Back to Journals » Drug Design, Development and Therapy » Volume 17
The Effect of Dezocine on the Median Effective Dose of Sufentanil-Induced Respiratory Depression in Patients Undergoing Spinal Anesthesia Combined with Low-Dose Dexmedetomidine
Authors Gui YK, Zeng XH, Xiao R , Xi WF, Zhang D, Liu Y, Zhu SH , Da X , Shi DW, Hu XD, Xu GH
Received 1 August 2023
Accepted for publication 19 November 2023
Published 7 December 2023 Volume 2023:17 Pages 3687—3696
DOI https://doi.org/10.2147/DDDT.S429752
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Georgios Panos
Yong-Kang Gui,1,2,* Xiao-Hui Zeng,1,2,* Rui Xiao,3 Wen-Feng Xi,1– 3 Dan Zhang,1– 3 Yang Liu,1,2 Si-Hui Zhu,1,2 Xin Da,1,2 De-Wen Shi,1,2 Xu-Dong Hu,1,2 Guang-Hong Xu1,2
1Department of Anesthesiology, the First Affiliated Hospital of Anhui Medical University, Hefei, Anhui, 230022, People’s Republic of China; 2Key Laboratory of Anesthesiology and Perioperative Medicine of Anhui Higher Education Institutes, Anhui Medical University, Hefei, Anhui, 230022, People’s Republic of China; 3Department of Anesthesiology, Fuyang Hospital of Anhui Medical University, Fuyang, Anhui, 236113, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Guang-Hong Xu, 230032, Tel +86 13856949535, Fax +86 0551 62923704, Email [email protected]
Purpose: The application of sedation and analgesia in spinal anesthesia has many benefits, but the risk of respiratory depression (RD) caused by opioids cannot be ignored. We aimed to observe the effect of dezocine, a partial agonist of μ-receptor, on the median effective dose (ED50) of sufentanil-induced RD in patients undergoing spinal anesthesia combined with low-dose dexmedetomidine.
Patients and Methods: Sixty-two patients were randomly assigned to dezocine group (DS) and control group (MS). After spinal anesthesia, mask oxygen (5 L/min) and dexmedetomidine (0.1 ug/kg) were given. Five minutes later, patients in the DS group received an Intravenous (IV) bolus of sufentanil and 0.05mg/kg dezocine, while patients in the MS group only received an IV bolus of sufentanil.
Results: ED50 of DS group was 0.342 ug/kg, 95% confidence interval (CI) was (0.269, 0.623) ug/kg, and the ED50 of MS group was 0.291 ug/kg, 95% CI was (0.257, 0.346) ug/kg. There was no difference in the type and treatment measures of RD and hemodynamic changes between the two groups, and no serious adverse reactions occurred in either group.
Conclusion: Dezocine can improve RD induced by sufentanil in patients with spinal anesthesia combined with low-dose dexmedetomidine, and increase the safety window of sufentanil use.
Keywords: spinal anesthesia, respiratory depression, median effective dose, sufentanil, dezocine
Introduction
Spinal anesthesia (SA) is a conventional technique in which a local anesthetic is administered in the cerebrospinal fluid (CSF) within the lumbar spine. This procedure effectively anesthetizes the nerves exiting the spinal cord, resulting in pain relief and subsequent muscle relaxation within the corresponding spinal innervation region. This technique is widely used in obstetrics and gynecology,1–7 pediatrics,8,9 orthopedics,10,11 and urology as well as in various surgical procedures.12,13 However, as individuals undergoing SA remain conscious throughout the procedure, this can often cause intraoperative anxiety. To address this issue, additional measures involving the use of analgesics and sedatives are warranted.
Sufentanil, a frequently used fentanyl drug in clinical settings, mainly exerts its effects on μ-receptors.14 Nevertheless, opioids exert an inherent respiratory depression (RD) effect,15 and when used improperly, they not induce adequate analgesia and sedation but lead to severe adverse reactions.16 In China, dezocine is currently the most widely used opioid. It is often administered in conjunction with other opioids to enhance its efficacy or mitigate the occurrence of adverse reactions.17 Dexmedetomidine, which is a novel selective α-2 adrenergic receptor agonist,18 can extend the duration of sensory block and delay the time of first remedial analgesia after SA. Therefore, it is frequently used as an adjunctive agent in the field of SA.19,20
The 50% effective dose (ED50) of sufentanil to induce RD when administered in a combination with dezocine and dexmedetomidine during SA remains unclear. Previous research has suggested that partial agonists of μ-receptors can mitigate opioid-induced RD.21,22 Hence, we postulated that dezocine, a partial agonist of μ-receptors, can mitigate sufentanil-induced RD and enhance the ED50 of sufentanil to induce RD when combined with a small dose of dexmedetomidine in patients undergoing SA.
Methods
This study was approved by the Ethics Committee of Fuyang Hospital of Anhui Medical University (KY202206, 2022/07/20), and was registered in the Chinese Clinical Trial Registry before enrollment (registration number: ChiCTR2200063386, 2022/09/05). This study was in full compliance with the Declaration of Helsinki.
Study Participants
The trial was conducted at Fuyang Hospital of Anhui Medical University from October 2022 to March 2023. Sixty patients, aged 18–60 years, American Society of Anesthesiologists (ASA) physical status I–II, body mass index (BMI) 18.5–27 kg/m2, scheduled for SA were enrolled in this study. Patients with long-term use of psychotropic drugs, a history of alcohol abuse, heart, lung, liver, kidney, neurological dysfunction, and severe drug allergy were not included. Patients with unsatisfactory spinal anesthesia level, severe adverse reactions, and incomplete records of the Case Report Form (CRF) were excluded.
Written informed consent was obtained from all patients participating in this experiment.
Randomization and Blinding
Patients were randomly assigned to the dezocine (DS) and control (MS) groups using the random number table method. The numbers were placed inside opaque envelopes, which were opened by the anesthesiologist once the patient entered the operating room and the drugs was prepared according to the numbers in the envelope.
To ensure unbiased evaluation, an investigator who was blinded to the group assignments performed respiratory monitoring for 10 min. This approach helped minimize potential observer bias.
Furthermore, all SA procedures in this study were performed by the same experienced anesthesiologist to ensure uniformity and reduce the impact of interoperator variability on the results.
Anesthesia Procedure
Blood pressure (BP), electrocardiogram, pulse oxygen saturation (SpO2), and end-tidal carbon dioxide partial pressure (PETCO2) were monitored on admission. Subarachnoid puncture was performed at the lumbar 2/3 interspace (L2/3) in the left lateral position. CSF reflux indicated a successful puncture. Next, 2 ml of 0.5% ropivacaine was injected within 10s and the sensory level was controlled in the supine position to the plane of the tenth thoracic vertebra (T10). After the plane was fixed, mask oxygen inhalation was administered at 5 L/min. The DS group was administered dexmedetomidine (0.1 µg/kg) for 5 min, followed by dezocine (0.05 mg/kg) and sufentanil. Conversely, the MS group received dexmedetomidine (0.1 µg/kg) for 5 min, followed by sufentanil. The dose of sufentanil was determined using the Dixon up–down method and the occurrence of RD. Based on the preliminary experiment, the first dose of sufentanil was selected (0.3 and 0.245 µg/kg in the DS and MS groups, respectively). The adjacent dose ratio was 1.1. If the patient exhibited RD within a span of 10 min after sufentanil infusion, the dose for the next patient was reduced by one level; otherwise, it was increased by one level. The experiment was terminated when there were seven crossovers of positive to negative alteration.
Criteria for Respiratory Depression
RD was diagnosed if one or more of the following conditions were observed: respiratory rate (RR) ≤ 8 times/min, apnea ≥ 15s, SpO2 ≤ 90%, PETCO2 ≥ 55 mmHg, arterial PaCO2 (partial pressure of carbon dioxide) ≥ 55 mmHg, and complaints of dyspnea.23
Management Measures After Respiratory Depression and Significant Hemodynamic Changes
When a patient experienced RD, the following treatments were gradually provided in the mentioned order until the resolution of RD or hypoxia symptoms: 1) increased oxygen flow (5 L/min), 2) language and physical stimulation, 3) lifting of the jaw, 4) mask-assisted ventilation, and 5) artificial airway implantation (laryngeal mask/tracheal intubation).
When the patient’s heart rate (HR) was <50 beats per minute and blood pressure (BP) was >30% of the baseline value, intravenous atropine and ephedrine were administered, respectively.
Observing Indicators
The primary outcome of this study was the ED50 of sufentanil in the two groups at the end of the study. The secondary outcomes were the dose of ropivacaine, level of anesthesia, Ramsay score, vital signs (such as BP, HR, RR, SpO2, and PETCO2), and incidence of adverse reactions (SA-related complications, HR and BP drop, dizziness, headache, nausea, vomiting, etc.) recorded during the trial.
Statistical Analysis
Based on the methods used by Wu and Oh TK et al,24,25 and a previous study involving 13 subjects, we determined that the mean dose of sufentanil required to induce RD in patients undergoing SA was 0.291 µg/kg (SD= 0.0495), when not combined with dezocine. We believe that a 10% sufentanil dose difference between the two groups indicates that the use of dezocine is meaningful for the antagonistic potency of sufentanil-induced RD. Assume a type I error of 0.05 (Alpha= 0.05) and a power of 0.9 (1-Beta= 0.9). Considering a dropout rate of 20%, we aimed to enroll 31 patients in each group.
Statistical analysis of the data was performed using the Statistical Package for the Social Sciences (SPSS Inc., Chicago, IL, USA) V26.0. The normality of distribution was assessed using the Shapiro–Wilk test. Patient’s characteristics were compared between the two groups using the independent-sample t-test for continuous variables with normal distribution and the Mann–Whitney U-test for those with nonnormal distribution. For categorical variables, Pearson’s χ2 test was used as appropriate.
Hemodynamic indices were tested via repeated measures analysis of variance. Normally distributed and skewed data were expressed as mean ± standard deviation and median (interquartile range), respectively. Count data were tested using the chi-squared test. The significance level for all statistical tests was set at P ≤ 0.05.
Results
A total of 62 eligible patients who provided written informed consent were evaluated. They were sequentially selected until seven crossovers were attained. One patient in the MS group and another in the DS group were excluded because of unsatisfactory block plane and incomplete experimental data recording, respectively. Thus, 51 patients (DS group, 24; MS group, 27) were included in the final analysis. (Figures 1-3). All SA procedures in this study were successfully performed by one same experienced anesthesiologist.
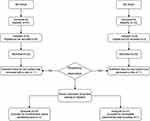 |
Figure 1 Flow diagram for the Dixon up-and-down method. Abbreviations: DS, dezocine group; MS, control group; positive, presence of respiratory depression; negative, absence of respiratory depression. |
 |
Figure 2 Response in DS Group. Abbreviations: DS, dezocine group; positive, presence of respiratory depression; negative, absence of respiratory depression. |
 |
Figure 3 Response in MS Group. Abbreviations: MS, control group; positive, presence of respiratory depression; negative, absence of respiratory depression. |
As shown in Table 1, the demographic characteristics of the two groups of patients were similar. The gender composition and the level of spinal anesthesia were similar between the two groups. Ramsay scores were similar in both groups. No significant difference was observed in the doses of dexmedetomidine and ropivacaine in the two groups.
 |
Table 1 Drugs and Social Demographic Variable of Participants |
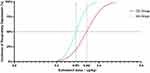
Using the Dixon up–down method,26,27 the ED50 values in the DS and MS groups were found to be 0.342 µg/kg (95% confidence interval [CI], 0.269–0.623) and 0.291 µg/kg (95% CI, 0.257–0.346), respectively. (Figure 4).
No significant difference was observed in the occurrence and treatment of RD, hemodynamic changes, and incidence of adverse reactions between the two groups the during the experimental observation period. (Tables 1–4).
 |
Table 2 The Types of Respiratory Depression |
 |
Table 3 The Management Measures of Respiratory Depression |
 |
Table 4 Hemodynamic Measures |
Discussion
SA is widely used in various surgeries. Patients undergoing SA remain conscious throughout the operation, which results in certain drawbacks such as needle-related apprehension and recollection of the surgical process.28,29 These are important sources of fear and anxiety for the patients,30 further highlighting the importance of sedation. An appropriate sedation can provide analgesia and amnesia as well as relieve anxiety and fear. Sedation can improve patient’s satisfaction with regional anesthesia and increase patient acceptance of the associated techniques.31 Consequently, the use of appropriate sedation and analgesia techniques can significantly improve the comfort of patients when undergoing SA, effectively alleviating their anxiety and fear.
Dezocine is the most extensively used opioid in China, accounting for 44% of the national opioid analgesic market.32 It has weaker RD effects than commonly used μ-receptor agonists, such as fentanyl. Furthermore, it exerts a “capping effect”.17,33–35 The receptor profile of dezocine differs from that of known pure μ-agonists; therefore, it is allowed to be used in a combination with other opioids to improve efficacy or reduce the occurrence of adverse effects.17 For example, dezocine can reduce the incidence of postoperative delirium when used during the induction of anesthesia36 and exert therapeutic effects on depression.37,38
Buprenorphine, a partial agonist of opioid receptors,39 can reverse opioid-induced RD in humans and animals.40,41 Because dezocine is also a partial agonist of μ-receptors, we used it in combination with sufentanil and concluded that dezocine could partially antagonize sufentanil-induced RD in SA. This effect may be attributed to the role of dezocine as a partial agonist of μ-receptors, where it may block or displace the binding of pure opioid receptor agonist to the μ-receptor when combined with dezocine. This mechanism is similar to that observed in a previous study and provides a plausible explanation for the observed increase in the ED50 in the DS group.17
The dose–response curve of a drug is usually S-shaped. The median ED50 is located at the midpoint of the curve, which indicates the dose that produces a positive response for 50% of individuals. Thus, it is often used as an index to reflect the potency of drug action.42
The difference in ED50 and amount of sufentanil used between the groups in this trial also confirmed that dezocine can decrease the ED50 of sufentanil-induced RD in patients undergoing SA. The implications of this finding within the field of SA are profound, as it demonstrates the potential of the combined use of sufentanil and dezocine to simultaneously minimize the incidence of RD while providing adequate sedation and analgesia. This undoubtedly expands the safety threshold of sufentanil administration during SA.
All patients with RD exhibited decreased RR, and similar observation was reported by Smart et al,43 and suggests that respiratory cycle monitoring can be used as a sensitive indicator of sufentanil-induced RD. suggesting that respiratory cycle monitoring can be used as a sensitive indicator of sufentanil-induced RD. When RD occurs, increased inspired oxygen concentration and speech stimulation could effectively improve the ventilation of patients. Similar to previous studies,44,45 only one patient in this study required jaw lifting to maintain satisfactory ventilation after the above treatments, but none required artificial airway implantation.
This study has several limitations. First, the results only apply to patients undergoing SA and are not a reference for the ED50 of sufentanil to induce RD under the same drug combination in patients in whom other anesthesia techniques are used. Second, this study was majorly conducted in the Han and Yellow race, and further experiments may be warranted to explore the ED50 of sufentanil under similar clinical conditions in other ethnic groups and races.
Conclusion
In conclusion, the use of dezocine can increase the ED50 of sufentanil to induce RD in patients undergoing SA combined with low-dose dexmedetomidine. This may be related to the competitive inhibition of μ-receptors by dezocine.
Data Sharing Statement
Under reasonable requirements, the data and material of this study can be obtained from the corresponding author. The data are not publicly available due to privacy restrictions.
Acknowledgments
The research team greatly appreciates the funding support, and the research participants for their cooperation and support.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81870837).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Abdallah FW, Halpern SH, Margarido CB. Transversus abdominis plane block for postoperative analgesia after caesarean delivery performed under spinal anesthesia? A systematic review and meta-analysis. Br J Anaesth. 2012;109(5):679–687. doi:10.1093/bja/aes279
2. Carney J, Finnerty O, Rauf J, Bergin D, Laffey JG, Mc Donnell JG. Studies on the spread of local anaesthetic solution in transversus abdominis plane blocks. Anaesthesia. 2011;66(11):1023–1030. doi:10.1111/j.1365-2044.2011.06855.x
3. Eslamian L, Jalili Z, Jamal A, Marsoosi V, Movafegh A. Transversus abdominis plane block reduces postoperative pain intensity and analgesic consumption in elective cesarean delivery under general anesthesia. J Anesth. 2012;26(3):334–338. doi:10.1007/s00540-012-1336-3
4. Kanazi GE, Aouad MT, Abdallah FW, et al. The analgesic efficacy of subarachnoid morphine in comparison with ultrasound-guided transversus abdominis plane block after cesarean delivery: a randomized controlled trial. Anesth Analg. 2010;111(2):475–481. doi:10.1213/ANE.0b013e3181e30b9f
5. Loane H, Preston R, Douglas MJ, Massey S, Papsdorf M, Tyler J. A randomized controlled trial comparing intrathecal morphine with transversus abdominis plane block for post-cesarean delivery analgesia. Int J Obstet Anesth. 2012;21(2):112–118. doi:10.1016/j.ijoa.2012.02.005
6. McDermott G, Korba E, Mata U, et al. Should we stop doing blind transversus abdominis plane blocks? Br J Anaesth. 2012;108(3):499–502. doi:10.1093/bja/aer422
7. Tan TT, Teoh WH, Woo DC, Ocampo CE, Shah MK, Sia AT. A randomised trial of the analgesic efficacy of ultrasound-guided transversus abdominis plane block after caesarean delivery under general anaesthesia. Eur J Anaesthesiol. 2012;29(2):88–94. doi:10.1097/EJA.0b013e32834f015f
8. Kachko L, Birk E, Simhi E, Tzeitlin E, Freud E, Katz J. Spinal anesthesia for noncardiac surgery in infants with congenital heart diseases. Paediatr Anaesth. 2012;22(7):647–653. doi:10.1111/j.1460-9592.2011.03769.x
9. Trifa M, Tumin D, Whitaker EE, Bhalla T, Jayanthi VR, Tobias JD. Spinal anesthesia for surgery longer than 60 min in infants: experience from the first 2 years of a spinal anesthesia program. J Anesth. 2018;32(4):637–640. doi:10.1007/s00540-018-2517-5
10. Brueckner S, Reinke U, Roth-Isigkeit A, Eleftheriadis S, Schmucker P, Siemens HJ. Comparison of general and spinal anesthesia and their influence on hemostatic markers in patients undergoing total hip arthroplasty. J Clin Anesth. 2003;15(6):433–440. doi:10.1016/s0952-8180(03)00082-5
11. Maurer SG, Chen AL, Hiebert R, Pereira GC, Di Cesare PE. Comparison of outcomes of using spinal versus general anesthesia in total hip arthroplasty. Am J Orthop. 2007;36(7):E101–6.
12. Alavi CE, Asgari SA, Falahatkar S, et al. Effectiveness of spinal anesthesia combined with obturator nerve blockade in preventing adductor muscle contraction during transurethral resection of bladder tumor. Turk J Urol. 2017;43(4):507–511. doi:10.5152/tud.2017.96992
13. Lie SA, Wong SW, Wong LT, Wong TGL, Chong SY. Practical considerations for performing regional anesthesia: lessons learned from the COVID-19 pandemic. Can J Anaesth. 2020;67(7):885–892. doi:10.1007/s12630-020-01637-0
14. Maciejewski D. Sufentanil in anaesthesiology and intensive therapy. Anaesthesiol Intensive Ther. 2012;44(1):35–41.
15. Dahan A, Aarts L, Smith TW. Incidence, reversal, and prevention of opioid-induced respiratory depression. Anesthesiology. 2010;112(1):226–238. doi:10.1097/ALN.0b013e3181c38c25
16. Boom M, Niesters M, Sarton E, Aarts L, Smith TW, Dahan A. Non-analgesic effects of opioids: opioid-induced respiratory depression. Curr Pharm Des. 2012;18(37):5994–6004. doi:10.2174/138161212803582469
17. Ye RR, Jiang S, Xu X, Lu Y, Wang YJ, Liu JG. Dezocine as a potent analgesic: overview of its pharmacological characterization. Acta Pharmacol Sin. 2022;43(7):1646–1657. doi:10.1038/s41401-021-00790-6
18. Keating GM. Dexmedetomidine: a review of its use for sedation in the intensive care setting. Drugs. 2015;75(10):1119–1130. doi:10.1007/s40265-015-0419-5
19. Abdallah FW, Abrishami A, Brull R. The facilitatory effects of intravenous dexmedetomidine on the duration of spinal anesthesia: a systematic review and meta-analysis. Anesth Analg. 2013;117(1):271–278. doi:10.1213/ANE.0b013e318290c566
20. Abdallah FW, Brull R. Facilitatory effects of perineural dexmedetomidine on neuraxial and peripheral nerve block: a systematic review and meta-analysis. Br J Anaesth. 2013;110(6):915–925. doi:10.1093/bja/aet066
21. Moss LM, Algera MH, Dobbins R, et al. Effect of sustained high buprenorphine plasma concentrations on fentanyl-induced respiratory depression: a placebo-controlled crossover study in healthy volunteers and opioid-tolerant patients. PLoS One. 2022;17(1):e0256752. doi:10.1371/journal.pone.0256752
22. Olofsen E, Algera MH, Moss L, et al. Modeling buprenorphine reduction of fentanyl-induced respiratory depression. JCI Insight. 2022;7(9). doi:10.1172/jci.insight.156973
23. Egan Talmage D, Warner David O. Miller’s Anesthesia. Anesthesiology. 2005;103(3):673. doi:10.1097/00000542-200509000-00044
24. Wu Y, Jin S, Zhang L, et al. Minimum alveolar concentration-awake of sevoflurane is decreased in patients with end-stage renal disease. Anesth Analg. 2019;128(1):77–82. doi:10.1213/ANE.0000000000003676
25. Oh TK, Eom W, Yim J, et al. The effect of chronic opioid use on end-tidal concentration of sevoflurane necessary to maintain a bispectral index below 50: a prospective, single-blind study. Anesth Analg. 2017;125(1):156–161. doi:10.1213/ANE.0000000000001791
26. Dixon WJ. Staircase bioassay: the up-and-down method. Neurosci Biobehav Rev. 1991;15(1):47–50. doi:10.1016/s0149-7634(05)80090-9
27. Pace NL, Stylianou MP, Warltier DC. Advances in and limitations of up-and-down methodology: a precis of clinical use, study design, and dose estimation in anesthesia research. Anesthesiology. 2007;107(1):144–152. doi:10.1097/01.anes.0000267514.42592.2a
28. Hu P, Harmon D, Frizelle H. Patient comfort during regional anesthesia. J Clin Anesth. 2007;19(1):67–74. doi:10.1016/j.jclinane.2006.02.016
29. Jenkins K, Grady D, Wong J, Correa R, Armanious S, Chung F. Post-operative recovery: day surgery patients’ preferences. Br J Anaesth. 2001;86(2):272–274. doi:10.1093/bja/86.2.272
30. Hohener D, Blumenthal S, Borgeat A. Sedation and regional anaesthesia in the adult patient. Br J Anaesth. 2008;100(1):8–16. doi:10.1093/bja/aem342
31. Wu CL, Naqibuddin M, Fleisher LA. Measurement of patient satisfaction as an outcome of regional anesthesia and analgesia: a systematic review. Reg Anesth Pain Med. 2001;26(3):196–208. doi:10.1053/rapm.2001.22257
32. Wang YX, Mao XF, Li TF, Gong N, Zhang MZ. Dezocine exhibits antihypersensitivity activities in neuropathy through spinal mu-opioid receptor activation and norepinephrine reuptake inhibition. Sci Rep. 2017;7(1):43137. doi:10.1038/srep43137
33. Liu R, Huang XP, Yeliseev A, Xi J, Roth BL. Novel molecular targets of dezocine and their clinical implications. Anesthesiology. 2014;120(3):714–723. doi:10.1097/ALN.0000000000000076
34. Ren BX, Zong J, Tang JC, et al. Effects of intravenous analgesia with combined dezocine and butorphanol on postoperative cognitive function in elderly patients. Genet Mol Res. 2015;14(2):5571–5576. doi:10.4238/2015.May.25.8
35. Wang YH, Chai JR, Xu XJ, et al. Pharmacological characterization of dezocine, a potent analgesic acting as a kappa partial agonist and mu partial agonist. Sci Rep. 2018;8(1):14087. doi:10.1038/s41598-018-32568-y
36. Wang L, Yi Q, Ye C, Luo N, Wang E. Effects of dezocine on the reduction of emergence delirium after laparoscopic surgery: a retrospective propensity score-matched cohort study. J Pers Med. 2023;13(4):590. doi:10.3390/jpm13040590
37. Chartoff E, Sawyer A, Rachlin A, Potter D, Pliakas A, Carlezon WA. Blockade of kappa opioid receptors attenuates the development of depressive-like behaviors induced by cocaine withdrawal in rats. Neuropharmacology. 2012;62(1):167–176. doi:10.1016/j.neuropharm.2011.06.014
38. Gillman KW, Parker MF, Silva M, et al. Design, optimization, and in vivo evaluation of a series of pyridine derivatives with dual NK1 antagonism and SERT inhibition for the treatment of depression. Bioorg Med Chem Lett. 2013;23(2):407–411. doi:10.1016/j.bmcl.2012.11.094
39. Bell J, Strang J. Medication treatment of opioid use disorder. Biol Psychiatry. 2020;87(1):82–88. doi:10.1016/j.biopsych.2019.06.020
40. Zamani N, Hassanian-Moghaddam H. Intravenous buprenorphine: a substitute for naloxone in methadone-overdosed patients? Ann Emerg Med. 2017;69(6):737–739. doi:10.1016/j.annemergmed.2016.12.024
41. Zamani N, Hassanian-Moghaddam H, Bayat AH, et al. Reversal of opioid overdose syndrome in morphine-dependent rats using buprenorphine. Toxicol Lett. 2015;232(3):590–594. doi:10.1016/j.toxlet.2014.12.007
42. Columb MO, Lyons G. Determination of the minimum local analgesic concentrations of epidural bupivacaine and lidocaine in labor. Anesth Analg. 1995;81(4):833–837. doi:10.1097/00000539-199510000-00030
43. Smart JA, Pallett EJ, Duthie DJ. Breath interval as a continuous measure of opioid effects of intravenous fentanyl and alfentanil. Eur J Anaesthesiol. 2003;20(6):498–500. doi:10.1017/s0265021503230805
44. Egan TD, Minto CF, Hermann DJ, Barr J, Muir KT, Shafer SL. Remifentanil versus alfentanil: comparative pharmacokinetics and pharmacodynamics in healthy adult male volunteers. Anesthesiology. 1996;84(4):821–833. doi:10.1097/00000542-199604000-00009
45. Ferguson LM, Drummond GB. Acute effects of fentanyl on breathing pattern in anaesthetized subjects. Br J Anaesth. 2006;96(3):384–390. doi:10.1093/bja/ael011
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.