Back to Journals » Cancer Management and Research » Volume 10
The effect of intravenous hydration strategy on plasma methotrexate clearance during intravenous high-dose methotrexate administration in pediatric oncology patients
Authors Traivaree C , Likasitthananon N, Monsereenusorn C , Rujkijyanont P
Received 24 April 2018
Accepted for publication 24 August 2018
Published 10 October 2018 Volume 2018:10 Pages 4471—4478
DOI https://doi.org/10.2147/CMAR.S172117
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Harikrishna Nakshatri
Chanchai Traivaree,1 Napakjira Likasitthananon,2 Chalinee Monsereenusorn,1 Piya Rujkijyanont1
1Division of Hematology/Oncology, Department of Pediatrics, Phramongkutklao Hospital and College of Medicine, Bangkok, Thailand; 2Department of Pediatrics, Lat Krabang Hospital, Bangkok, Thailand
Background: High-dose methotrexate (HD-MTX) is widely used as a standard chemotherapeutic agent in pediatric cancers. Most research studies have confirmed the therapeutic efficacy of HD-MTX; however, strategies to prevent side effects vary among institutions, especially in developing countries, with limited monitoring of plasma methotrexate level.
Objective: To evaluate the effect of intravenous hydration during HD-MTX administration on plasma methotrexate clearance in pediatric oncology patients.
Materials and methods: This study retrospectively reviewed 165 courses of HD-MTX administered to children with acute lymphoblastic leukemia (ALL), non-Hodgkin’s lymphoma (NHL), or osteosarcoma. Demographic data of patients were collected. Adverse complications related to HD-MTX and 72-hour plasma methotrexate level were analyzed between patients receiving intravenous hydration ≥3,000 mL/m2/day and those receiving hydration <3,000 mL/m2/day.
Results: Among 56 HD-MTX (1.5 g/m2) courses in ALL, delayed methotrexate clearance was only found in one course administered with hydration <3,000 mL/m2/day. However, no correlation was observed between adverse complications and methotrexate levels. Of 34 HD-MTX (1.5–3 g/m2) courses in NHL, no significant correlation was observed between methotrexate levels and intravenous hydration. However, increased adverse complications were found in the course with delayed methotrexate clearance. Interestingly, among 75 HD-MTX (10–12 g/m2) courses in osteosarcoma, normal methotrexate clearance was successfully achieved in all courses administered with hydration ≥3,000 mL/m2/day compared with those administered with hydration <3,000 mL/m2/day (P=0.007). Furthermore, the courses administered with hydration <3,000 mL/m2/day and had delayed methotrexate clearance were more likely to develop adverse complications.
Conclusion: Intravenous hydration of ≥3,000 mL/m2/day during HD-MTX administration is essentially required in osteosarcoma and can be considered as optional in ALL with HD-MTX <1.5 g/m2, especially in developing countries with limited monitoring of plasma methotrexate level.
Keywords: high-dose methotrexate, hydration, children, cancer, osteosarcoma, leukemia, lymphoma
Introduction
Methotrexate is a chemotherapeutic agent that is practically used in cancer treatment, with additional indications for autoimmune and rheumatological diseases.1,2 A methotrexate dose of >500 mg/m2 is generally considered a high dose, for which careful monitoring of drug clearance and extended supportive care to prevent adverse complications are essentially needed.3–6 In the pediatric oncology population, high-dose methotrexate (HD-MTX) is commonly used as a part of treatment protocols among patients with acute lymphoblastic leukemia (ALL),7–9 non-Hodgkin’s lymphoma (NHL),10,11 and osteosarcoma,12–15 with the dose ranging from 1 to 12 g/m2. With the integration of HD-MTX in standard chemotherapeutic regimens, the current survival rates of those patients with ALL, NHL, and osteosarcoma have dramatically improved compared with those reported previously.
Methotrexate is a synthetic folate antimetabolite and cell cycle specific for S phase which inhibits DNA synthesis, repair, and cellular replication. The action of methotrexate is caused by irreversible competitive inhibition of the dihydrofolate reductase enzyme, resulting in the interference of active tetrahydrofolate synthesis and later in inhibited purine and thymidylic acid synthesis, and thus inhibiting DNA synthesis, repair, and cellular replication.16–19 Therefore, actively proliferative tissues are more susceptible to the effects of this chemotherapeutic agent. When methotrexate is used in a higher dose, the drug produces several serious complications. The most common adverse complications of high-dose treatment include mucositis or ulcerative stomatitis, hepatotoxicity, nephrotoxicity, and bone marrow suppression, predisposing to severe infection and bleeding complications.20 An additional rare but important complication is the reaction of the central nervous system to methotrexate, especially when it is administered using an intrathecal route, including leukoencephalopathies and myelopathies.21
The risk of developing adverse toxicities of HD-MTX can be prevented by careful monitoring of plasma methotrexate level, aggressive intravenous hydration with alkalinization of urine, and concurrent use of leucovorin or folinic acid at the appropriate timing according to plasma methotrexate level nomogram.3,4 Since methotrexate can penetrate third-space fluids, such as pleural effusion or ascites, and exits slowly from these compartments as well as excretes through urine, delayed methotrexate clearance can cause serious adverse complications among patients with kidney dysfunction or third-space fluid accumulation or those receiving inadequate hydration. Evidence suggests that a less aggressive hydration regimen (1,500 mL/m2/day) was associated with higher plasma methotrexate levels at the end of infusion of HD-MTX (8 g/m2) among patients with osteosarcoma compared with a higher aggressive hydration regimen (2,000 mL/m2/day); however, there was no difference in the incidence of late eliminated methotrexate between the two hydration regimens.22 Many of the current pediatric protocols recommend a higher hydration strategy during HD-MTX administration, with at least 2 hours of aggressive hydration of a minimum of 200 mL/m2 hourly or 2,400–3,600 mL/m2/day beginning 12 hours before the start of HD-MTX and continuing for 24–48 hours or longer;3 however, the benefits of this aggressive hydration remain unclear.
Although most research studies have focused on the efficacy of HD-MTX in cancer treatment, only a few reports have described the hydration strategy in preventing adverse complications of this chemotherapy.22–25 The strategies for intravenous hydration among patients receiving HD-MTX vary among different institutions and treatment protocols. In addition, some institutions, especially in developing countries, have limited monitoring of plasma methotrexate level, making the intravenous hydration management difficult, which, in turn, results in clinical symptoms of volume overload due to overhydration or HD-MTX-related toxicities due to underhydration. Our study aimed to evaluate the effect of intravenous hydration during HD-MTX administration on plasma methotrexate clearance, as well as adverse complications of HD-MTX among pediatric oncology patients. In addition, potential adverse events from aggressive hydration, including clinical symptoms of volume overload, were also reviewed.
Patients and methods
Patient selection
Thirty-seven pediatric patients diagnosed with ALL, NHL, or osteosarcoma, undergoing 165 courses of HD-MTX containing a chemotherapy regimen at the Division of Hematology/Oncology, Department of Pediatrics, Phramongkutklao Hospital, Bangkok, Thailand, from January 2010 to December 2016, were retrospectively reviewed. Written informed consent and assent forms to review medical records were obtained from all participants including children and their parents or guardians prior to enrollment in the study. The study protocol was approved by the Institutional Review Board of Phramongkutklao Hospital and College of Medicine and adhered to the ethical principles of the Declaration of Helsinki of 1975 and its revision. The inclusion criteria included patients who were diagnosed with ALL, NHL, or osteosarcoma, were younger than 18 years, received intravenous hydration during their hospital admission for HD-MTX administration, and had plasma methotrexate levels measured at least one time point at 72 hours after the initiation of HD-MTX. Patients who had incomplete data, including progress clinical summary notes during their admission for HD-MTX administration, HCO3 level assessment, and evaluation of urine specific gravity, and had no plasma methotrexate level recorded at 72 hours after initiating HD-MTX were excluded from the study.
HD-MTX regimens
Patients with ALL were treated according to the Thai Pediatric Oncology Group (ThaiPOG) protocols, in which treatment is stratified according to risk groups. For low-risk ALL protocol (ThaiPOG ALL-01-05), HD-MTX is administered during the consolidation phase at a dose of 1.5 g/m2 over 24 hours every 2 weeks for a total of four cycles on days 1, 15, 29, and 43 of treatment. For high-risk ALL protocol (ThaiPOG ALL-02-05), HD-MTX is administered during the consolidation phase at a dose of 1.5 g/m2 over 24 hours every 2 weeks for a total of four cycles on days 29, 43, 57, and 71 of treatment. Patients with NHL were treated according to the ThaiPOG protocols depending on lymphoma subtypes. Following mature B-cell lymphoma protocol (ThaiPOG NHL-04-05) for diffuse large B-cell lymphoma and Burkitt lymphoma, HD-MTX is administered during induction, consolidation, and maintenance phases at a dose of 3 g/m2 over 4 hours on the first day of each cycle for a total of five cycles. Patients with lymphoblastic lymphoma were treated with HD-MTX 1.5 g/m2 as in ThaiPOG ALL protocol. Patients with osteosarcoma are treated according to ThaiPOG protocol consisting of HD-MTX 10–12 g/m2 given over 4 hours on weeks 4, 5, 9, 10, 13, 16, 19, 22, 25, and 28 of treatment. Regarding HD-MTX-containing chemotherapy regimens for ALL, NHL, and osteosarcoma in this study, the starting dose of leucovorin (folinic acid) in all regimens was similar at 15 mg/m2/dose administered every 6 hours. The timing to start leucovorin rescue in NHL and osteosarcoma regimens was at 24 hours after the initiation of HD-MTX, and the timing to start leucovorin rescue in ALL regimen was at 36 hours after the initiation of HD-MTX. All patients enrolled in this study were given intravenous leucovorin at the exact time point without delay. In addition, the dose and frequency of intravenous leucovorin (folinic acid) administration were adjusted according to plasma methotrexate nomogram.
Intravenous hydration strategy
According to our institutional methotrexate administration and monitoring guidelines, the amount of aggressive intravenous hydration administered was between 2,500 and 3,500 mL/m2/day depending on individual oncologist’s decisions based on patients’ clinical status at the time of HD-MTX administration; therefore, patients received a different exact amount of intravenous fluid within the instructed range between 2,500 and 3,500 mL/m2/day on each course of HD-MTX regimen. In this study, the intravenous hydration administrations were grouped as <3,000 and ≥3,000 mL/m2/day. In addition, potential adverse events from aggressive hydration including clinical symptoms of volume overload were also reviewed.
Methotrexate levels and laboratory monitoring during HD-MTX administration
All patients treated with HD-MTX were carefully followed up according to our institutional methotrexate administration and monitoring guidelines including adjusting of the dose and monitoring the frequency of intravenous leucovorin (folinic acid) administration based on the methotrexate level obtained at different time points (plasma methotrexate nomogram). In addition, the bicarbonate in intravenous fluid was adjusted based on serial urine pH monitoring to keep urine pH between 7 and 8 at all times. Plasma methotrexate levels collected at 72 hours after the initiation of HD-MTX from all patients were reviewed and analyzed. A 72-hour methotrexate level of <0.1 μmol/L was considered complete methotrexate clearance and a level ≥0.1 μmol/L indicated delayed methotrexate clearance. In addition, blood HCO3 and urine specific gravity during HD-MTX administration were reviewed and their average levels were used to evaluate the usefulness of serving as predictive factors for delayed methotrexate clearance.
Adverse complications from HD-MTX administration
Adverse complications such as mucositis, bone marrow suppression, renal toxicity, and liver toxicity were reviewed among all patients during and after each course of the HD-MTX regimen. Bone marrow suppression was defined as an absolute neutrophil count of <500 cell/mm3 within 3 days and/or platelet count of <75,000 cell/mm3 within 1 week after the course of HD-MTX regimen. Renal toxicity was defined as an elevated serum creatinine level of >2 SD of the upper limit of normal in the reference population of the same age. Liver toxicity was defined as an elevated level of serum aspartate aminotransferase and/or serum alanine aminotransferase of >2 SD of the upper limit of normal in the reference population of the same age.
Statistical analysis
Baseline values of selected variables were calculated as mean and SD or as median (range) for continuous variables and were expressed using frequency and percentage for categorical variables. The chi-squared test and Fisher’s exact test were used to analyze the categorical variables for data with a parametric distribution and nonparametric distribution, respectively. SPSS Version 23 Software (IBM, Armonk, NY, USA) was used for statistical analysis, and P-values <0.05 were considered significant.
Results
Patient characteristics
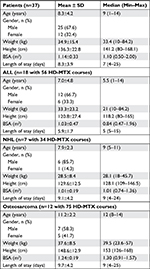
Patient characteristics including age, gender, weight, height, body surface area (BSA), and length of hospital stay are summarized in Table 1. The patients’ age in this study was consistent with common age groups at the diagnosis of each cancer type, such as younger patients diagnosed with ALL and older patients diagnosed with osteosarcoma. The median weight, height, and BSA were appropriate for the patient’s age in all disease types. Males were more prominent than females at a ratio of 2:1. Of the 165 HD-MTX-containing chemotherapy regimens during the study period at our institution, the most common cancer-specific HD-MTX regimen was the osteosarcoma regimen followed by ALL and NHL regimens.
Intravenous hydration was given during each cancer-specific HD-MTX course, as shown in Table 2. The intravenous hydration administrations were grouped as <3,000 and ≥3,000 mL/m2/day. Although the number of intravenous hydrations ≥3,000 mL/m2/day given was less than hydrations <3,000 mL/m2/day, none of the patients in both groups developed clinical signs or symptoms of volume overload from aggressive hydration.
The correlation between plasma methotrexate level at 72 hours and associated factors in cancer-specific HD-MTX regimens
The correlation between 72-hour plasma methotrexate levels and associated factors in disease-specific HD-MTX regimens was analyzed and is shown in Table 3. Of 56 courses of HD-MTX regimen for ALL, elevated 72-hour plasma methotrexate level of ≥0.1 μmol/L was noted in one course of treatment. However, this abnormally elevated methotrexate level was not significantly associated with any factors including patients’ gender, intravenous hydration, HCO3, urine specific gravity, and duration of methotrexate infusion. Adverse complications including mucositis, bone marrow suppression, and liver toxicity were found in 5, 5, and 1 course of HD-MTX treatment, respectively.
Of 34 courses of HD-MTX regimen for NHL, 14 courses were given with intravenous hydrations <3,000 mL/m2/day and the 20 remaining courses were given with intravenous hydrations ≥3,000 mL/m2/day. Four of 14 courses were given at <3,000 mL/m2/day hydration, and seven of 20 courses were given at ≥3,000 mL/m2/day hydration and were associated with an elevated 72-hour plasma methotrexate level of ≥0.1 μmol/L; however, the above association between different intravenous hydrations and 72-hour plasma methotrexate levels was not statistically significant. In addition, the correlation between 72-hour plasma methotrexate levels and other associated factors including patients’ gender, HCO3, urine specific gravity, and duration of methotrexate infusion was analyzed and revealed no statistical significance. Of note, adverse complications were found more in courses having elevated 72-hour plasma methotrexate levels ≥0.1 μmol/L (mucositis [45.5%], bone marrow suppression [27.3%], renal toxicity [9.1%], and liver toxicity [9.1%]) than courses having 72-hour plasma methotrexate levels <0.1 μmol/L (mucositis [13.0%], bone marrow suppression [13.0%], renal toxicity [0%], and liver toxicity [4.3%]).
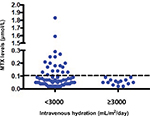
Of 75 HD-MTX courses for osteosarcoma, 62 courses were given with intravenous hydrations <3,000 mL/m2/day and the 13 remaining courses were given with intravenous hydrations ≥3,000 mL/m2/day. Twenty-three of 62 courses (59.0%) given with <3,000 mL/m2/day hydration were associated with elevated 72-hour plasma methotrexate levels ≥0.1 μmol/L. Interestingly, none of the HD-MTX courses given with ≥3,000 mL/m2/day hydration had elevated 72-hour plasma methotrexate levels (Figure 1). The above association between intravenous hydration and 72-hour plasma methotrexate levels was significant with a P-value of 0.007. In addition, the association between 72-hour plasma methotrexate levels and other associated factors including patients’ gender, HCO3, and urine specific gravity was analyzed and revealed no statistical significance. Similar to NHL, adverse complications were found more often in courses having elevated 72-hour plasma methotrexate levels ≥0.1 μmol/L (mucositis [8.7%] and liver toxicity [26.1%]) than in courses having 72-hour plasma methotrexate levels <0.1 μmol/L (mucositis [0%], bone marrow suppression [1.9%], and liver toxicity [15.4%]).
Discussion
HD-MTX has been widely used as a key element in treating various cancer types. In childhood malignancies, this agent has been added to standard chemotherapeutic regimens for ALL, NHL, and osteosarcoma, and the outcomes of these cancer types have been dramatically improved compared with the pre-HD-MTX era.7,8,10–13 Methotrexate is a cell cycle-specific chemotherapeutic agent and can be administered by infusion over few hours or continuous drip over 24 hours.17,26 The adverse complications of HD-MTX administration have been well recognized, such as various degrees of mucositis, bone marrow suppression, and renal and liver toxicities. Several methods have been introduced to prevent these complications, including the use of leucovorin in HD-MTX-containing regimens and the plasma methotrexate level-monitoring guidelines to optimize the dose and frequency of administered leucovorin. Aggressive hydration is another method used in HD-MTX regimens, but the optimal amount of intravenous hydration required to prevent its adverse complications remains unclear. Most research studies have mainly focused on the efficacy of the HD-MTX-containing regimens in cancer treatment; however, only a few of these have specifically looked at the safety of this chemotherapeutic agent and especially the method to prevent associated adverse complications. Herein, we have conducted a study specifically focusing on the most effective use of the intravenous strategy during HD-MTX administration, especially for institutions in developing countries with limited access to monitoring of plasma methotrexate level.
Thirty-seven pediatric oncology patients undergoing 165 courses of HD-MTX-containing chemotherapeutic regimens at Phramongkutklao Hospital were retrospectively reviewed. The patients were diagnosed with various cancer types requiring HD-MTX in their standard chemotherapeutic treatment regimens, including ALL, NHL, and osteosarcoma. All patients were given differing amounts of aggressive hydration and had plasma methotrexate level monitored. Important data were collected and analyzed, including 72-hour plasma methotrexate level, amounts of intravenous hydration, HCO3 level, urine specific gravity, and duration of methotrexate infusion (4 vs 24 hours). The intravenous hydrations were administered between 2,400 and 3,600 mL/m2/day according to the recommendation from current pediatric protocols and were grouped and divided as <3,000 and ≥3,000 mL/m2/day.
In our study, patients with ALL tended to tolerate HD-MTX better than patients with NHL and osteosarcoma. Almost all patients with ALL had 72-hour plasma methotrexate levels <0.1 μmol/L consistent with complete clearance of HD-MTX. Only one patient presented a 72-hour plasma methotrexate level ≥0.1 μmol/L; however, the elevation of methotrexate level was not significantly correlated with patients’ gender, amount of intravenous hydration, HCO3, urine specific gravity, or duration of methotrexate infusion. In addition, this patient did not experience adverse complications from delayed methotrexate clearance. One possible explanation could be that the dose of HD-MTX in ALL protocols (1.5 g/m2) being lower than NHL and osteosarcoma protocols (3–12 g/m2) more easily caused the drug clearance among patients with ALL than among those with NHL and osteosarcoma. Although one-third of the patients with NHL experienced delayed clearance of HD-MTX, we could not identify a significant correlation between elevated 72-hour plasma methotrexate levels and other associated factors comprising patients’ gender, amount of intravenous hydration, HCO3, urine specific gravity, or duration of methotrexate infusion. Although our observation was not able to identify predictive factors for delayed methotrexate clearance, it revealed increased numbers of adverse complications including mucositis, bone marrow suppression, and renal toxicity among those patients experiencing delayed clearance of HD-MTX. This finding indicated the importance of monitoring plasma methotrexate levels among patients with NHL receiving HD-MTX regimen. All HD-MTX courses following osteosarcoma protocols were infused over 4 hours. The dose of HD-MTX was also noted to be higher than ALL and NHL protocols. Thirty percent of HD-MTX courses in osteosarcoma were associated with delayed methotrexate clearance. Interestingly, we found a significant association between intravenous hydration of <3,000 mL/m2/day and delayed methotrexate clearance. This finding indicated the importance of aggressive hydration ≥3,000 mL/m2/day in the HD-MTX course following osteosarcoma protocols. Furthermore, our observation again revealed the increased numbers of adverse complications, including mucositis and liver toxicity, among patients experiencing delayed clearance of HD-MTX. This observational finding also addressed the need to monitor plasma methotrexate levels among patients with osteosarcoma receiving HD-MTX regimens. Of note, no differences were observed in toxicities related to the timing in starting leucovorin rescue. Five (8.9%) patients who developed mucositis and five (8.9%) patients who experienced bone marrow suppression received ALL regimen (leucovorin started at 36 hours). However, 10 (9.1%) patients and 7 (6.4%) patients receiving NHL or osteosarcoma regimen (leucovorin started at 24 hours) developed mucositis and bone marrow suppression, respectively.
One limitation was that our study employed a retrospective design with a small sample size and the possibility of having other unknown confounding factors; therefore, a future randomized study enrolling a larger sample size might be of greater interest to affirm the usefulness of aggressive intravenous hydration among patients receiving HD-MTX regimens.
In summary, our study emphasized the requirement of aggressive intravenous hydration ≥3,000 mL/m2/day during HD-MTX administration following osteosarcoma protocols to achieve complete clearance of HD-MTX. In patients with osteosarcoma, aggressive hydration ≥3,000 mL/m2/day is highly essential. Moreover, this amount of fluid hydration is strongly recommended in institutions where plasma methotrexate levels are not available or results are not provided on the same day. No adverse complications were noted following ALL protocols with HD-MTX 1.5 g/m2. In addition, the administration of HD-MTX at this dose or lower might not require aggressive hydration ≥3,000 mL/m2/day as well as monitoring of plasma methotrexate level. None of the patients receiving aggressive hydration ≥3,000 mL/m2/day experienced signs or symptoms of volume overload; therefore, for all patients receiving this higher regime to increase the safety of HD-MTX administration would be more sensible.
Acknowledgments
This study was supported, in part, by grants from the Phramongkutklao Hospital and College of Medicine, Royal Thai Army. The authors would like to thank the patients and families for participating in the study.
Disclosure
The authors report no conflicts of interest in this work.
References
Herfarth HH. Methotrexate for Inflammatory Bowel Diseases - New Developments. Dig Dis. 2016;34(1-2):140–146. | ||
Weinblatt ME. Methotrexate in rheumatoid arthritis: a quarter century of development. Trans Am Clin Climatol Assoc. 2013;124:16–25. | ||
Howard SC, Mccormick J, Pui CH, Buddington RK, Harvey RD. Preventing and Managing Toxicities of High-Dose Methotrexate. Oncologist. 2016;21(12):1471–1482. | ||
Stoller RG, Hande KR, Jacobs SA, Rosenberg SA, Chabner BA. Use of plasma pharmacokinetics to predict and prevent methotrexate toxicity. N Engl J Med. 1977;297(12):630–634. | ||
Mitchell MS, Wawro NW, Deconti RC, Kaplan SR, Papac R, Bertino JR. Effectiveness of high-dose infusions of methotrexate followed by leucovorin in carcinoma of the head and neck. Cancer Res. 1968;28(6):1088–1094. | ||
Abrey LE, Deangelis LM, Yahalom J. Long-term survival in primary CNS lymphoma. J Clin Oncol. 1998;16(3):859–863. | ||
Moe PJ, Holen A. High-dose methotrexate in childhood all. Pediatr Hematol Oncol. 2000;17(8):615–622. | ||
Cooper SL, Brown PA. Treatment of pediatric acute lymphoblastic leukemia. Pediatr Clin North Am. 2015;62(1):61–73. | ||
Pui CH, Relling MV, Evans WE. Is mega dose of methotrexate beneficial to patients with acute lymphoblastic leukemia? Leuk Lymphoma. 2006;47(12):2431–2432. | ||
PDQ® Pediatric Treatment Editorial Board. Childhood Non-Hodgkin Lymphoma Treatment (PDQ(R)): Health Professional Version. PDQ Cancer Information Summaries. Bethesda, MD: National Cancer Institute; 2002. | ||
Woessmann W, Seidemann K, Mann G, et al. The impact of the methotrexate administration schedule and dose in the treatment of children and adolescents with B-cell neoplasms: a report of the BFM Group Study NHL-BFM95. Blood. 2005;105(3):948–958. | ||
Choeyprasert W, Pakakasama S, Sirachainan N, et al. Comparative outcome of Thai pediatric osteosarcoma treated with two protocols: the role of high-dose methotrexate (HDMTX) in a single institute experience. Asian Pac J Cancer Prev. 2014;15(22):9823–9829. | ||
Isakoff MS, Bielack SS, Meltzer P, Gorlick R. Osteosarcoma: Current Treatment and a Collaborative Pathway to Success. J Clin Oncol. 2015;33(27):3029–3035. | ||
Patiño-García A, Zalacaín M, Marrodán L, San-Julián M, Sierrasesúmaga L. Methotrexate in pediatric osteosarcoma: response and toxicity in relation to genetic polymorphisms and dihydrofolate reductase and reduced folate carrier 1 expression. J Pediatr. 2009;154(5):688–693. | ||
Holmboe L, Andersen AM, Mørkrid L, Slørdal L, Hall KS. High dose methotrexate chemotherapy: pharmacokinetics, folate and toxicity in osteosarcoma patients. Br J Clin Pharmacol. 2012;73(1):106–114. | ||
Fleisher M. Antifolate analogs: mechanism of action, analytical methodology, and clinical efficacy. Ther Drug Monit. 1993;15(6):521–526. | ||
Goodsell DS. The molecular perspective: methotrexate. Stem Cells. 1999;17(5):314–315. | ||
Rajagopalan PT, Zhang Z, Mccourt L, Dwyer M, Benkovic SJ, Hammes GG. Interaction of dihydrofolate reductase with methotrexate: ensemble and single-molecule kinetics. Proc Natl Acad Sci U S A. 2002;99(21):13481–13486. | ||
Longo-Sorbello GS, Bertino JR. Current understanding of methotrexate pharmacology and efficacy in acute leukemias. Use of newer antifolates in clinical trials. Haematologica. 2001;86(2):121–127. | ||
Khan ZA, Tripathi R, Mishra B. Methotrexate: a detailed review on drug delivery and clinical aspects. Expert Opin Drug Deliv. 2012;9(2):151–169. | ||
Bhojwani D, Sabin ND, Pei D, et al. Methotrexate-induced neurotoxicity and leukoencephalopathy in childhood acute lymphoblastic leukemia. J Clin Oncol. 2014;32(9):949–959. | ||
Ferrari S, Orlandi M, Avella M, et al. Effects of hydration on plasma concentrations of methotrexate in patients with osteosarcoma treated with high doses of methotrexate. Minerva Med. 1992;83(5):289–293. | ||
Karremann M, Sauerbier J, Meier C, et al. The impact of prehydration on the clearance and toxicity of high-dose methotrexate for pediatric patients. Leuk Lymphoma. 2014;55(12):2874–2878. | ||
Mikkelsen TS, Mamoudou AD, Tuckuviene R, Wehner PS, Schroeder H. Extended duration of prehydration does not prevent nephrotoxicity or delayed drug elimination in high-dose methotrexate infusions: a prospectively randomized cross-over study. Pediatr Blood Cancer. 2014;61(2):297–301. | ||
Kinoshita A, Kurosawa Y, Kondoh K, et al. Effects of sodium in hydration solution on plasma methotrexate concentrations following high-dose methotrexate in children with acute lymphoblastic leukemia. Cancer Chemother Pharmacol. 2003;51(3):256–260. | ||
Treon SP, Chabner BA. Concepts in use of high-dose methotrexate therapy. Clin Chem. 1996;42(8 Pt 2):1322–1329. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.