Back to Journals » Journal of Pain Research » Volume 15
The Effect of Long-Term Menstrual Pain on Large-Scale Brain Network in Primary Dysmenorrhea Patients
Authors Yi SJ, Chen RB, Zhong YL, Huang X
Received 13 March 2022
Accepted for publication 1 July 2022
Published 28 July 2022 Volume 2022:15 Pages 2123—2131
DOI https://doi.org/10.2147/JPR.S366268
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Jinlei Li
Si-Jie Yi,1,* Ri-Bo Chen,2,* Yu-Lin Zhong,3 Xin Huang3
1Department of Gynecology and Obstetrics, Jiangxi Provincial People’s Hospital, The First Affiliated Hospital of Nanchang Medical College, Nanchang, 330006, People’s Republic of China; 2Department of Radiology, Jiangxi Provincial People’s Hospital, The First Affiliated Hospital of Nanchang Medical College, Nanchang, 330006, People’s Republic of China; 3Department of Ophthalmology, Jiangxi Provincial People’s Hospital, The First Affiliated Hospital of Nanchang Medical College, Nanchang, 330006, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xin Huang, Department of Ophthalmology, Jiangxi Provincial People’s Hospital, No. 152, Ai Guo Road, Dong Hu District, Nanchang, Jiangxi, 330006, People’s Republic of China, Tel +86 15879215294, Email [email protected]
Purpose: Primary dysmenorrhea (PD) is a common gynecological disease, characterized by crampy and suprapubic pain occurring with menses. Growing evidences demonstrated that PD patients were associated with abnormalities in brain function and structure. However, little is known regarding whether the large-scale brain network changes in PD patients. The purpose of this study was to investigate the effect of long-term menstrual pain on large-scale brain network in PD patients using independent component analysis (ICA) method.
Methods: Twenty-eight PD patients (female, mean age, 24.25± 1.00 years) and twenty-eight healthy controls (HCs) (mean age, 24.46± 1.31 years), closely matched for age, sex, and education, underwent resting-state magnetic resonance imaging scans. ICA was applied to extract the resting-state networks (RSNs) in two groups. Then, two-sample t-tests were conducted to investigate different intranetwork FCs within RSNs and interactions among RSNs between two groups.
Results: Compared to the HC group, PD patients showed significant increased intra-network FCs within the auditory network (AN), sensorimotor network (SMN), right executive control network (RECN). However, PD patients showed significant decreased intra-network FCs within ventral default mode network (vDMN) and salience network (SN). Moreover, FNC analysis showed increased VN-AN and decreased VN-SMN functional connectivity between two groups.
Conclusion: Our study highlighted that PD patients had abnormal brain networks related to auditory, sensorimotor and higher cognitive network. Our results offer important insights into the altered large-scale brain network neural mechanisms of pain in PD patients.
Keywords: primary dysmenorrhea, independent component analysis, resting-state networks
Introduction
Primary dysmenorrhea (PD) is a common gynecological disease characterized by crampy pain located in the lower abdomen, which often occurs before or after the onset of the menstrual bleeding. According to the most recent survey, the prevalence of primary dysmenorrhea is 41.7% among female university students in China.1 Meanwhile, the prevalence of primary dysmenorrhea is 7.7% with non-pathological dysmenorrhea.2 There are several risk factors for PD, including biological, psychological, social, and lifestyle factors.3–5 The main pathophysiology of PD comprises uterine ischemia modulated by prostaglandin synthesis, in association with other factors that affect pain perception and severity. There is increasing evidence that PD patients have both abdominal pain and psychological problems.6,7 Meanwhile, the kinesio taping and lifestyle can improve pain symptoms of dysmenorrhea patients.8
Recently, neuroimaging studies have revealed brain functional and structural changes in PD patients. Zhang et al reported that PD patients had decreased amplitude of low-frequency fluctuation (ALFF) in several pain-related brain regions including the right cerebellum posterior lobe, right middle temporal gyrus, and right parahippocampal gyrus.9 Additionally, Jin et al found that PD patients in the menstrual phase had increased regional homogeneity (ReHo) values in the left midbrain, hippocampus, right posterior cingulate cortex (PCC), insula, and middle temporal cortex (MTC); they had decreased ReHo values in the left dorsolateral prefrontal cortex and right medial prefrontal cortex (mPFC).10 Moreover, PD patients exhibited brain network dysfunction. Liu et al found that PD patients showed abnormal functional connectivity in the default mode network (DMN).11 Liu et al reported that abnormal anterior cingulate cortex (ACC) connectivity may be associated with PD-related disturbances in pain perception, modulation, and affection.12 Dun et al found that PD patients had lower gray matter density in the left anterior insula (aINS), along with hypoconnectivity between the aINS and medial prefrontal cortex (mPFC); these findings were negatively associated with the VAS during menstruation.13 Thus far, studies have demonstrated that PD patients exhibit abnormal pain-related is minimal information concerning whether large-scale brain networks are altered in PD patients.
In the human brain, functional activities can be divided into several functional networks. Previous neuroimaging studies demonstrated that RSNs can be divided into perceptual networks (visual, sensorimotor, and auditory), higher-order cognitive networks (default mode, executive, and salience), and other subcortical networks (basal ganglia network [BGN]).14,15 Independent component analysis (ICA) is a powerful data-driven approach for identifying multiple RSNs, then investigating intra- and inter-network functional connectivity (FC) in vivo.16,17 There are several advantages of ICA method compared to other fMRI methods. The ICA method does not require a preset seed point, compared with resting-state functional connectivity-based seed points. Besides, the ICA method was applied to independent components (ICs) using the Infomax algorithm and ICASSO algorithm to assess the repeatability or stability of ICs. Thus, the ICA method has the advantage of good repeatability. However, it is generally unknown whether alterations of large-scale brain networks in PD patients can be identified using the ICA method. Thus, we hypothesized that PD patients would exhibit large-scale brain network dysfunction when observed by ICA.
Based on this hypothesis, we performed this study to determine whether large-scale brain network dysfunction occurs in PD patients. Our findings might provide new insights into the neural mechanisms that underlie long-term menstrual pain in PD patients.
Research Design and Methods
Participants
This is a retrospective cohort study. Twenty-eight PD patients during their menstruation period with pain phase (28 women; mean age, 24.25±1.00 years) and 28 HCs (28 women; mean age, 24.46±1.31 years), matched for age, sex, and education, participated in this study. All participants enrolled in the study met the following criteria: 1) they had no cardiac pacemaker or implanted metal devices and could undergo magnetic resonance imaging; 2) they did not have heart disease or claustrophobia; 3) they did not have cerebral diseases, as determined by high-resolution T1-weighted images assessed by an experienced radiologist.
The diagnostic criteria of PD patients were:1 childless, right-handed women between the ages of 16 and 30 years;2 a regular menstrual cycle of 21–35 days;3 a course of menstrual pain lasting longer than 6 months;4 no receipt of medication or other treatment for menstrual cramps in the three months prior to participation in the study;6 an average visual analogue score (VAS) of menstrual pain of greater than or equal to 40 (0 = no pain sensation, 100 = the worst pain sensation) in the three months prior to participation in the study. The exclusion criteria of PD patients included the presence of pelvic organic diseases; severe life-threatening disease; psychiatric disorder; a history of drug use, such as any oral contraceptives, analgesics or antidepressants within 6 months of participation in the study;
All HCs met the following criteria:1 a pain score between 0 and 1 in VAS for the last 3 months.;2 presence of pelvic organic diseases;3 no mental disorders.
Ethical Statement
The research protocol adhered to the tenets of the Declaration of Helsinki and was approved by the institutional review board of Jiangxi Provincial People’s Hospital. Ethics committee decision number is 202210012. All subjects provided written informed consent to participate in the study.
MRI Acquisition
MRI scanning was performed on a 3-tesla magnetic resonance scanner (Discovery MR 750W system; GE Healthcare, Milwaukee, WI, USA) with eight-channel head coil. Whole-brain T1 weights were obtained with three-dimensional brain volume imaging (3D-BRAVO) MRI with the following parameters: repetition time [TR]/echo time [TE] = 8.5/3.3, thickness = 1.0 mm, no intersection gap, acquisition matrix = 256 × 256, field of view = 240×240 mm2, and flip angle = 12°.
Functional images were obtained by using a gradient-echo-planar imaging sequence with the following parameters: TR/TE = 2000 ms/25 ms, thickness = 3.0 mm, gap = 1.2 mm, acquisition matrix = 64 × 64, flip angle = 90°, field of view = 240×240 mm2, voxel size = 3.6 × 3.6×3.6 mm3, and 35 axial slices. All the subjects were instructed to rest quietly with their eyes closed and relaxed without thinking about anything in particular or falling asleep. PD patients during their menstruation period with pain phase. The MRI scanning time is 8 minutes.
Data Analysis
All preprocessing was performed using the toolbox for Data Processing & Analysis of Brain Imaging (DPABI, http://www.rfmri.org/dpabi),18 which is based on Statistical Parametric Mapping (SPM12) (http://www.fil.ion.ucl.ac.uk) implemented in MATLAB 2013a (MathWorks, Natick, MA, USA) and briefly the following steps: 1) DICOM format images converted to NIFTI format and remove first ten volumes. 2) The BOLD images were corrected for slice timing effects, motion corrected. For head motion parameters, more than 2 mm or for whom rotation exceeded 1.5°during scanning were excluded.19 3) Individual 3D-BRAVO images were registered to the mean fMRI data, then resulting aligned T1-weighted images were segmented using the diffeomorphic anatomical registration through Exponentiated Lie Algebra (DARTEL) toolbox to improve spatial precise in the normalization of fMRI data.20 Normalized data (in Montreal Neurological Institute [MNI] 152 space) were re-sliced at a resolution of 3 × 3 × 3 mm3. 4) spatial smoothing by convolution with an isotropic Gaussian kernel of 6× 6 × 6 mm full width at half maximum.
Group ICA Analysis
Group ICA was performed to decompose the data into independent components (ICs) using the GIFT toolbox (http://icatb.sourceforge.net/, version 3.0b). First, 25 IC maps were estimated in this study using the minimum description length criterion to adjust for spatial correlation. Second, the ICs for each subject were derived from the group ICA back-reconstruction step and were converted into z-scores.21 Components retained for further analysis among the 25 estimated ICs were selected based on the largest spatial correlation with specific RSN templates.22,23 The IC time-courses and spatial maps for each subject were transformed to z-scores. Thirteen RSNs were identified in this study. We selected thirteen meaningful ICs by using the following criteria: (a) peak coordinates of spatial maps located primarily in the gray matter, (b) no spatial overlap with vascular, ventricular, or susceptibility artifacts, and (c) time courses dominated by low-frequency signals (ratio of powers below 0.1 Hz to 0.15–0.25 Hz in the frequency spectrum).
Statistical Analysis
Spatial Maps for Each of the RSNs
The ICs corresponding to nine RSNs were extracted from all subjects and one-sample t-tests were performed for the spatial maps of each RSN by using SPM12 software. Statistical significance thresholds were set at P < 0.001 (false discovery rate [FDR]-corrected).
Intranetwork Functional Connectivity Analysis
Two-sample t-tests were used to compare differences between the two groups in the intra-network FC within RSN maps; the Gaussian random field method was used to correct for multiple comparisons and regressed covariates of age, sex using SPM12 software. Group comparisons were masked to the voxels within corresponding RSNs (two-tailed, voxel-level P < 0.01, Gaussian random field correction, cluster-level P < 0.05).
Internetwork Functional Connectivity Analysis
The FNC method was used to calculate temporal relationships between RSNs. Two-sample t-tests were used to compare distinct temporal relationships between RSNs between the two groups (P < 0.01, uncorrected).
Results
Demographics and Visual Measurements
There were no significant differences in the gender, age, weight between the groups. Meanwhile, the clinical characteristics of the history of PD, the duration of PD, and possible received treatment of PD are shown in Table 1.
 |
Table 1 Demographics and Visual Measurements Between Two Groups |
Spatial Pattern of RSNs in Each Group
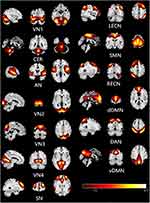
The typical spatial patterns in each RSN of both PD and HC groups, as illustrated in Figure 1. Thirteen of these components coincided with RSNs included: visual network (VN1-4), cerebellum network (CER), auditory network (AN), left executive control network (lECN), right executive control network (RECN), sensorimotor network (SMN), dorsal default mode network (dDMN), Dorsal attention network (DAN), ventral default mode network (vDMN), salience network (SN).
Altered RSNs in the PD Group
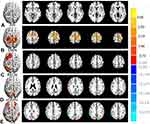
Significant increased intra-network FC within RSNs were identified in PD group relative to HC group (Figure 2 and Table 2). Compared with the HC group, the PD group showed increased intra-network FC in the left superior temporal gyrus of the AN (Figure 2A), the left supplementary motor area of the SMN (Figure 2B), the left superior frontal gyrus of the RECN (Figure 2C). The PD group showed decreased intra-network FC in the left superior parietal lobule of vDMN (Figure 2D), the right inferior parietal lobule of the SN (Figure 2E). (two-tailed, voxel-level P < 0.01, GRF correction, cluster-level P < 0.05).
 |
Table 2 Different Intra-Network FC of RSNs Between Two Group |
FNC Analysis
FNC analysis showed increased VN-AN (Figure 3A) and decreased VN-SMN (Figure 3B) functional connectivity between two groups (P < 0.01, uncorrected).
Discussion
To our knowledge, this is the first study to investigate the effects of long-term menstrual pain on large-scale brain network alterations in PD patients. Compared with the HC group, PD patients showed significantly increased intra-network FCs within the auditory network (AN), sensorimotor network (SMN), and right executive control network (RECN). However, PD patients showed significantly decreased intra-network FCs within the ventral default mode network (vDMN) and salience network (SN). Moreover, FNC analysis revealed increased VN-AN and decreased VN-SMN functional connectivity between the two groups. There are two main pathways in pain neural pathways that process pain information. A neural pathway is that it projects from the medial thalamus to the anterior cingulate and guide cortex, which is responsible for processing the emotional motivational components. Another neural pathway is that it projects from the lateral thalamus to the primary somatosensory central cortex and the guide lobe cortex, which processes the discrimination of pain sensations. In the study, we hypothesized that PD patients might be accompanied by abnormalities in sensory and emotional brain networks. The AN is located in the temporal lobe, which has an important role in auditory information processing. Bingren Zhang et al demonstrated that PD patients showed higher functional and emotional scale scores, along with stronger auditory evoked potentials.24 Pin-Shiuan Lee et al reported that PD patients had increased theta activity in the left middle/inferior temporal gyrus during pain processing.25 Fan et al also demonstrated that chronic pain patients have a faster auditory memory trace decay, compared with healthy controls.26 Thus, PD patients show increased intra-network FCs in the left superior temporal gyrus of the AN, suggesting that auditory function is impaired in PD patients.
The SMN is located in the precentral and postcentral gyri, which have important roles in motion control and pain processing. Zhang et al reported that migraine patients showed decreased FC between the S1 and brain areas within the pain intensity and spatial discrimination pathways and trigemino-thalamo-cortical nociceptive pathway.27 Bhatt et al found that pain in localized provoked vulvodynia (PVD) patients involved a greater volume of gray matter in sensorimotor cortices.28 The primary somatosensory cortex (SI) is a critical component of the neural substrate that underlies interindividual differences in pain sensitivity.29 There is increasing neuroimaging evidence that PD patients exhibit SMN dysfunction. Yu et al demonstrated that PD patients had abnormal FC in the SMN.30 Furthermore, Tu et al found that decreased activity was mainly present in sensorimotor regions of the left hemisphere at onset of PD, compared to offset of PD.31 Consistent with these findings, our PD patients had increased intra-network FCs in the left supplementary motor area of the SMN, which implies sensorimotor dysfunction in PD patients.
The ECN is involved in goal-directed selection of stimuli and responses, as well as cognitive control. In our study, PD patients showed increased intra-network FCs in the left superior frontal gyrus of the RECN. Keogh et al demonstrated that tasks requiring higher-order processes (eg, executive control) are susceptible to pain interference.32 Pei et al also found that patients with low-back-related leg pain had abnormal hyperconnectivity between the S1 cortex and the executive control network.33 Overall, previous studies have demonstrated that chronic pain can alter higher cognitive control functions. Thus, we speculated that persistent chronic pain in PD patients might contribute to hyperconnectivity in the left superior frontal gyrus of the RECN.
The DMN is regarded as an endogenous neural network that demonstrates consistently higher blood oxygenation level-dependent activity at rest. Previous neuroimaging studies demonstrated that the DMN has an important role in clinical pain.34 Jones et al found that increasing levels of pain are associated with potential desegregation of the DMN and the prefrontal cortex, which are important for cognitive control; increasing levels of pain are also associated with novel patterns of connectivity between the DMN and cerebellum.35 A previous neuroimaging study showed that PD patients have DMN-related abnormalities; that finding might provide insights concerning disease pathophysiology.11 Consistent with the previous findings, our study showed that PD patients had decreased intra-network FC in the left superior parietal lobule of the vDMN.
The SN is involved in processing several internal and external stimuli to identify the most relevant stimulus for current behavior; this network consists of the dorsal anterior cingulate and anterior insula, which are involved in switching between the ECN and DMN. Isenburg et al reported that chronic low back pain (cLBP) patients had abnormal salience network (SLN) connectivity.36 van Ettinger-Veenstra et al demonstrated that chronic widespread pain (CWP) patients had decreased connectivity in the inferior posterior cingulate cortex (PCC) in the DMN, along with increased connectivity in the left anterior insula/superior temporal gyrus in the SN.37 Lin-Chien Lee et al demonstrated that the global and regional network metrics and modular structure of the resting-state brain functional networks were not altered in young PDM females.38 In our study, we found that PD patients had decreased intra-network FC in the right inferior parietal lobule of the SN. Therefore, we hypothesize that PD patients exhibit impaired attention and working memory.
Furthermore, FNC analysis revealed increased VN-AN and decreased VN-SMN functional connectivity between the two groups. Figley et al demonstrated that interactions among the VN, AN, and SMN are involved in multisensory tasks.39 Thus, we speculate that increased functional connectivity in the VN-AN and decreased functional connectivity in the VN-SMN reflect RSN compensation in PD patients with persistent pain. In conclusion, our results suggest that PD patients have alterations in auditory, sensorimotor, and higher cognitive brain networks. The findings in this study offer important insights into the altered large-scale brain network neural mechanisms that underlie pain in PD patients.
Some limitations should be mentioned in the study. First, the sample size of PD patients in our study was small. Second, the lack of psychological tests prevented us from investigating the relationship between RSNs and psychological characteristics in these PD patients. Third, blood oxygenation level-dependent (BOLD) signals would still be affected by physiological noise.
Acknowledgments
We acknowledge the assistance provided by the Natural Science Foundation of Jiangxi Province (20212BAB216058), Jiangxi Provincial Health Technology Project (202210012).
Disclosure
The authors in this work declare no conflict of interest.
References
1. Hu Z, Tang L, Chen L, et al. Prevalence and risk factors associated with primary dysmenorrhea among Chinese female university students: a cross-sectional study. J Pediatr Adolesc Gynecol. 2020;33:15–22. doi:10.1016/j.jpag.2019.09.004
2. Bakhsh H, Algenaimi E, Aldhuwayhi R, AboWadaan M. Prevalence of dysmenorrhea among reproductive age group in Saudi Women. BMC Womens Health. 2022;22:78. doi:10.1186/s12905-022-01654-9
3. Fernandez-Martinez E, Onieva-Zafra MD, Parra-Fernandez ML, Palazón-Bru A. Lifestyle and prevalence of dysmenorrhea among Spanish female university students. PLoS One. 2018;13:e0201894. doi:10.1371/journal.pone.0201894
4. Zurawiecka M, Wronka I. Association of primary dysmenorrhea with anthropometrical and socio-economic factors in Polish university students. J Obstet Gynaecol Res. 2018;44:1259–1267. doi:10.1111/jog.13645
5. Hailemeskel S, Demissie A, Assefa N. Primary dysmenorrhea magnitude, associated risk factors, and its effect on academic performance: evidence from female university students in Ethiopia. Int J Womens Health. 2016;8:489–496. doi:10.2147/IJWH.S112768
6. Gagua T, Tkeshelashvili B, Gagua D, et al. Assessment of anxiety and depression in adolescents with primary dysmenorrhea: a case-control study. J Pediatr Adolesc Gynecol. 2013;26(6):350–354. doi:10.1016/j.jpag.2013.06.018
7. Balik G, Ustuner I, Kagitci M, et al. Is there a relationship between mood disorders and dysmenorrhea? J Pediatr Adolesc Gynecol. 2014;27:371–374. doi:10.1016/j.jpag.2014.01.108
8. Doğan H, Eroğlu S, Akbayrak T. The effect of kinesio taping and lifestyle changes on pain, body awareness and quality of life in primary dysmenorrhea complement. Ther Clin Pract. 2020;39:101120. doi:10.1016/j.ctcp.2020.101120
9. Zhang YN, Huo JW, Huang YR, et al. Altered amplitude of low-frequency fluctuation and regional cerebral blood flow in females with primary dysmenorrhea: a resting-state fMRI and arterial spin labeling study. J Pain Res. 2019;12:1243–1250. doi:10.2147/JPR.S177502
10. Jin L, Yang X, Liu P, et al. Dynamic abnormalities of spontaneous brain activity in women with primary dysmenorrhea. J Pain Res. 2017;10:699–707. doi:10.2147/JPR.S121286
11. Liu P, Liu Y, Wang G, et al. Aberrant default mode network in patients with primary dysmenorrhea: a fMRI study. Brain Imaging Behav. 2017;11:1479–1485. doi:10.1007/s11682-016-9627-1
12. Liu P, Liu Y, Wang G, et al. Changes of functional connectivity of the anterior cingulate cortex in women with primary dysmenorrhea. Brain Imaging Behav. 2018;12:710–717. doi:10.1007/s11682-017-9730-y
13. Dun WH, Yang J, Yang L, et al. Abnormal structure and functional connectivity of the anterior insula at pain-free periovulation is associated with perceived pain during menstruation. Brain Imaging Behav. 2017;11:1787–1795. doi:10.1007/s11682-016-9646-y
14. Damoiseaux JS, Rombouts SA, Barkhof F, et al. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103:13848–13853. doi:10.1073/pnas.0601417103
15. Smith SM, Fox PT, Miller KL, et al. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009;106:13040–13045. doi:10.1073/pnas.0905267106
16. van de Ven VG, Formisano E, Prvulovic D, et al. Functional connectivity as revealed by spatial independent component analysis of fMRI measurements during rest. Hum Brain Mapp. 2004;22:165–178. doi:10.1002/hbm.20022
17. Beckmann CF, DeLuca M, Devlin JT, et al. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 2005;360(1457):1001–1013. doi:10.1098/rstb.2005.1634
18. Yan CG, Wang XD, Zuo XN, et al. DPABI: data processing & analysis for (Resting-State) brain imaging. Neuroinformatics. 2016;14:339–351. doi:10.1007/s12021-016-9299-4
19. Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59:431–438. doi:10.1016/j.neuroimage.2011.07.044
20. Goto M, Abe O, Aoki S, et al. Diffeomorphic anatomical registration through exponentiated lie algebra provides reduced effect of scanner for cortex volumetry with atlas-based method in healthy subjects. Neuroradiology. 2013;55:869–875. doi:10.1007/s00234-013-1193-2
21. Zuo XN, Kelly C, Adelstein JS, et al. Reliable intrinsic connectivity networks: test-retest evaluation using ICA and dual regression approach. Neuroimage. 2010;49:2163–2177. doi:10.1016/j.neuroimage.2009.10.080
22. Wang D, Qin W, Liu Y, et al. Altered resting-state network connectivity in congenital blind. Hum Brain Mapp. 2014;35:2573–2581. doi:10.1002/hbm.22350
23. Shirer WR, Ryali S, Rykhlevskaia E, et al. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb Cortex. 2012;22(1):158–165. doi:10.1093/cercor/bhr099
24. Zhang B, Xu Y, He W, et al. Intensity dependence of auditory evoked potentials in primary dysmenorrhea. J Pain. 2017;18:1324–1332. doi:10.1016/j.jpain.2017.06.009
25. Lee PS, Low I, Chen YS, et al. Encoding of menstrual pain experience with theta oscillations in women with primary dysmenorrhea. Sci Rep. 2017;7:15977. doi:10.1038/s41598-017-16039-4
26. Fan L, Sun YB, Sun ZK, et al. Modulation of auditory sensory memory by chronic clinical pain and acute experimental pain: a mismatch negativity study. Sci Rep. 2018;8:15673. doi:10.1038/s41598-018-34099-y
27. Zhang J, Su J, Wang M, et al. The sensorimotor network dysfunction in migraineurs without aura: a resting-state fMRI study. J Neurol. 2017;264:654–663. doi:10.1007/s00415-017-8404-4
28. Bhatt RR, Gupta A, Rapkin A, et al. Altered gray matter volume in sensorimotor and thalamic regions associated with pain in localized provoked vulvodynia: a voxel-based morphometry study. Pain. 2019;160:1529–1540. doi:10.1097/j.pain.0000000000001532
29. Niddam DM, Wang SJ, Tsai SY. Pain sensitivity and the primary sensorimotor cortices: a multimodal neuroimaging study. Pain. 2021;162:846–855. doi:10.1097/j.pain.0000000000002074
30. Yu S, Xie M, Liu S, et al. Resting-state functional connectivity patterns predict acupuncture treatment response in primary dysmenorrhea. Front Neurosci. 2020;14:559191. doi:10.3389/fnins.2020.559191
31. Tu CH, Niddam DM, Chao HT, et al. Abnormal cerebral metabolism during menstrual pain in primary dysmenorrhea. Neuroimage. 2009;47:28–35. doi:10.1016/j.neuroimage.2009.03.080
32. Keogh E, Moore DJ, Duggan GB, et al. The disruptive effects of pain on complex cognitive performance and executive control. PLoS One. 2013;8(12):e83272. doi:10.1371/journal.pone.0083272
33. Pei Y, Zhang Y, Zhu Y, et al. Hyperconnectivity and high temporal variability of the primary somatosensory cortex in low-back-related leg pain: an fMRI Study of static and dynamic functional connectivity. J Pain Res. 2020;13:1665–1675. doi:10.2147/JPR.S242807
34. Loggia ML, Kim J, Gollub RL, et al. Default mode network connectivity encodes clinical pain: an arterial spin labeling study. Pain. 2013;154:24–33. doi:10.1016/j.pain.2012.07.029
35. Jones SA, Morales AM, Holley AL, et al. Default mode network connectivity is related to pain frequency and intensity in adolescents. Neuroimage Clin. 2020;27:102326. doi:10.1016/j.nicl.2020.102326
36. Isenburg K, Mawla I, Loggia ML, et al. Increased salience network connectivity following manual therapy is associated with reduced pain in chronic low back pain patients. J Pain. 2021;22:545–555. doi:10.1016/j.jpain.2020.11.007
37. van Ettinger-Veenstra H, Lundberg P, Alfoldi P, et al. Chronic widespread pain patients show disrupted cortical connectivity in default mode and salience networks, modulated by pain sensitivity. J Pain Res. 2019;12:1743–1755. doi:10.2147/JPR.S189443
38. Lee L-C, Chen Y-H, Lin C-S, et al. Unaltered intrinsic functional brain architecture in young women with primary dysmenorrhea. Sci Rep. 2018;8:12971. doi:10.1038/s41598-018-30827-6
39. Figley TD, Mortazavi Moghadam B, Bhullar N, et al. Probabilistic white matter atlases of human auditory, basal ganglia, language, precuneus, sensorimotor, visual and visuospatial networks. Front Hum Neurosci. 2017;11:306. doi:10.3389/fnhum.2017.00306
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.