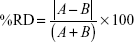Back to Journals » Drug Design, Development and Therapy » Volume 12
The effect of food and liquid pH on the integrity of enteric-coated beads from cysteamine bitartrate delayed-release capsules
Authors Pavloff N, Hauser TA, Williams C, Isbell SL, Cadieux B, Johnson M
Received 22 May 2018
Accepted for publication 13 June 2018
Published 6 September 2018 Volume 2018:12 Pages 2795—2804
DOI https://doi.org/10.2147/DDDT.S174928
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Nadine Pavloff,1 Terry A Hauser,1 Chris Williams,2 Sara Louis Isbell,1 Ben Cadieux,1 Mark Johnson1
1Horizon Pharma, Inc., Lake Forest, IL, USA; 2Alcami Corporation, Wilmington, NC, USA
Background: Cysteamine bitartrate delayed-release (DR) capsule (Procysbi®) is approved for treatment of nephropathic cystinosis in the USA, Canada, and the EU. The capsules contain cysteamine bitartrate beads that are enteric coated with acid-resistant Eudragit L 30 D-55, preventing drug release in the acidic stomach environment while allowing dissolution of the beads in the more alkaline environment of the small intestine. Patients who have difficulty swallowing capsules can open capsules, sprinkle beads onto 4 ounces of a suitable food or liquid, gently mix, and consume the entire content within 30 minutes. Foods found to be suitable for administration, and described in the Procysbi US labeling, include fruit juices (except grapefruit juice), applesauce, and berry jelly; there are minor variations in the foods and liquids recommended by regulatory authorities in other countries. This study aimed to assess the stability of enteric-coated beads exposed to additional foods at different conditions to expand the list of suitable foods for drug administration.
Methods: For each test condition, beads from eight opened 75 mg cysteamine bitartrate DR capsules were gently mixed with test food and maintained at a prespecified temperature and duration; remaining undissolved beads were then recovered from the food. The recovered beads were split into two portions: one assayed for remaining drug content and the other subjected to dissolution testing to assess the effect on the drug-release profile.
Results: The results show that bead integrity was maintained when mixed with foods at pH values <5.5 at all time points when refrigerated (2°C–8°C) and at room (20°C–22°C) and lukewarm (37°C–41°C) temperatures. Bead integrity was not maintained when mixed with foods at pH values of ≥5.5 at room temperature.
Conclusion: The results from this in vitro dissolution study help in identifying additional foods that may be used for the administration of cysteamine bitartrate DR beads from opened capsules using the sprinkle method.
Keywords: nephropathic cystinosis, cysteamine, delayed-release, dissolution
Corrigendum for this paper has been published
Introduction
Depletion of cystine with delayed-release (DR) cysteamine bitartrate (Procysbi®; Horizon Pharma USA, Inc., Lake Forest, IL, USA, and Chiesi Farmaceutici S.p.A., Parma, Italy) is the mainstay of therapy for nephropathic cystinosis,1,2 a rare genetic disease characterized by accumulation of cystine in lysosomes,3 and is approved for the treatment of nephropathic cystinosis (the US indication is for the treatment of patients aged 1 year and older). Nephropathic cystinosis is associated with kidney failure and progressive multiorgan damage, unless treatment is initiated early and maintained for life.4 However, poor treatment compliance has been observed and is expected to negatively affect patient outcomes.5 Unlike immediate-release cysteamine bitartrate, which requires 6-hourly dosing around the clock, the DR formulation allows for dosing every 12 hours6 and is expected to greatly improve compliance.
The DR formulation is administered in the form of encapsulated cysteamine bitartrate beads. These beads are enteric coated with Eudragit L 30 D-55, an acid-resistant polymer that allows the bead to bypass dissolution in the acidic environment of the stomach while allowing dissolution in the more alkaline environment of the small intestine (pH >5.5).7 This results in a predictable and reproducible pharmacokinetic profile that allows for more convenient dosing every 12 hours.1,6 This coating has been used on a number of other medications, including esomeprazole magnesium trihydrate, tamsulosin hydrochloride, ranitidine hydrochloride, and diclofenac sodium.7
Oral ingestion of intact capsules is the recommended mode of administration for DR cysteamine bitartrate. Patients with swallowing difficulties (a common pathological characteristic of cystinosis) may successfully receive adequate amounts of the drug when capsule contents (beads) are sprinkled onto 4 ounces of suitable foods or delivered via a gastronomy tube; this is the recommended amount of food or liquid stated in the US prescribing information.1,8 Foods previously found to be suitable for administration and described in the US Procysbi® labeling include fruit juices (except grapefruit juice), applesauce, and berry jelly. There is some variation in the foods and liquids recommended by regulatory authorities in other countries; for example, in the EU, ~100 grams of apple compote or berry jelly is recommended. Patients are advised to swallow the bead/food mixture within 30 minutes of preparation; however, additional information is frequently requested by patients and prescribers about the suitability of other foods and the effects of food temperature and exposure time on the integrity of the enteric coating.
The purpose of this in vitro study was to assess the stability of enteric-coated beads exposed to a variety of foods and liquids of varying acidity/pH and temperatures to expand the list of potential foods and liquids deemed suitable for the administration of the drug product. The bioequivalence between administration of intact cysteamine bitartrate DR capsules and beads from opened capsules mixed with food has been demonstrated.9 This study did not evaluate the effects of various foods and liquids on halitosis, which is a known transient side effect of cysteamine via its metabolite dimethyl sulfide. A halitosis sub-study of a Phase III clinical trial with DR cysteamine bitartrate associated that formulation with a 26% reduction in exhaled dimethyl sulfide in comparison with immediate-release cysteamine bitartrate.7 Neither this study nor the halitosis sub-study assessed halitosis as a factor in therapy compliance.
Methods
Overview of experimental approach
A variety of foods and liquids were chosen for evaluation (six liquids, eight solids; Table 1) based on patient feedback, with pH values ranging from 3.17 to 6.70; pH values were <5.5 for 13 foods and liquids and >5.5 for three of the foods and liquids tested. Prior to mixing with the beads, the foods and liquids were exposed to various temperatures: cold (2°C–8°C), lukewarm (37°C–41°C), and room temperature (20°C–22°C). Detailed procedures for preparing the foods, mixing with beads, and bead recovery are described in the Supplementary materials.
  | Table 1 Foods and liquids tested: supplier, pH, and temperature are listed |
Separate methods were used to evaluate the integrity of the bead coating (dissolution testing) and the strength of the recovered active ingredient (cysteamine bitartrate assay). For both the dissolution and assay methods, the following three samples were analyzed: 1) treated sample, consisting of DR beads mixed with food or liquid, followed by washing and subsequent recovery; 2) control sample, consisting of DR beads not exposed to food or liquid but prepared in the same manner as the treated sample; and 3) untreated sample to serve as the control, consisting of DR beads not prepared per the procedures for treated or control samples and not exposed to food or liquid. All beads were derived from the same source supply of capsules.
For each food or liquid tested, findings from the control samples were compared with those of the untreated and treated samples. If the control samples met the specified acceptance criteria listed for each test when compared with untreated samples, it implied that the procedure for preparing the beads for administration did not affect the integrity or strength of the beads. If a treated sample did not meet acceptance criteria when compared with results from a control sample, it implied that the exposure to the test food or liquid affected the integrity and drug-release properties of the cysteamine bitartrate DR beads, and those foods and liquids were considered incompatible for drug product administration.
Dissolution testing
The US Food and Drug Administration-recommended dissolution method for cysteamine bitartrate DR capsules is a two-stage process; the first is an acid stage representative of conditions in the stomach, and the second is a buffer stage representative of intestinal fluid.10 The USA/Canada and the EU have different acceptance criteria based on the countries’ regulations (buffer stage is analyzed at 20 minutes for USA/Canada and 30 minutes for the EU). Dissolution testing of the cysteamine bitartrate beads was performed first under acidic conditions (pH ~1–3; acid stage, 0.1 N HCl), and then under near-neutral pH conditions (pH 6.8; buffer stage, 0.05 M sodium phosphate). This study used an internal protocol-defined method to adjust pH using a 10 N sodium hydroxide solution.
Beads from eight opened 75 mg DR cysteamine bitartrate capsules (considered a typical patient’s dose, supplied by Horizon Pharma USA, Inc., lot number 3118115) were exposed and recovered from the food or liquid at each time point tested, as described in the Supplementary materials. After exposure, washing, and recovery, the composite of wet beads was weighed in a drying dish to determine the average capsule fill weight of the wet beads. The amount corresponding to the calculated fill weight of one capsule was transferred to an Apparatus 1 dissolution basket2,11 and analyzed. The total content of the six capsules was analyzed.
The following acceptance criteria were used: for the acid stage, the food exposed and control beads met the drug product release specification limit of 10% or lower dissolved; for the buffer stage, the percentage relative deviation (%RD) (calculated as below) between the treated sample (A) and control sample (B) at the 20- and 30-minute pull points was 6% or lower.
|
Assay testing
For each food or liquid and time point tested, beads equivalent to eight 75 mg capsules were prepared, exposed, and recovered. For the treated samples, the beads were allowed to dry for 90 minutes in a drying dish in an oven at 60°C with a 100 mmHg vacuum. After determination of the final weight of the dried composite, the dried beads were ground to a fine powder in a ball mill. Ground beads, equivalent to one 75 mg capsule fill weight, were prepared in duplicate, diluted, and analyzed by high-performance liquid chromatography. Control and untreated samples were prepared in the same manner as the treated samples (cysteamine bitartrate reference standard, lot numbers CBT-1213-09 and CBT-0314-10). To meet acceptance criteria, the average assay result (from two preparations) for beads exposed to liquid or food must have been 90%–110% of the average assay result obtained from the untreated/control beads. These methods were previously validated for the release of the drug product on the market.
Results
Dissolution study
Control samples and untreated samples
Two preparations of untreated and control samples were used for comparison to all solids and liquids tested. Untreated sample 1 and control sample 1 were tested for comparison against all foods except smoothies and warm sweet potato; untreated sample 2 and control sample 2 were tested for comparison with smoothies and warm sweet potato. All of the control samples and untreated samples met the acceptance criteria of the release of the drug product for dissolution at both the acid and buffer stages. In the acid stage, the percentage dissolved was 1% or lower for all samples, whereas the mean percentage relative SD in the buffer stage was <6% for all samples (Table 2).
  | Table 2 Dissolution results for untreated samples and control samples |
Food-exposed samples vs control samples
Results are reported for the acid stage and the buffer stage of the dissolution process. The difference in dissolution between food-exposed samples and control samples was <6% for most food items at all time points tested at the buffer stage, meeting the acceptance criteria for suitability. Exceptions were baby formula (%RD: 94% and 48%–49% at 1 and 2 hours of food exposure, respectively), unflavored oral rehydration solution (%RD: 19%, 39%–40%, and 29%–31% at 30 minutes, 1 hour, and 2 hours, respectively), and bottled water (%RD: 11% at 2 hours of food exposure for the 20-minute buffer stage; Table 3). For all of these samples, the percentage dissolved was <80% of the label claim, indicating the enteric coating of the beads had disintegrated before exposure to the buffer-stage dissolution medium. Figure 1 shows the dissolution profiles of cysteamine bitartrate exposed to controls and food for 30, 60, and 120 minutes. Figure 2 shows the dissolution pattern in the acid stage. Samples exposed to unflavored oral rehydration solution and bottled water (exposed for 1 and 2 hours) had >10% dissolution in the acid stage, suggesting that the enteric coating of the beads likely dissolved before the acid dissolution phase began.
Beads exposed to mashed potatoes could not be analyzed using acid- or buffer-stage dissolution because of challenges in separating the beads from the food after exposure. The mashed potatoes compromised the coating on the beads, rendering them soft and enlarged upon visual inspection. No results could be reported, and thus mashed potatoes were deemed incompatible for administration of the enteric-coated beads.
Assay study
Control samples and untreated samples
Similar to the dissolution study, two preparations of untreated and control samples were used, first (sample 1, control 1) in comparison with samples treated with all foods except for smoothies and warm sweet potato, and second (sample 2, control 2) in comparison with samples treated with smoothies and warm sweet potato.
Food-exposed samples vs control samples
Average assay results were within 90%–110% of the control average assay result for most food-exposed samples analyzed at all time points tested. Exceptions were baby formula, for which the average assay results fell to 87% and 43% of the treated control at 1 and 2 hours, respectively, and unflavored oral rehydration solution, for which the average assay results fell to 86%, 45%, and 55% of the treated control at 30 minutes, 1 hour, and 2 hours, respectively (Table 4).
Discussion
The cysteamine bitartrate beads are formulated with an enteric coating to bypass dissolution in the acidic environment of the stomach while allowing dissolution in the more alkaline environment of the small intestine (pH >5.5), thereby imparting the DR attribute to the drug product. Premature dissolution of the cysteamine bitartrate DR beads can affect drug bioavailability. Therefore, selection of compatible foods is an important consideration for patients, particularly those with swallowing difficulties, who prefer to administer cysteamine bitartrate DR beads mixed with food instead of ingesting whole capsules. Previously, only a few foods and liquids (applesauce, berry jelly, fruit juices except grapefruit) had been evaluated as part of the clinical studies to ensure optimal drug bioavailability; these are listed in the approved US drug product labeling.1 To help potentially expand food and liquid options for the administration of this drug, we investigated bead stability and integrity from a list of preferred foods and liquids suggested by patients.
The recommended dissolution method for cysteamine bitartrate DR beads is a two-stage process, first in acid and then in near-neutral buffer (pH 6.8), to mimic conditions in the stomach and intestine, respectively. Findings from our in vitro analyses confirmed the suitability of several foods and liquids in maintaining the drug-release properties of cysteamine bitartrate DR beads, including fruit juices, yogurts, pureed fruits, and yogurt-based smoothies. Exposure to these foods and liquids for up to 2 hours did not negatively affect the enteric coating of the beads. The strength of the recovered active ingredient, in terms of the percentage of cysteamine bitartrate compared with the label claim, decreased with longer food exposure times. The regulatory authority-approved use of cysteamine bitartrate DR beads suggests that food (applesauce, berry jelly, or fruit juice) should be consumed within a maximum of 30 minutes (US prescribing information)1 or 2 hours (EU summary of product characteristics) of mixing with the drug.2 The results of this study corroborated the caution against the use of longer food exposure times.
Foods determined to be incompatible for drug product administration were baby formula, unflavored oral rehydration solution, and bottled water (at exposure times >1 hour), all of which caused the enteric coating of the beads to dissolve prior to exposure to the buffer-stage solution. Of note, the treated samples that did not meet the acceptance criteria (or could not be tested, such as the mashed potatoes) were mixed with foods with a pH >5.5, except for bottled water at pH 5.3 (failed only at 1-hour acid stage). This finding is consistent with the known pH-dependent dissolution properties of the enteric coating (Eudragit L 30 D-55); at a higher pH, the enteric coating is expected to disintegrate. These findings suggest that the beads are likely to prematurely release active drug upon exposure to these liquids prior to reaching the site of absorption in the small intestine, thus disrupting the DR properties of the drug product. Temperature did not impact suitability of cysteamine bitartrate beads in foods and liquids tested at cold, lukewarm, or room temperatures. Exposure of the beads to mashed potatoes caused the beads to become soft and enlarged, preventing the completion of dissolution tests. Therefore, this food was also determined to be incompatible with the sprinkle method of administration.
These findings indicate that certain additional foods identified in this study may be suitable for administration of enteric-coated cysteamine bitartrate DR beads via the sprinkle method from opened capsules or through a gastrostomy tube. Furthermore, the results suggest that beads may be mixed with compatible foods up to 2 hours prior to ingestion with minimal impact on the enteric coating, thus expanding options for administration.
Conclusion
The results from this in vitro study confirm the pH-dependent compatibility of cysteamine bitartrate DR beads with foods. Experimentally tested foods with pH values below 5.5 (ie, plain or Greek yogurt, strawberry yogurt, pureed bananas, pureed mangoes, and pickle juice, as well as smoothies of yogurt, strawberries, and bananas) preserved bead integrity during mixing and exposure periods up to 2 hours. The dissolution and assay results of this study align with the compatible foods and liquids listed on the US and EU prescribing information for Procysbi (berry jelly, applesauce, and fruit juice). This study also showed that beads may be added to refrigerated or lukewarm foods and liquids, and may be kept up to 2 hours after mixing, if needed, prior to administration. A warning from this study is that some foods might cause the enteric-coated beads to soften and and/or cause premature dissolution of the beads, particularly foods with pH values (eg, mashed potatoes, baby formula, and unflavored oral rehydration solution). Our findings may offer expanded food and liquid options for patients with nephropathic cystinosis, thus helping to reduce the heavy burden associated with this disease and increasing the potential options for administration.
Acknowledgments
The abstract of this paper was presented as a poster presentation with interim findings at the American Pharmacists Association (APHA) Annual Meeting and Exposition 2017; March 24–27, 2017; San Francisco, CA, USA. The poster’s abstract was published in APhA2017 abstracts of contributed papers. JAPhA. 2017;57(3):e1–e142.
Writing and editorial support for the preparation of this manuscript was provided by Sabrina L Maurer, PharmD, CMPP, of Excel Scientific Solutions; funding for the support was provided by Horizon Pharma USA, Inc.
This study was supported by Raptor Pharmaceuticals (now Horizon Pharma USA, Inc.). Employees of Raptor designed the study and participated in the analysis and interpretation of the data.
Disclosure
At the time this study was conducted, N Pavloff, TA Hauser, SL Isbell, B Cadieux, and M Johnson were full-time employees of Horizon Pharma USA, Inc. C Williams is a full-time employee of Alcami Corporation. The authors report no other conflicts of interest in this work.
References
Horizon Pharma USA, Inc. PROCYSBI® (cysteamine bitartrate) delayed-release capsules for oral use [prescribing information]; 2017. Available from: http://hznp.azureedge.net/public/Procysbi-Prescribing-Info.pdf. Accessed August 14, 2018. | ||
The United States Pharmacopeial Convention. The United States Pharmacopeia and The National Formulary (USP–NF), General Chapter 711 Dissolution. Available from: https://www.usp.org/sites/default/files/usp/document/harmonization/gen-method/q01_pf_ira_33_4_2007.pdf. Accessed December 27, 2018. | ||
Gahl WA, Thoene JG, Schneider JA. Cystinosis. N Engl J Med. 2002;347(2):111–121. | ||
Emma F, Nesterova G, Langman C, et al. Nephropathic cystinosis: an international consensus document. Nephrol Dial Transplant. 2014;29(Suppl 4):iv87–iv94. | ||
Brodin-Sartorius A, Tête MJ, Niaudet P, et al. Cysteamine therapy delays the progression of nephropathic cystinosis in late adolescents and adults. Kidney Int. 2012;81(2):179–189. | ||
Langman CB, Greenbaum LA, Grimm P, et al. Quality of life is improved and kidney function preserved in patients with nephropathic cystinosis treated for 2 years with delayed-release cysteamine bitartrate. J Pediatr. 2014;165(3):528–533. | ||
Greenbaum L, Deschênes G, Levtchenko E, et al. SP026 quantification of dimethyl sulfide associated with cysteamine bitartate-induced halitosis using breath analysis in cystinosis patients treated with delayed-release and immediate-release cysteamine bitartrate. Nephrol Dial Transplant. 2017;32(Suppl 3):iii114. | ||
Dohil R, Cabrera BL, Gangoiti J, Rioux P. The effect of food on cysteamine bitartrate absorption in healthy participants. Clin Pharmacol Drug Dev. 2012;1(4):170–174. | ||
Dohil R, Rioux P. Pharmacokinetic studies of cysteamine bitartrate delayed-release. Clin Pharmacol Drug Dev. 2013;2(2):178–185. | ||
US Food and Drug Administration. Dissolution methods database. Available from: https://www.accessdata.fda.gov/scripts/cder/dissolution/dsp_getallData.cfm. Accessed December 27, 2017. | ||
The United States Pharmacopeial Convention. The United States Pharmacopeia and The National Formulary (USP–NF), General Chapter 711 Dissolution. Available from: https://www.usp.org/sites/default/files/usp/document/harmonization/gen-method/q01_pf_ira_33_4_2007.pdf. Accessed December 27, 2017. |
Supplementary materials
Food preparation
Smoothie
One-third cup of plain or Greek yogurt, one-third cup of strawberries, and half of a medium-sized firm banana were processed in a blender for 30–45 seconds or until smooth. Orange or apple juice could be added, if needed, to achieve desired consistency (volumes recorded).
Sweet potatoes
Sweet potatoes were placed in a microwave-safe bowl or dish and heated in the microwave in 15-second increments at 50% power until the temperature reached 36.5°C–41.5°C. The sweet potatoes were heated in the microwave for 15 seconds at 50% power and stirred to ensure homogeneous heat distribution. The temperature was measured prior to adding cysteamine bitartrate DR beads.
Bead/food mixing
Approximately 113 g (equivalent to 4 ounces) of food or liquid was placed into three separate 1 L media bottles. The weights of eight intact cysteamine bitartrate DR capsules were recorded, the capsules were opened, and the beads were added to the food. The weights of the empty capsule shells were recorded to determine the average capsule fill weight. This step was repeated for each of the three bottles. The beads were carefully mixed with the food or liquid and incubated for three different lengths of time: 30 minutes, 1 hour, or 2 hours. The smoothie was kept cold (2°C–8°C) or at room temperature (20°C–22°C). The sweet potato was heated to prespecified temperatures at 0 minutes, 30 minutes, 1 hour, and 2 hours before the beads were added.
Bead recovery
Once the specified food exposure time was met, 750 mL of diluent (0.1 N HCl) was added to each container and mixed well. A stir bar was added and solutions were allowed to stir at 75 rpm for 1 hour at 37°C ± 0.5°C. The stir bar was then removed, and liquids and any floating solids were then decanted (importantly, the decanted liquids/solids were rinsed with the diluent as many times as necessary to ensure that none of the beads were trapped). Next, ~50 mL of diluent was added to the media bottle to rinse and transfer the beads to a beaker, thus making decanting easier. At least 300 mL of diluent was added to the beaker containing the beads in aliquots of 50 mL, swirling and decanting after each aliquot.
Beads were rinsed with a vacuum filter using a wire screen but no filter membrane. With the vacuum on, diluent was added to the beaker containing the beads, and contents of the beaker were swirled (including the beads) and poured onto the screen. The beads were carefully rinsed on the filter screen with at least 50 mL of the diluent until clean. After rinsing, the beads were transferred to a drying dish. For the dissolution analysis, the weight of the wet bead composite was determined to avoid the development of variation in acid resistance during the drying process; control experiments were conducted to determine the wet weight of beads equivalent to one capsule. The equivalence of one capsule fill weight was transferred to an Apparatus 1 dissolution basket and analyzed as per the current United States Pharmacopeia method at 75 rpm (50 mL of 0.2 M monobasic potassium phosphate into a 200 mL flask and then add 22.4 mL of 0.2 M sodium hydroxide and dilute to volume with water). Dissolution sample from acid-stage dissolution was analyzed at 2 hours. Dissolution sample from buffer-stage dissolution was analyzed at 20 minutes (US and Canada specifications) and 30 minutes (EU specifications). For the assay analysis, the beads were dried for at least 90 minutes at 60°C, before being allowed to cool to room temperature, and weighed with the lid. The beads were transferred to a ball mill cup and ground into a powder. The powder was diluted and analyzed by high-performance liquid chromatography using a Xbridge C18 column with 210 nm UV detection. A comparison of treated samples to the treated control and untreated control was made to ensure that the rinsing process did not affect bead stability.
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.