randomized, active- and placebo-controlled clinical trial. Minerva Anestesiol. 2016;82(10):1069–1076.
24. Lyketsos CG. Prevention of unnecessary hospitalization for patients with dementia: the role of ambulatory care. JAMA. 2012;307(2):197–198. doi:10.1001/jama.2011.2005
25. Tsai TL, Sands LP, Leung JM. An update on postoperative cognitive dysfunction. Adv Anesth. 2010;28(1):269–284. doi:10.1016/j.aan.2010.09.003
26. Wang Y, Sands LP, Vaurio L, et al. The effects of postoperative pain and its management on postoperative cognitive dysfunction. Am J Geriatr Psychiatry. 2007;15(1):50–59. doi:10.1097/01.JGP.0000229792.31009.da
27. O’Brien H, Mohan H, Hare CO, et al. Mind over matter? The hidden epidemic of cognitive dysfunction in the older surgical patient. Ann Surg. 2017;265(4):677–691. doi:10.1097/sla.0000000000001900
28. Gao B, Zhu B, Wu C. Preoperative serum 25-Hydroxyvitamin D level, a risk factor for postoperative cognitive dysfunction in elderly subjects undergoing total joint arthroplasty. Am J Med Sci. 2019;357(1):37–42. doi:10.1016/j.amjms.2018.10.012
29. Zhang Y, Bao HG, Lv YL, et al. Risk factors for early postoperative cognitive dysfunction after colorectal surgery. BMC Anesthesiol. 2019;19(1):6. doi:10.1186/s12871-018-0676-4
30. Monk TG, Weldon BC, Garvan CW, et al. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108(1):18–30. doi:10.1097/01.anes.0000296071.19434.1e
31. Evered L, Scott DA, Silbert B, et al. Postoperative cognitive dysfunction is independent of type of surgery and anesthetic. Anesth Analg. 2011;112(5):1179–1185. doi:10.1213/ANE.0b013e318215217e
32. Abelha FJ, Fernandes V, Botelho M, et al. Apolipoprotein E e4 allele does not increase the risk of early postoperative delirium after major surgery. J Anesth. 2012;26:412–421. doi:10.1007/s00540-012-1326-5
33. Nadelson MR, Sanders RD, Avidan MS. Perioperative cognitive trajectory in adults. Br J Anaesth. 2014;112(3):440–451. doi:10.1093/bja/aet420
34. Li YL, Huang HF, Le Y. Risk factors and predictive value of perioperative neurocognitive disorders in elderly patients with gastrointestinal tumors. BMC Anesthesiol. 2021;21(1):193. doi:10.1186/s12871-021-01405-7
35. Scholz AF, Oldroyd C, McCarthy K, et al. Systematic review and meta-analysis of risk factors for postoperative delirium among older patients undergoing gastrointestinal surgery. Br J Surg. 2016;103(2):e21–8. doi:10.1002/bjs.10062
36. Schmitz A, Weiss M, Kellenberger C, et al. Sedation for magnetic resonance imaging using propofol with or without ketamine at induction in pediatrics-A prospective randomized double-blinded study. Paediatr Anaesth. 2018;28(3):264–274. doi:10.1111/pan.13315
37. Tu W, Yuan H, Zhang S, et al. Influence of anesthetic induction of propofol combined with esketamine on perioperative stress and inflammatory responses and postoperative cognition of elderly surgical patients. Am J Transl Res. 2021;13(3):1701–1709.
38. Moaddel R, Abdrakhmanova G, Kozak J, et al. Sub-anesthetic concentrations of (R,S)-ketamine metabolites inhibit acetylcholine-evoked currents in α7 nicotinic acetylcholine receptors. Eur J Pharmacol. 2013;698(1–3):228–234. doi:10.1016/j.ejphar.2012.11.023
39. Domino EF, Warner DS. Taming the ketamine tiger. 1965. Anesthesiology. 2010;113(3):678–684. doi:10.1097/ALN.0b013e3181ed09a2
40. Sessler DI, Bloomstone JA, Aronson S, et al. Perioperative quality initiative consensus statement on intraoperative blood pressure, risk and outcomes for elective surgery. Br J Anaesth. 2019;122(5):563–574. doi:10.1016/j.bja.2019.01.013
41. Siepe M, Pfeiffer T, Gieringer A, et al. Increased systemic perfusion pressure during cardiopulmonary bypass is associated with less early postoperative cognitive dysfunction and delirium. Eur J Cardiothorac Surg. 2011;40(1):200–207. doi:10.1016/j.ejcts.2010.11.024
42. Milner B, Squire LR, Kandel ER. Cognitive neuroscience and the study of memory. Neuron. 1998;20(3):445–468. doi:10.1016/s0896-6273(00)80987-3
43. Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44(1):5–21. doi:10.1016/j.neuron.2004.09.012
44. Işik B. Postoperative cognitive dysfunction and Alzheimer disease. Turk J Med Sci. 2015;45(5):1015–1019. doi:10.3906/sag-1405-87
45. Uchino H, Nagashima F, Nishiyama R, et al. Pathophysiology and mechanisms of postoperative cognitive dysfunction. Masui. 2014;63(11):1202–1210.
46. Zhang Q, Li Y, Bao Y, et al. Pretreatment with nimodipine reduces incidence of POCD by decreasing calcineurin mediated hippocampal neuroapoptosis in aged rats. BMC Anesthesiol. 2018;18(1):42. doi:10.1186/s12871-018-0501-0
47. Gill H, Gill B, Rodrigues NB, et al. The effects of ketamine on cognition in treatment-resistant depression: a systematic review and priority avenues for future research. Neurosci Biobehav Rev. 2021;120:78–85. doi:10.1016/j.neubiorev.2020.11.020
48. Wu CL, King AB, Geiger TM, et al. American society for enhanced recovery and perioperative quality initiative joint consensus statement on perioperative opioid minimization in opioid-naïve patients. Anesth Analg. 2019;129(2):567–577. doi:10.1213/ane.0000000000004194
49. Orena EF, King AB, Hughes CG. The role of anesthesia in the prevention of postoperative delirium: a systematic review. Minerva Anestesiol. 2016;82(6):669–683.
50. Monk TG, Price CC. Postoperative cognitive disorders. Curr Opin Crit Care. 2011;17(4):376–381. doi:10.1097/MCC.0b013e328348bece
51. Steiner LA. Postoperative delirium. Part 1: pathophysiology and risk factors. Eur J Anaesthesiol. 2011;28(9):628–636. doi:10.1097/EJA.0b013e328349b7f5
52. Rudolph JL, Marcantonio ER. Review articles: postoperative delirium: acute change with long-term implications. Anesth Analg. 2011;112(5):1202–1211. doi:10.1213/ANE.0b013e3182147f6d
53. Escallier KE, Nadelson MR, Zhou D, et al. Monitoring the brain: processed electroencephalogram and peri-operative outcomes. Anaesthesia. 2014;69(8):899–910. doi:10.1111/anae.12711
54. Quan C, Chen J, Luo Y, et al. BIS-guided deep anesthesia decreases short-term postoperative cognitive dysfunction and peripheral inflammation in elderly patients undergoing abdominal surgery. Brain Behav. 2019;9(4):e01238. doi:10.1002/brb3.1238
55. Farag E, Chelune GJ, Schubert A, et al. Is depth of anesthesia, as assessed by the Bispectral Index, related to postoperative cognitive dysfunction and recovery? Anesth Analg. 2006;103(3):633–640. doi:10.1213/01.ane.0000228870.48028.b5
56. Ballesteros JJ, Huang P, Patel SR, et al. Dynamics of ketamine-induced loss and return of consciousness across primate neocortex. Anesthesiology. 2020;132(4):750–762. doi:10.1097/aln.0000000000003159
57. Amat-Foraster M, Jensen AA, Plath N, et al. Temporally dissociable effects of ketamine on neuronal discharge and gamma oscillations in rat thalamo-cortical networks. Neuropharmacology. 2018;137:13–23. doi:10.1016/j.neuropharm.2018.04.022
58. Li J, Wang Z, Wang A, et al. Clinical effects of low-dose esketamine for anaesthesia induction in the elderly: a randomized controlled trial. J Clin Pharm Ther. 2022. doi:10.1111/jcpt.13604
59. Roach GW, Newman MF, Murkin JM, et al. Ineffectiveness of burst suppression therapy in mitigating perioperative cerebrovascular dysfunction. Multicenter Study of Perioperative Ischemia (McSPI) Research Group. Anesthesiology. 1999;90(5):1255–1264. doi:10.1097/00000542-199905000-00006
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


