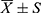Back to Journals » Drug Design, Development and Therapy » Volume 17
The Effect of Different Doses of Ciprofol in Patients with Painless Gastrointestinal Endoscopy
Authors Chen L, Xie Y, Du X , Qin W, Huang L, Dai J, Qin K, Huang J
Received 11 April 2023
Accepted for publication 7 June 2023
Published 12 June 2023 Volume 2023:17 Pages 1733—1740
DOI https://doi.org/10.2147/DDDT.S414166
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Lini Chen,* Yongguo Xie,* Xueke Du, Weiyong Qin, Lifu Huang, Junmin Dai, Ke Qin,* Jianfeng Huang*
Department of Anesthesiology, The Second Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ke Qin; Jianfeng Huang, Department of Anesthesiology, The Second Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi, 530007, People’s Republic of China, Tel/Fax +8613878103578 ; +8613978896039, Email [email protected]; [email protected]
Background: Ciprofol is currently used for painless gastrointestinal endoscopy and anesthesia induction. However, whether it is superior to propofol and its optimal dose remains unknown.
Methods: A total of 149 patients, 63 males and 86 females, aged 18– 80 years, BMI 18– 28 kg/m2, ASA I–III, were divided randomly into four groups: propofol group (group P, n = 44), ciprofol 0.2mg/kg group (group C2, n = 38), ciprofol 0.3mg/kg group (group C3, n = 36) and ciprofol 0.4 mg/kg group (group C4, n = 31). Groups C2, C3 and C4 had injected IV with ciprofol 0.2, 0.3 and 0.4 mg/kg, respectively. Group P had injected IV with propofol 1.5mg/kg. The time for disappearance of the eyelash reflex, gastrointestinal endoscopy time, recovery time, and the Modified Observer’s Assessment of Alertness/Sedation (MOAA/S) score at awakening (T1), 15 minutes after awakening (T2) and 30 minutes after awakening (T3) were recorded.
Results: Compared with group P, the time to fall asleep was significantly shortened, and the incidence of nausea and vomiting and injection pain was significantly lower in groups C2, C3 and C4 (P < 0.05). There was no significant difference in recovery time and recovery quality between each group (P > 0.05). Compared with group P and C4, the incidence of hypotension and respiratory depression was significantly lower in groups C2 and C3 (P < 0.05).
Conclusion: The appropriate dose of ciprofol for painless gastrointestinal endoscopy is more advantageous than propofol in hemodynamics and respiratory stability, with less injection pain and nausea and vomiting, which is worthy of clinical promotion.
Keywords: ciprofol, propofol, painless gastrointestinal endoscopy
Introduction
With the rapid development of medicine and the promotion of comfort medicine, painless gastrointestinal endoscopy has been widely used in clinics. As one of the most commonly used drugs for sedation, propofol has pharmacokinetic properties such as fast onset and rapid elimination. In painless gastrointestinal endoscopies, it is however limited by their high incidence of hypotension, respiratory depression, and injection pain. The latest 2,6-disubstituted phenol derivative, ciprofol, was independently developed in China and is currently used for painless gastrointestinal endoscopy and anesthesia induction.1 Although ciprofol has a similar sedative effect as propofol, it has a clear absorption, distribution, metabolism and excretion processes, a low incidence of hypotension and respiratory depression, and a high level of safety.2 Therefore, the aim of this study was to compare different doses of ciprofol and propofol used in painless gastrointestinal endoscopy in order to determine the appropriate dose of ciprofol, improve the sedation mode of painless gastrointestinal endoscopy, reduce the incidence of related complications, and provide reference for painless gastrointestinal endoscopy.
Patients and Methods
Ethical Approval
This was a prospective, single-blinded, randomized controlled clinical trial. It was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Second Affiliated Hospital of Guangxi medical University (KY-2022-0502). It was registered at the Chinese Clinical Trials Registry (ChiCTR2200063410). Signed, informed consent to participate in the study was obtained from the patients and their families before the operation.
Design and Patients
The study was a prospective, randomized, single-blind study conducted on 160 patients who underwent elective painless gastrointestinal endoscopy, and was aged 18–80 years, with a body mass index (BMI) of 18–28 kg/m2 and an American Society of Anesthesiology physical status (ASA PS) of I–III. Patients were excluded according to the following criteria: patients with mental and nervous system diseases, heart failure, respiratory failure, long-term use of sedatives or antidepressants, pregnant or lactating women, and unable to communicate or cooperate. Patients were withdrawn from the study according to the following criteria: endoscopic operation fails, gastroscopy combined with colonoscopy examination, change to tracheal intubation or laryngeal mask anesthesia. A total of 149 patients were selected and included based on the above criteria, they were divided randomly into four groups: propofol group (group P, n = 44), ciprofol 0.2mg/kg group (group C2, n = 38), ciprofol 0.3mg/kg group (group C3, n = 36) and ciprofol 0.4 mg/kg group (group C4, n = 31).
Interventions
All patients were routinely taken 10 mL of dyclonine hydrochloride mucilage before entering the operating room. After entering the room, patients were placed in the left lateral position and a nasal catheter was used for oxygen inhalation at 2 L/min, an electrocardiogram (ECG) was performed, non-invasive blood pressure (NIBP), peripheral oxygen saturation (SpO2), and heart rate (HR) were monitored routinely. The peripheral vein of an upper limb was opened and all groups received an IV injection of fentanyl at a dose of 2 μg/kg. Group C2, group C3 and group C4 were received an IV injection of ciprofol (batch number: H20200013, Liaoning Haisike Pharmaceutical Co., Ltd.) at a dose of 0.2, 0.3 and 0.4mg/kg, and administration time over 30s, while group P received an IV injection of propofol (batch number: JX20160026, Fissenius Carbi Pharmaceutical Co. Ltd., Beijing) at a dose of 1.5 mg/kg. Endoscopy was performed when patients disappeared the eyelash reflex. Additional 0.1 mg/kg of ciprofol or 0.5 mg/kg of propofol was added according to the actual situation of the patient during gastrointestinal endoscopy (body movement, eye opening, elevated blood pressure, increased heart rate, etc.). All examinations were performed by experienced anesthesiologist and endoscopists. During the operation, the jaw was lifted for opening the airway when the SpO2 was lower than 95%, if the SpO2 was lower than 90%, the patients inhaled pure oxygen using a mask immediately following endoscope withdrawal, and balloon-assisted ventilation was given if necessary. If the heart rate (HR) was <50 beats/min, intravenous atropine 0.5 mg was administered. If the systolic blood pressure (SBP) decreased more than 30% as compared with the baseline value, intravenous ephedrine 5 mg was administered. Patients were transferred to the post-anesthesia care unit (PACU) for at least 30 min at the end of the endoscopy. Patients will leave the PACU with their families when Aldrete score >9.
Outcome Measures
HR, SBP, DBP and SpO2 were recorded after entering the endoscopy room and during endoscopic operation. The time for disappearance of the eyelash reflex, gastrointestinal endoscopy time, recovery time, and the Modified Observer’s Assessment of Alertness/Sedation (MOAA/S) score at awakening (T1), 15 minutes after awakening (T2) and 30 minutes after awakening (T3) were recorded. Adverse reactions such as hypotension, respiratory depression, injection pain, nausea and vomiting were recorded.
Statistical Analysis
Data were analyzed using R (R version R 4.21), Measurement data were expressed as mean ± standard deviation, and One‐way ANOVA was employed for the comparison of multiple groups. If a significant difference was detected among the groups, then pairwise comparisons were made by using Bonferroni test. Categorical data was described by rate or ratio and chi-square test or Fisher’s exact probability method was used to compare the differences between groups. The Bonferroni test was performed for pair-wise comparisons between groups. All hypothesis tests will be two-sided and P < 0.05 considered statistically significant.
 |
Figure 1 Flow diagram of the study. |
Results
A total of 160 patients were screened, and 149 were randomized into four groups (Figure 1). As shown in Table 1, there was no significant difference in general data of patients among the four groups (P > 0.05).
 |
Table 1 Comparison of General Conditions in Patients Among the Four Groups ( |
As shown in Table 2, compared with group P, the time for disappearance of eyelash reflex was obviously shortened in group C2, C3 and C4 (P < 0.001). There was no significant difference in time of awakening among the four groups (P > 0.05).
 |
Table 2 Comparison of Sedative Effects in Patients Among the Four Groups ( |
As shown in Table 3, compared with group P, the incidence of hypotension and bradycardia were significantly shortened in group C2 and C3 (P < 0.05). Compared with group C4, the incidence of hypotension was significantly shortened in group C2 and C3 (P < 0.05).
 |
Table 3 SBP, DBP, HR and SPO2 at Different Time in Patients Among Four Groups ( |
As shown in Table 4, compared with group P, the incidence of nausea and vomiting and injection pain were significantly shortened in group C2, C3 and C4 (P < 0.05), and respiratory depression was significantly decreased in group C2 and C3 (P < 0.05).
 |
Table 4 Incidence of Adverse Reactions in Patients Among Four Groups (n, %) |
As shown in Figure 2, there was no significant difference in recovery quality in patients among four groups at T1, T2, T3 (P > 0.05).
Discussion
Pathological diagnosis through gastrointestinal endoscopy is the gold standard for the diagnosis of malignant tumors of the digestive tract and the most direct means for the diagnosis of upper gastrointestinal diseases.3 Painless gastrointestinal endoscopy can not only significantly reduce the incidence of adverse reactions during the examination but also alleviate the psychological and physiological discomfort caused by the operation.4 At present, propofol or etomidate is mostly used as intravenous agents for painless gastrointestinal endoscopy in China.5 For ASA I–III outpatients, the use of traditional drugs can achieve good sedative effect, but there were still some patients developed respiratory and circulatory depression, obvious injection pain and postoperative nausea and vomiting and other adverse reactions.6 Studies have shown that propofol intravenous anesthesia for painless gastrointestinal endoscopy can cause hemodynamic fluctuations.7 As it can cause hemodynamic inhibition and peripheral vascular dilation, resulting in hypotension, injection pain, and the propofol infusion syndrome.8–10 Injection pain affects the comfort experience of patients with anesthesia induction. Etomidate is relatively stable during anesthesia, but it is prone to muscle fibrillation and postoperative nausea and vomiting, which affects the process of gastrointestinal endoscopy and the patients’ comfortable experience of anesthesia.
This study indicates that in terms of sleep time, compared with propofol, the three doses of ciprofol were significantly shortened, while the wake up time and recovery quality had no obvious advantage. This is consistent with previous research. Ciprofol is a new drug for intravenous anesthesia and sedation. It binds to the gamma-aminobutyric acid-A (GABAA) receptor l.11 By enhancing the GABA receptor mediated Clˉ influx, GABA ergic neurons are activated and the nerve cell membrane supersized, resulting in central nervous system inhibition to achieve sedative or anesthetic effects.12 Phase I trials have shown that a single intravenous injection of ciprofol in healthy volunteers over a dose range of 0.15–0.90 mg/kg was well tolerated and showed non-linear pharmacokinetic characteristics in the dose range of 0.40–0.90 mg/kg.13,14 The results of the Phase II clinical trial showed that the recommended initial maintenance dose of ciprofol was 0.8 mg·kg−1·h−1.15 According to the completed clinical trials of sedation or anesthesia and general anesthesia induction of gastrointestinal endoscopy, the sedative effect of ciprofol was accurate, with lower doses producing a satisfactory general anesthetic effect.16 The results of phase II and Phase III clinical trials showed that ciprofol had a rapid onset, rapid recovery, a high titer, and less injection pain, characteristics that are suitable mainly for sedation in gastrointestinal endoscopy and induction of general anesthesia in adult patients.16,17
The results of this study showed that compared with propofol, ciprofol can better stabilize respiratory and hemodynamic parameters. Both doses of 0.2 mg/kg and 0.3 mg/kg can significantly reduce the incidence of respiratory depression and hypotension, which was similar to the results of Bian et al.2 However, the incidence of respiratory depression and hypotension in the 0.4 mg/kg dose group was higher than that in the other two dose groups, which may be related to the higher dose of ciprofol. The patients in the four groups were satisfied with the depth of anesthesia and sedation, the diagnosis and treatment of gastrointestinal endoscopy were successful, and there was no difference in the satisfaction of endoscopic doctors, which was similar to the results of Luo et al.18 It may be due to the similar molecular structure and pharmacokinetics of ciprofol and propofol.19
In recent years, the anti-nausea and vomiting effect of propofol has been demonstrated.20 This study showed that the incidence of nausea and vomiting in the ciprofol group was significantly lower than that in the propofol group, which was similar to the effect of propofol. The occurrence of nausea and vomiting is considered to be related to the combined use of opioids. Previous studies have shown that a high concentration of propofol in the aqueous phase of emulsion will cause injection pain.21 In this study, the incidence of injection pain of ciprofol was significantly lower than that of propofol. The mechanism may be that ciprofol is insoluble in water and is prepared into oil-in-water emulsion. In addition, higher hydrophobicity and lower plasma concentration of ciprofol compared to propofol may lead to reduced injection pain.22,23 The reduction of injection pain is conducive to alleviating patients’ emotional tension and fear, reducing hemodynamic fluctuations, and improving the comfort experience of patients undergoing gastrointestinal endoscopy.
Limitations
The study had some limitations, including the fact that no comparison of blood concentration monitoring of ciprofol and propofol was conducted, and individualized medication was not achieved in combination with BIS monitoring. In addition, there are few articles on the study of ciprofol at present, which cannot provide reference for the experimental sample size, and all the experimental sample size cannot be accurately calculated. Finally, this study is a small sample, single center clinical study that needs further confirmation from large sample, multicenter clinical studies.
Conclusion
In summary, the appropriate dose of ciprofol for painless gastrointestinal endoscopy is more advantageous than propofol in maintaining the stability of respiratory and hemodynamic parameters, and reducing the incidence of adverse reactions such as nausea, vomiting and injection pain. In this study, 0.2 mg/kg and 0.3 mg/kg of ciprofol can better improve the patients’ comfortable medical experience. With the increase in propofol dose, the respiratory and circulatory inhibition increases.
Data Sharing Statement
The datasets generated from the current study are available from the corresponding author, Jianfeng Huang, upon reasonable request.
Ethics Statement
This trial was approved by the Ethics Committee of the Second Affiliated Hospital of Guangxi medical University (KY-2022-0502) before beginning the study. The trial was registered in the Chinese Clinical Trial Registry (https://www.chictr.org.cn, Lini Chen) on September 6, 2022 (registration no. ChiCTR2200063410), before patient recruitment. Written informed consent was obtained from each patient before enrollment. The research protocol complied with the Consolidated Standards of Reporting Trials (CONSORT) statement and the Helsinki Declaration.
Acknowledgments
The authors would like to express their gratitude to EditSprings (www.editsprings.cn) for the expert linguistic services provided.
Author Contributions
Lini Chen and Yongguo Xie contributed equally to this work and are joint first authors. Ke Qin and Jianfeng Huang contributed equally to this work and are joint corresponding authors. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The study was supported by the Guangxi Medical and health key discipline construction project. This study was also supported by the Research Guangxi Zhuang Autonomous Region funded by the Chinese Medicine Board for the “Effects of isoliquiritigenin on ischemia-reperfusion injury of lower limbs in rats” (grant number: GZZC2020186). The funder was not involved in the study design, collection, analysis or interpretation of the data, the writing of this article, or the decision to submit it for publication.
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Qin L, Ren L, Wan S, et al. Design, synthesis, and evaluation of novel 2,6-disubstituted phenol derivatives as general anesthetics. J Med Chem. 2017;60(9):3606–3617. doi:10.1021/acs.jmedchem.7b00254
2. Bian Y, Zhang H, Ma S, et al. Mass balance, pharmacokinetics and pharmacodynamics of intravenous HSK3486, a novel anaesthetic, administered to healthy subjects. Br J Clin Pharmacol. 2021;87(1):93–105. doi:10.1111/bcp.14363
3. Wang F, Zhou Q, Shen M, et al. Efficacy and safety of remimazolam in procedural sedation and analgesia: a protocol for systematic review and meta analysis. Medicine. 2020;99(27):e20765. doi:10.1097/md.0000000000020765
4. Xu C, He L, Ren J, et al. Efficacy and safety of remimazolam besylate combined with alfentanil in painless gastroscopy: a randomized, single-blind, parallel controlled study. Contrast Media Mol Imaging. 2022;2022:7102293. doi:10.1155/2022/7102293
5. Liu G, Xiong Y. Analysis of stress response and analgesic effect of remazolam combined with etomidate in painless gastroenteroscopy. Contrast Media Mol Imaging. 2022;2022:4863682. doi:10.1155/2022/4863682
6. Zhan Y, Liang S, Yang Z, et al. Efficacy and safety of subanesthetic doses of esketamine combined with propofol in painless gastrointestinal endoscopy: a prospective, double-blind, randomized controlled trial. BMC Gastroenterol. 2022;22(1):391. doi:10.1186/s12876-022-02467-8
7. Xing D, Li Q, Lin G, et al. The protective effects of propofol against renal ischemia-reperfusion injury are potentiated by norisoboldine treatment via inhibition of oxidative stress pathways. J Biochem Mol Toxicol. 2022;36(1):e22937. doi:10.1002/jbt.22937
8. Sneyd JR, Absalom AR, Barends CRM, et al. Hypotension during propofol sedation for colonoscopy: a retrospective exploratory analysis and meta-analysis. Br J Anaesth. 2022;128(4):610–622. doi:10.1016/j.bja.2021.10.044
9. Lee JS, Kim ES, Cho KB, et al. Pain intensity at injection site during esophagogastroduodenoscopy using long- and medium-chain versus long-chain triglyceride propofol: a randomized controlled double-blind study. Gut Liver. 2021;15(4):562–568. doi:10.5009/gnl20243
10. Dehesa-López E, Irizar-Santana SS, Claure-Del Granado R, et al. Propofol infusion syndrome in the postoperative period of a kidney transplant. Case Rep Nephrol. 2019;2019:7498373. doi:10.1155/2019/7498373
11. Lu M, Liu J, Wu X, et al. Ciprofol: a novel alternative to propofol in clinical intravenous anesthesia? Biomed Res Int. 2023;2023:7443226. doi:10.1155/2023/7443226
12. Kim JJ, Gharpure A, Teng J, et al. Shared structural mechanisms of general anaesthetics and benzodiazepines. Nature. 2020;585(7824):303–308. doi:10.1038/s41586-020-2654-5
13. Hu C, Ou X, Teng Y, et al. Sedation effects produced by a ciprofol initial infusion or bolus dose followed by continuous maintenance infusion in healthy subjects: a phase 1 trial. Adv Ther. 2021;38(11):5484–5500. doi:10.1007/s12325-021-01914-4
14. Teng Y, Ou M, Wang X, et al. Efficacy and safety of ciprofol for the sedation/anesthesia in patients undergoing colonoscopy: phase IIa and IIb multi-center clinical trials. Eur J Pharm Sci. 2021;164:105904. doi:10.1016/j.ejps.2021.105904
15. Zeng Y, Wang DX, Lin ZM, et al. Efficacy and safety of HSK3486 for the induction and maintenance of general anesthesia in elective surgical patients: a multicenter, randomized, open-label, propofol-controlled Phase 2 clinical trial. Eur Rev Med Pharmacol Sci. 2022;26(4):1114–1124. doi:10.26355/eurrev_202202_28101
16. Wang X, Wang X, Liu J, et al. Effects of ciprofol for the induction of general anesthesia in patients scheduled for elective surgery compared to propofol: a Phase 3, multicenter, randomized, double-blind, comparative study. Eur Rev Med Pharmacol Sci. 2022;26(5):1607–1617. doi:10.26355/eurrev_202203_28228
17. Li J, Wang X, Liu J, et al. Comparison of ciprofol (HSK3486) versus propofol for the induction of deep sedation during gastroscopy and colonoscopy procedures: a multi-centre, non-inferiority, randomized, controlled phase 3 clinical trial. Basic Clin Pharmacol Toxicol. 2022;131(2):138–148. doi:10.1111/bcpt.13761
18. Luo Z, Tu H, Zhang X, et al. Efficacy and safety of HSK3486 for anesthesia/sedation in patients undergoing fiberoptic bronchoscopy: a multicenter, double-blind, propofol-controlled, randomized, phase 3 study. CNS Drugs. 2022;36(3):301–313. doi:10.1007/s40263-021-00890-1
19. Liao J, Li M, Huang C, et al. Pharmacodynamics and pharmacokinetics of HSK3486, a novel 2,6-disubstituted phenol derivative as a general anesthetic. Front Pharmacol. 2022;13:830791. doi:10.3389/fphar.2022.830791
20. Gan TJ, Diemunsch P, Habib AS, et al. Consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2014;118(1):85–113. doi:10.1213/ane.0000000000000002
21. Desousa KA. Pain on propofol injection: causes and remedies. Indian J Pharmacol. 2016;48(6):617–623. doi:10.4103/0253-7613.194845
22. Chen X, Guo P, Yang L, et al. Comparison and clinical value of ciprofol and propofol in intraoperative adverse reactions, operation, resuscitation, and satisfaction of patients under painless gastroenteroscopy anesthesia. Contrast Media Mol Imaging. 2022;2022:9541060. doi:10.1155/2022/9541060
23. Nair A, Seelam S. Ciprofol- a game changing intravenous anesthetic or another experimental drug! Saudi J Anaesth. 2022;16(2):258–259. doi:10.4103/sja.sja_898_21
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.