Back to Journals » Journal of Pain Research » Volume 10
The effect of local/topical analgesics on incisional pain in a pig model
Authors Castel D, Sabbag I, Meilin S
Received 27 June 2017
Accepted for publication 15 August 2017
Published 4 September 2017 Volume 2017:10 Pages 2169—2175
DOI https://doi.org/10.2147/JPR.S144949
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Katherine Hanlon
David Castel,1 Itai Sabbag,2 Sigal Meilin3
1The Neufeld Cardiac Research Institute, Sheba Medical Centre, Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, 2Lahav Research Institute, Kibutz Lahav, Negev, 3Neurology R&D Division, MD Biosciences, Nes-Ziona, Israel
Abstract: Interest in the development of new topical/local drug administration for blocking pain at peripheral sites, with maximum drug activity and minimal systemic effects, is on the rise. In the review article by Kopsky and Stahl, four critical barriers in the process of research and development of topical analgesics were indicated. The active pharmaceutical ingredient (API) and the formulation are among the major challenges. The road to the development of such drugs passes through preclinical studies. These studies, if planned correctly, should serve as guidance for choosing the right API and formulation. Although rodent models for pain continue to provide valuable data on the mechanisms driving pain, their use in developing topical and localized treatment approaches is limited for technical (intraplate injection area is small) as well as mechanical reasons (non-similarity to human skin and innervation). It has been previously shown that pigs are comparable to humans in ways that make them a better choice for evaluating topical and local analgesics. The aim of this study was to summarize several experiments that used pigs for testing postoperative pain in an incisional pain model (skin incision [SI] and skin and muscle incision [SMI]). At the end of the surgery, the animals were treated with different doses of bupivacaine solution (Marcaine®), bupivacaine liposomal formulation (Exparel®) or ropivacaine solution (Naropin). Von Frey testing demonstrated a decrease in the animals’ sensitivity to mechanical stimulation expressed as an increase in the withdrawal force following local treatment. These changes reflect the clinical condition in the level as well as in the duration of the response. These data indicate a good resemblance between pig and human skin and suggest that use of these animals in the preclinical phase of developing topical analgesics can, to some extent, release the bottleneck.
Keywords: postoperative pain, de-risk clinical trials, local treatment, swine
Introduction
An interesting review article that discusses the bottleneck in the development of topical analgesics was recently published by Kopsky and Stahl.1 The authors suggested four barriers to the development of such drugs. The first two are related to the choice of the active pharmaceutical ingredient (API) and the API formulation. The other two are related to the design and execution of clinical trials. It is suggested that using a pig for the preclinical development of such a drug candidate can not only serve as a solution for the two major obstacles stated by the authors but also de-risk the clinical trials. As an introduction, a summary of the relevance of pigs for such studies is provided by reviewing the main points in which the pig anatomy and physiology resemble those of humans, focusing on the skin structure and function. This study used the postoperative pain model in the pig as an example for its resemblance to humans.
Comparison between human and porcine skin structure, function, penetration and innervation
A comprehensive review describing the similarity between the human skin and pig skin and its relevance to wound healing was published in 2001.2 The authors pointed to several similarities that make pigs the best choice for studying wound and incision healing. 1) Both humans and pigs have a thick epidermis. However, since the epidermis thickness varies across different sites on the body, the authors mentioned the dermal/epidermal ratio as the more important and relevant parameter. This ratio ranges between 10:1 and 13:1 in both species. 2) The similarity in the collagen chemistry of humans and pigs is so significant that in some products pig collagen is used to treat human injuries. Pig xenografts and matrix implantation in treating severe human dermal diseases were also reported.3,4 3) Humans and pigs do not have loose skin, as do mice and rats. 4) The dermal blood vessel size, orientation and distribution are similar. 5) Humans and pigs are unique in the fact that both species heal through re-epithelization rather than contraction. An additional study suggests a similarity between the pig and the human skin immune system.3 This aspect is very relevant in cases of chronic pain such as the complex regional pain syndrome (CRPS).
Skin permeability is a major component in the development of a drug for topical administration. A study that compared the skin permeability of the rat, rabbit, pig and human showed the greatest similarity in permeability characteristics for the tested compounds for the skins of miniature swine and humans, already in 1972.4 Untill now, Monkey, pig, rat, rabbit, guinea pig, hairless rodents (such as hairless rat, hairless mouse and hairless guinea pig) and hairless dog are among the most widely used animals for testing skin permeability of drugs. A comprehensive review discussing the in vitro and in vivo methods for assessing permeability in these animals suggested that monkey, pig and hairless guinea pig are more predictive of human skin absorption/penetration.5
The innervation of human and pig skins is composed mainly of unmyelinated and slowly conducting C-nociceptors.6,7 Obreja and Schmelz8 wrote that:
Nociceptive and non-nociceptive fiber classes found in pig correlate with human fiber classes, in both distribution and axonal excitability changes. It is suggested that the pig is an attractive model for studying […] C-fiber classes that correspond to those in man
Different molecules participate in driving pain. Nerve growth factor (NGF) is one of the components whose involvement in pain was studied extensively in humans and was proven to be involved in sensitization of nociceptive processing in acute and chronic pain in humans. In a study using the same protocol of NGF dermal stimulation in pigs and humans, the authors showed that NGF increased conduction velocity and reduced activity-dependent slowing (ADS) and propagation failure in mechano-insensitive nociceptors. These changes were not related to any changes in dermal nerve density.9 Petersson et al10 further investigated the contribution of mechano-sensitive (CM) and mechano-insensitive (CMi) fibers to chronic pain and found that the same molecular mechanism underlying the switch in the electrical signature of CMi fibers following exposure to NGF rectifier exists in humans and pigs. Specifically, they suggested that the potassium channel Kdr, the voltage-gated sodium channel NaV1.7 and the Na+/K+ pump are the most relevant ion channels in chronification of pain in both humans and pigs. However, the neuronal system is not the sole system responsible for the chronification of pain. In an in vitro study that included dorsal root ganglion (DRG) neurons from pig and human keratinocytes, it was shown that the cross talk between dermal fibroblast and keratinocytes is highly involved in skin innervation and plays a major role in atopic eczema.11 Further a study suggested that keratinocytes are major contributors to sustained pain conditions in humans.12
Overall, the similarity between human and pig skin structure, function, innervation and cross talk between different systems in the skin strongly suggests that better use of these animals in the development of local and topical analgesics will shorten the “time to market” in the development of topical analgesics. This study presents an example of such a use, employing the incision model which shows that the activity of known local analgesics in this model resembles the activity known in humans.
Materials and methods
Animals
Danish Landrace × Large White crossbred pigs from the domestic herd at Lahav Labs (Negev, Israel) were used in this study. All procedures and experiments were approved by the Institutional Animal Care and Use Committee (MD Biosciences) and by the Israeli National Animal Care and Use Committee. The studies were designed to reduce numbers and undue suffering according to the International Association for the Study of Pain (IASP) guidelines.15 Prior to the beginning of the study, all animals were kept under conventional pig production conditions. The animals were housed in open pens (1.4 m × 2.4 m) on a 12 h:12 h (light–dark cycle: on at 07:00 and off at 19:00) for 7 days prior to study initiation. Feeding was given three times daily using special pig food (Dry Sows; Ct # 5420; Milobar, zip code 7880, Oshrat, Israel). The pigs were provided opportunities to root and chew for enrichment. Fresh water was provided ad libitum by an automated system. In this study, six male animals were used. The number of animals was determined based on variability in the withdrawal force responses within each group observed in previous work using this model.15,16
Surgeries
The skin incision (SI) model differs from an incision involving muscle (skin and muscle incision [SMI]). The difference applies to the level of different markers that are expressed in the incision area.13,14 Both methods are therefore described in this study. Animals underwent either a full SI or full SMI as previously described by Castel et al.15 Briefly, prior to the surgery, the low back of the pigs was shaved and swabbed with 70% ethanol and antiseptic liquid polidine solution (Polysept solution; Rekah Pharmaceutical Industry Ltd., Holon, Israel), and the non-operated areas were covered with sterile sheets. Blood O2 saturation was monitored throughout the anesthesia (Spacelab Medical, Snoqualmie, WA, USA). A 6–7 cm incision was made through either the skin/fascia or the skin fascia and muscle on one side of the lower back of the animal, toward the caudal end. Immediately after the incision, drugs were applied to the SI layers or to the muscle area (SMI). Following drug administration, the incisions were closed using 3–0 silk sutures (Assut medizinische, Produkte GmbH, Altenbergerstrasse, Steinfurt, Germany). Following the incision, all pigs received the antibiotic marbofloxacin (10% w/v; Marbocyl®; Vétoquinol UK Ltd., Buckingham, UK) at a total dose of 0.5 mL per pig through intramuscular (IM) injection into the neck muscle. The animals were then returned to their home pen for recovery.
Drugs
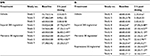
Handling of all drugs was as per the manufacturer’s instructions. Exparel, 233 mg/mL bupivacaine, was dosed in a volume of 1.3 mL to achieve a total dose of 300 mg/animal. To achieve a dose of 100 mg/animal, Exparel was diluted with saline to a concentration of 100 mg/mL and 1 mL was dosed. Marcaine was delivered as 5 mg/mL. To dose 30 mg/animal, the animals were dosed with 6 mL. A volume of 2 mL were dosed to animals that were treated with 10 mg/animal. To achieve different dosage range the solution concentration was unchanged and the dosing volume was changed. In order to dose 50 mg/animal, the animal was injected with 5 mL. Animals that were treated with 25 mg/animal were dosed with 2.5 mL (Table 1).
  | Table 1 Details of drug concentration, dosing volume and final dose per animal |
Assessment of mechanical sensitivity
Mechanical sensitivity was assessed using von Frey filaments (Touch Test [von Frey] Sensory Evaluator Kit, model 58011; Stoelting Co., Wood Dale, IL, USA). Von Frey filaments ranging from a minimum of 1 g to a maximum of 60 g were used as described previously.15,16 Each filament was applied three times with a 5–10 s interval between applications. If withdrawal was not achieved, a thicker filament was applied. If withdrawal was achieved, a thinner filament was applied. The force required to achieve a withdrawal reaction was determined by alternating the filaments. All tests were conducted at the same time in all groups (vehicle-treated and drug-treated animals).
Study design
The results presented in this article are the sum of several studies. In principle, all studies followed the same outline. Before surgery, the animals underwent habituation and handling as described by Castel et al.15,16 Then, prior to surgery, the baseline response to the von Frey test of all animals was recorded. On study day 0, the animals were operated using the SI or SMI procedure as described earlier, followed by local application of the various treatments. The animals were then retested using the von Frey test at different time points as described in Table 2. Testing time points were selected based on several factors: animals are wakening following anesthesia, and they are completely awake and interacting with their home pen and are responsive to researchers already after 30 min post SI model, thus enabling a testing time point after 1 h. However, the SMI surgery is a slightly longer, and monitoring the withdrawal force at 1 h post surgery is not reliable. Therefore, the first time point selected was 3 h post SMI surgery. Since evaluation of the duration of activity of the tested drugs was also a goal of these studies, the animals were introduced to the von Frey tests every 2 h. The assumption of the study was that the duration of activity of Exparel, the liposomal formulation of bupivacaine, would be the longest. A time point of 12 h was therefore added to the study in which dose-related activity of Exparel was assessed (SMI model). Since this time point is problematic (morning monitoring vs. evening monitoring), it was not added to the SI model in which drugs with an expected shorter duration of activity were tested. Instead, a 7 h testing time point was added to capture the expected decrease in the activity of all tested drugs. In both models, a time point of 24 h was added to evaluate long-lasting activity or lack of activity of all tested drugs.
  | Table 2 Study design, study day 0 is the surgery day Abbreviations: SI, skin incision; SMI, skin and muscle incision. |
Statistical analysis
One-way ANOVA followed by a multi-comparison Tukey test was used to assess the significance of the different treatments vs. saline-treated animals. p<0.05 was considered significant.
Results
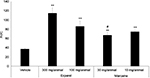
The current data are a set of data collected over the past 3 years of working with the postoperative pain model in pigs and using different local anesthetics. Table 3 provides an example of three studies of the SMI study and three studies of the SI studies. The data presented in the table suggest that both procedures are reproducible. No difference was found between studies in the withdrawal following either vehicle or drug treatment. In addition, a statistically significant higher withdrawal force was required in all cases following treatment with the various drugs vs. vehicle-treated groups. Further analysis of the data suggests a significant analgesic effect of ropivacaine and two different bupivacaine formulations (Marcaine, a free bupivacaine solution; Exparel, a liposomal formulation) currently available on the market. Following the SMI model, animals treated with Exparel showed an increase in withdrawal force in a dose-related manner (Figure 1A). The effect was significant for 12 h (300 mg/animal Exparel 22.18±4.26 g vs. 1.85±0.24 g for the vehicle-treated group; p<0.01). At 24 h, there was no difference between the vehicle-treated group and Exparel-treated groups. Treatment with the Marcaine, bupivacaine solution, also resulted in a significant increase in withdrawal force. However, this increase was not dose related and was smaller compared to the Exparel treatment (Figure 1B). At 5 h post dosing, the mean withdrawal force recorded for the Exparel-treated animals (300 mg/animal) was 34.27±5.08 g vs. 11.50±3.2 g recorded following treatment with Marcaine at 30 mg/animal (p<0.05). Both treatments (Exparel and Marcaine) resulted in a significantly higher withdrawal force response vs. Saline treated animals. (1.85±0.22; p<0.01). Calculation of the area under the curve for analyzing the sum of analgesic effect during the 24 h of the study period suggests a superior effect of Exparel vs. Marcaine (Figure 2).
Following treatment with Naropin, a ropivacaine solution, an increase in withdrawal force vs. saline treated animals was recored. (Figure 3). The increase in withdrawal force lasted for 7 h. The effect was dose dependent. At 7 h post dosing, the withdrawal force following ropivacaine treatment was 24.67 5.10 g for the 50 mg/animal group (p<0.01 vs. vehicle) and 7.27±2.38 g for the 25 mg/animal group (nonsignificant vs. saline).
Discussion
This work refers to the similarity between the human and pig skin structure and function when discussing the development of topical analgesics. The current data were collected from different preclinical studies on postoperative pain performed over a period of 3 years and suggest the following:
- As previously shown, pigs are sensitive to von Frey stimulation following an incision, similar to humans.
- The analgesic effect of bupivacaine, Exparel and ropivacaine resembles the human postoperative condition, with the exception of cesarean surgeries which might be due to sex differences in pain.
- The difference between long-term analgesics such as liposomal bupivacaine (Exparel) and bupivacaine solution resembles the difference in activity reported in humans.
Full SI resulted in a decrease in withdrawal force that is in line with a previous study.16 A significant increase in the withdrawal force following incisional infiltration with ropivacaine has been reported previously, suggesting a decrease in the animals’ sensitivity to the stimuli. This was correlated with the level of ropivacaine found in the wounds.15
Using a ropivacaine infusion postoperatively as a pain control is successful in reducing pain as long as the infusion continues.17 When ropivacaine was dosed locally without infusion, the duration of activity lasted only for the first few hours post surgery.18
Using ropivacaine in different surgeries was compared to the use of morphine and other systemic pain killers, and it suggests a significant ropivacaine activity as a pain reliever. In most cases, the activity was lower than when using the systemic approach.19 This is in line with previous findings in pigs. When treating the animals with systemic administration of morphine,16 there is a complete block of the animal’s sensitivity to tactile stimuli using the von Frey filaments. However, when dosing ropivacaine, the analgesic activity was observed to a much lesser degree.
The activity of ropivacaine in cesarean surgeries is controversial. Nguyen et al20 showed a beneficial activity of ropivacaine, whereas Reinikainen et al21 showed no effect on need for opioids and it had no impact on pain scores or patient satisfaction. Another article suggested that local treatment with ropivacaine reduces the use of opioids in hysterectomy surgery.22 This difference in the activity reported in postoperative pain in women can be related to sex differences in pain. Sex differences between female and male pigs should be investigated further.
In patients, bupivacaine 0.25% wound infiltration had an onset of action within 4±2 min. Percentage of pain relief was 60–80%, depending on the type of surgery. The action continued for 6–8 h.23 Comparing the activity of bupivacaine and Exparel in humans, it was suggested that Exparel has superior activity over bupivacaine and that the duration of action for Exparel was 10–19 h and for bupivacaine it was only 3–8 h.24 These data are in line with the data described in this study when either bupivacaine or Exparel was administered to pigs that had undergone an SMI procedure.
This study shows that the activity of three analgesics which are commonly used for postoperative analgesia in the clinic, i.e., ropivacaine, bupivacaine and Exparel, exhibited high similarity to humans when tested in a post-incisional model in pigs. The resemblance exhibited in this study includes three major points: the level of activity, the duration of activity and the superiority expected from treatment with Exparel vs. bupivacaine. These data, and understanding the resemblance between the pig skin and human skin, suggest that use of these animals in the preclinical phase of developing topical analgesics can accelerate research and development and can to some extent release the bottleneck mentioned by Kopsky and Stahl. Further study should be conducted in neuropathic pain models in the pigs using topical treatments to evaluate the activity of topical analgesics in these models as well.
Conclusion
These data presented in this article, indicate a good resemblance between pig and human in respect of incision pain and the analgesic activity of local treatments. Further studies should be conducted to assess the activity of local and topical treatments in neuropathic pain in the pigs.
Disclosure
The authors report no conflicts of interest in this work.
References
Kopsky DJ, Stahl SM. Bottlenecks in the development of topical analgesics : molecule, formulation, dose-finding, and phase III design. J Pain Res. 2017;10:635–641. | ||
Sullivan TP, Eaglstein WH, Davis SC, Mertz P. The pig as a model for human wound healing. Wound Repair Regen. 2001;9(2):66–76. | ||
Marquet F, Bonneau M, Pascale F, et al. Characterization of dendritic cells subpopulations in skin and afferent lymph in the swine model. PLoS One. 2011;6(1):1–8. | ||
Bartek MJ, Labudde JA, Maibach HI. Skin permeability in vivo: comparison in rat, rabbit, pig and man. J Invest Dermatol. 1972;58(3):114–123. | ||
Jung EC, Maibach HI. Animal models for percutaneous absorption. J Appl Toxicol. 2015;35(1):1–10. | ||
Schmidt R, Schmelz M, Forster C, Ringkamp M, Torebjörk E, Handwerker H. Novel classes of responsive and unresponsive C nociceptors in human skin. J Neurosci. 1995;15(1 pt 1):333–341. | ||
Schmidt R, Schmelz M. Mechano-insensitive nociceptors encode pain evoked by tonic pressure to human skin. Neuroscience. 2000;98(4):793–800. | ||
Obreja O, Schmelz M. Single-fiber recordings of unmyelinated afferents in pig. Neurosci Lett. 2010;470(3):175–179. | ||
Hirth M, Rukwied R, Gromann A, et al. Nerve growth factor induces sensitization of nociceptors without evidence for increased intraepidermal nerve fiber density. Pain. 2013;154(11):2500–2511. | ||
Petersson ME, Obreja O, Lampert A, Carr RW, Schmelz M, Franse E. Differential axonal conduction patterns of mechano- sensitive and mechano-insensitive nociceptors – a combined experimental and modelling study. PLoS One. 2014;9(8):1–11. | ||
Roggenkamp D, Falkner S, Sta F, Petersen M, Schmelz M, Neufang G. Atopic keratinocytes induce increased neurite outgrowth in a coculture model of porcine dorsal root ganglia neurons and human skin cells. J Invest Dermatol. 2012;32(7):1892–1900. | ||
Rice FL, Albrecht PJ, Wymer JP, et al. Sodium channel Nav1. 7 in vascular myocytes, endothelium, and innervating axons in human skin. Mol Pain. 2015;11:26–38. | ||
Wu C, Erickson MA, Xu J, Wild KD, Brennan TJ. Expression profile of nerve growth factor after muscle incision in the rat. Anesthesiology. 2009;110(1):140–149. | ||
Carvalho B, Clark DJ, Yeomans D, Angst MS. Collecting and measuring wound exudate biochemical mediators in surgical wounds. J Vis Exp. 2012;(68). | ||
Castel D, Naveh M, Aharon A, Doron O, Meilin S. Prolonged analgesic effect of PRF-108 and PRF-110 on post-operative pain in pigs. Pain Ther. 2015;5(1):29–42. | ||
Castel D, Willentz E, Doron O, Brenner O, Meilin S. Characterization of a porcine model of post-operative pain. Eur J Pain. 2014;18(4):496–505. | ||
Fustran N, Dalmau A, Ferreres E, Camprub I, Sanzol R, Redondo S. Postoperative analgesia with continuous wound infusion of local anaesthesia vs saline : a double-blind randomized, controlled trial in colorectal surgery. Colorectal Dis. 2015;17(4):342–350. | ||
Karaman S, Kocaba S, Ergun S, Firat V, Uyar M, Şendağ F. Intraperitoneal ropivacaine or ropivacaine plus meperidine for laparoscopic gynecological procedures. Agri. 2012;24(2):56–62. | ||
Bjørnholdt KT, Jensen JM, Bendtsen TF, Søballe K. Local infiltration analgesia versus continuous interscalene brachial plexus block for shoulder replacement pain : a randomized clinical trial. Eur J Orthop Surg Traumatol. 2015;25(8):1245–1252. | ||
Nguyen NK, Landais A, Barbaryan A, et al. Analgesic efficacy of pfannenstiel incision infiltration with ropivacaine 7.5 mg/mL for caesarean section. Anesthesiol Res Pract. 2010;2010:542375. | ||
Reinikainen M, Syväoja S, Hara K. Continuous wound infiltration with ropivacaine for analgesia after caesarean section: a randomised, placebo-controlled trial. Acta Anaesthesiol Scand. 2014;58(8):973–979. | ||
Hayden JM, Oras J. Post-operative pain relief using local infiltration analgesia during open abdominal hysterectomy : a randomized, double-blind study. Acta Anaesthesiol Scand. 2017;61:539–548. | ||
Akhtar MI, Saleem M, Zaheer J. Wound infiltration with Bupivacaine versus Ketorolac for postoperative pain relief in minor to moderate surgeries. J Pak Med Assoc. 2009;59(6):385–388. | ||
Lambrechts M, Brien MJO, Savoie FH, You Z. Liposomal extended-release bupivacaine for postsurgical analgesia. Patient Prefer Adherence. 2013;7:885–890. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.