Back to Journals » Journal of Inflammation Research » Volume 16
The Effect of Decrease in Serum Urate for the Risk of Gout Flares During Urate-Lowering Therapy Initiation Among Chinese Male Gout Patients: A Prospective Cohort Study
Authors Pang L, Xue X, He Y, Wang C, Han L, Li M, Qi H, Li C, Lu J
Received 8 June 2023
Accepted for publication 1 September 2023
Published 8 September 2023 Volume 2023:16 Pages 3937—3947
DOI https://doi.org/10.2147/JIR.S424820
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Monika Sharma
Lei Pang,1– 4,* Xiaomei Xue,1– 4,* Yuwei He,1– 4 Can Wang,1– 4 Lin Han,1– 4 Maichao Li,1– 4 Han Qi,1– 4 Changgui Li,1– 4 Jie Lu1– 4
1Shandong Provincial Clinical Research Center for Immune Diseases and Gout, the Affiliated Hospital of Qingdao University, Qingdao, 266003, People’s Republic of China; 2Shandong Provincial Key Laboratory of Metabolic Diseases and Qingdao Key Laboratory of Gout, the Affiliated Hospital of Qingdao University, Qingdao, 266003, People’s Republic of China; 3Department of Endocrinology and Metabolism, the Affiliated Hospital of Qingdao University, Qingdao, 266003, People’s Republic of China; 4Institute of Metabolic Diseases, Qingdao University, Qingdao, 266003, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jie Lu; Changgui Li, Shandong Provincial Key Laboratory of Metabolic Diseases and Qingdao Key Laboratory of Gout, the Affiliated Hospital of Qingdao University, Qingdao, 266003, People’s Republic of China, Tel +86 17853297395, Fax +86 532-82912019, Email [email protected]; [email protected]
Purpose: Higher baseline serum urate or higher initial urate-lowering medication dose increased risk of gout flares during urate-lowering therapy (ULT) initiation. The decrease in serum urate may play a crucial role in this process. Therefore, we aim to explore the relationship between decrease in serum urate and the risk of gout flares during ULT initiation.
Patients and Methods: A 12-week prospective cohort study of Chinese male gout patients was conducted at Shandong Provincial Clinical Research Center for Immune Diseases and Gout in China. Patients were grouped by baseline serum urate (7– 7.9 mg/dL, 8– 8.9 mg/dL and ≥ 9 mg/dL). All patients received febuxostat 20 mg daily during weeks 0– 4, then escalated to 40mg during weeks 4– 12 if serum urate > 6mg/dL. The main outcomes were the number of gout flares and the decrease in serum urate. Poisson regression was performed.
Results: A total of 282 participants were enrolled, of whom 260 completed (84, 87 and 89 in each group) from March 2021 to December 2021. A 44.2% of all participants experienced at least one gout flare. In the multivariate Poisson regression 1, Δ serum urate 0– 12 weeks (IRR 1.184, 95% CI, 1.062– 1.320; P=0.002), the number of gout flares before treatment 1 year (1.017, 1.010– 1.024; P< 0.001) and tophus (1.580, 1.023– 2.440; P=0.039) were independently associated with the number of gout flares. While in the multivariate Poisson regression 2, baseline serum urate (1.256, 1.050– 1.503; P=0.013) and the number of gout flares before treatment 1 year (1.014, 1.007– 1.022; P< 0.001) were independently associated with the number of gout flares, Δ serum urate 0– 12 weeks (1.055, 0.923– 1.207; P=0.433) was no longer a risk factor.
Conclusion: ULT-induced gout flares depend on the degree of decrease in serum urate, which is affected by baseline serum urate. Higher baseline serum urate and greater decrease in serum urate lead to higher risk of gout flares.
Keywords: baseline serum urate, decrease in serum urate, gout flares, febuxostat
Introduction
Gout is a common metabolic disorder caused by the deposition of monosodium urate (MSU) crystals in the joints and other tissues. Gout clinically manifests as follows: recurrent acute arthritis, chronic joint swelling, tophus. In severe cases, it may lead to joint deformity and renal failure. The prevalence of gout has risen in China, with a weighted prevalence rate of 3.2% in adults (males for 4.4%, females for 2.0%).1 Urate-lowering therapy (ULT) is used to reduce serum urate levels to prevent the formation of new MSU crystals and dissolve existing ones, thus decreasing harm to the body.2
However, gout flares are more likely to occur during the first few months of ULT, leading to poor medication adherence, increased healthcare resource utilization and work productivity loss.3–5 What is more, cardiovascular events are more likely to occur within days 61 to 120 after a gout flare.6
Thus, it is essential to identify the risk factors for gout flares during ULT initiation and optimize the treatment regimen accordingly. In clinical practice, we have noted that patients with elevated baseline serum urate levels face an increased risk of gout flares during ULT. Furthermore, a higher initial dosage of urate-lowering medication is associated with an elevated incidence of gout flares.7 The degree of decrease in serum urate may significantly influence the occurrence of gout flares. However, to the best of our knowledge, there is limited existing research on the association between the decrease in serum urate and gout flares in response to ULT.8,9 Moreover, the few available studies did not investigate the decrease in serum urate and gout flares in detail during the first 12 weeks of initiating ULT, which is a critical period for gout flare occurrence. In addition, the scarce existing research did not adjust for some potential confounding factors that may affect the risk of gout flares, such as hypertension, diabetes, tophus, the number of gout flares before treatment 1 year, and body mass index (BMI). These factors may be associated with both serum urate level and the risk of gout flares, and if not adjusted for, they may also confound the relationship between decrease in serum urate level and the risk of gout flares.
Therefore, we conducted a prospective cohort study among Chinese male gout patients during ULT initiation. We aimed to explore the relationship between decrease in serum urate and the risk of gout flares during ULT initiation.
Materials and Methods
Study Design and Patients
A prospective cohort study was conducted at the Shandong Provincial Clinical Research Center for Immune Diseases and Gout from March 2021 to December 2021. Male gout patients aged between 18 and 70 years, who met the 2015 American College of Rheumatology/European League Against Rheumatism diagnostic criteria for gout,10 were eligible to participate in the study. Exclusion criteria included serum urate <7 mg/dL, estimated glomerular filtration rate (eGFR) <60 mL/min/1.73m2, rheumatoid arthritis, serum alanine aminotransferase (ALT) or aspartate aminotransferase (AST) >80 U/L, allergic to febuxostat. On the one hand, we contacted potential participants who met the study criteria from the medical record system of the Shandong Provincial Clinical Research Center for Immune Diseases and Gout, and invited them to join the study. On the other hand, we recruited potential participants by advertising our study through posters and social media.
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Affiliated Hospital of Qingdao University (Permit Number: QYFYWZLL26144) and registered at the China Clinical Trial Registration Center (Registration number: ChiCTR2100043573). All participants provided informed consent and enrolled voluntarily.
All eligible patients underwent a two-week washout period, during which they stopped taking urate-lowering drugs and followed a low-purine diet. Then, we tested their baseline serum urate. Patients were assigned to three groups (7–7.9 mg/dL, 8–8.9 mg/dL and ≥9 mg/dL) based on their baseline serum urate. All participants were administered febuxostat at a dose of 20 mg once daily for 0–4 weeks. If serum urate was >6 mg/dL on the fourth weekend, the dose was escalated to 40 mg for 4–12 weeks. Anti-inflammatory prophylaxis was not administered. Anti-inflammatory drugs were only used during a gout flare, defined as an attack with more than two symptoms/signs of joint erythema, swelling, warmth and tenderness, which was considered to require treatment with anti-inflammatory medications by the subjects and the doctors.11 If a gout flare occurred during the study period, participants were given additional etoricoxib 120 mg daily for 3–5 days. Polyene phosphatidylcholine was administered as an adjuvant treatment for patients with elevated liver enzymes 1.5 times the normal upper limit. Participants failing to take the medication for three consecutive days were terminated from the study. Patients were followed up every four weeks at the Shandong Provincial Clinical Research Center for Immune Diseases and Gout.
Data Variables and Collection
Following is a definition of the study variables. The gout duration was the time since the first incident of gout flare.12 The presence of one or more relatives who were diagnosed with gout in the third degree or closer was considered to be a positive family history of gout. According to the diagnostic standards,13,14 hypertension and type 2 diabetes were defined.
The following baseline data were obtained: age, onset age of gout, BMI, systolic blood pressure (SBP), diastolic blood pressure (DBP), gout duration, positive family history, the number of gout flares before treatment 1 year, subcutaneous tophus presence/absence, hypertension and type 2 diabetes. Fasting serum laboratory assessment included ALT, AST, glucose, triglyceride (TG), total cholesterol (TC), serum creatinine, blood urea nitrogen (BUN), low-density lipoprotein-cholesterol (LDL), serum urate, albumin and total bilirubin. The biochemical parameters were repeatedly measured at each visit, using an automatic biochemical analyzer (Cobas C 501, Roche Diagnostics, Switzerland). eGFR was calculated using the CKD-EPI equation.15 The decrease in serum urate (Δ serum urate) = previous visit’s serum urate – current visit’s serum urate. The follow-up method consisted of regular visits to the Clinical Research Center, where patients recorded number of gout flares that occurred in the previous four weeks, underwent fasting blood tests for biochemical analysis, and reported their adherence to prescribed medications and any potential discomfort experienced after taking medications. These data were obtained every four weeks by trained field doctors using the same equipment and testing protocol throughout the study period.
Outcomes
The primary outcomes were the number of gout flares and the decrease in serum urate over a period of 12 weeks. The secondary efficacy outcome was the proportion of participants who achieved the target serum urate ≤6 mg/dL at week 12, the proportion of participants achieving serum urate ≤5 mg/dL at week 12.
Sample Size Estimation
Sample size was estimated via PASS 2021 version 21.0.3, based on the proportion of participants who experienced at least one gout flare after 12 weeks ULT. According to the results of our pre-experiment, the proportions of participants experienced at least one gout flare were 30%, 44%, 55% in the baseline serum urate groups of 7–7.9mg/dL, 8–8.9mg/dL, and ≥9mg/dL, respectively, during 12 weeks ULT. Consistently, 40% proportion of gout flare as estimated by the reports for general gout patients.16 We calculated a sample size of 94 participants in each group at a ratio of 1:1:1, with a 20% drop-out rate considered, using a 5% two-sided significance level and 80% power to detect differences in gout flares among the three groups.
Statistical Analyses
The data was analyzed using IBM SPSS 25.0 (Chicago, USA) software, with a two-sided P value of <0.05 considered statistically significant. Categorical variables were summarized as frequencies (percent), and quantitative variables as mean (standard deviations) if normally distributed, or median (interquartile range) if not normally distributed, based on their distributions. ANOVA was used for normally distributed quantitative variables, while Kruskal–Wallis’s tests were used for non-normally distributed variables. The chi-squared test was used for comparisons of proportions. The significance level was corrected using the Bonferroni method.
To explore the relationship between decrease in serum urate and the risk of gout flares, a Poisson regression analysis was performed. In the multivariate Poisson regression analysis, any variables with a P-value less than 0.05 in the univariate Poisson regression analysis were included. The incident rate ratio (IRR) and 95% confidence intervals (CI) were calculated.
Results
Participants and Baseline Characteristics
A total of 335 participants were screened and 282 eligible participants were enrolled. During the follow-up, 22 participants dropped out, with 13 withdrawing their consent and 9 taking medicine irregularly (Figure 1).
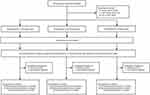 |
Figure 1 Flow chart. |
Table 1 presents the baseline demographic, comorbidities, and laboratory characteristics of the participants. The median (IQR) age of the participants was 48 (39, 58) years. The median (IQR) BMI was 26.91 (24.75, 28.62) kg/m2. The median (IQR) gout duration was 7 (3, 12) years. A total of 19 (7.3%) patients had subcutaneous tophus, 73 (28.1%) had hypertension, and 19 (7.3%) had type 2 diabetes. The median (IQR) baseline serum urate was 8.49 (7.80, 9.28) mg/dL, and the mean (SD) eGFR was 92.47 (15.60) mL/min/1.73m2. Baseline characteristics were compared among three groups. The median (IQR) onset age of gout in ≥9 mg/dL groups [36 (31, 44)] and the 8–8.9 mg/dL groups [39 (32, 47)] were both significantly earlier than that in the 7–7.9 mg/dL group [41 (36, 51.75)] (P=0.001). The proportion of positive family history of gout was significantly higher in the baseline serum urate ≥9 mg/dL group (30%) than in the 8–8.9 mg/dL group (14%) (P=0.026). The number of gout flares before treatment 1 year was more in the baseline serum urate ≥9 mg/dL group [3 (1, 5)] than in both the 8–8.9 mg/dL group [1 (1, 3)] and 7–7.9 mg/dL group [2 (1, 2)] (P<0.001). The TG in the baseline serum urate ≥9 mg/dL group [1.72 (1.29, 2.42)] were significantly higher than those in the 7–7.9 mg/dL group [1.40 (1.15, 2.00)] (P=0.033).
 |
Table 1 Baseline Characteristics Based on Baseline Serum Urate |
Gout Flares Over the 12-week Study Period
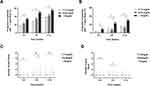
During 12 weeks, 44.2% of all participants experienced at least one gout flare. Notably, the proportion of participants who experienced at least one gout flare was significantly higher in baseline serum urate ≥9 mg/dL group than in the 7–7.9 mg/dL group during the 0–4 week period (33.7% vs 14.3%, P<0.05), 0–8 week period (49.4% vs 25%, P<0.05), and 0–12 week period (55.1% vs 34.5%, P <0.05) (Figure 2A). Additionally, 18.1% of participants experienced at least two gout flares during the 0–12 week period. The proportion of participants who experienced at least two gout flares was significantly higher in the baseline serum urate ≥9 mg/dL group than in the 8–8.9 mg/dL group during the 0–4 week period (9% vs 0%, P<0.05), 0–8 week period (22.6% vs 8%, P<0.05), and 0–12 week period (29.2% vs 12.6%, P<0.05). Meanwhile, more participants in the baseline serum urate ≥9 mg/dL group experienced at least two gout flares than in the 7–7.9 mg/dL group during the 0–8 week period (22.6% vs 7.1%, P<0.05) and 0–12 week period (29.2% vs 11.9%, P<0.05) (Figure 2B). The number of gout flares in the baseline serum urate ≥9 mg/dL group was more than in the 7–7.9 mg/dL group during the 0–4 week period, 0–8 week period, and 0–12 week period (Figure 2C). Meanwhile, the number of gout flares in the baseline serum urate ≥9 mg/dL group was more than in the 7–7.9 mg/dL group during the 0–4 week period (P<0.01) and 4–8 week period (P<0.05). Notably, there was no significant difference in the number of gout flares among the three groups during the 8–12 week period (Figure 2D).
Decrease in Serum Urate
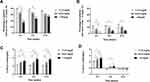
The highest Δ serum urate was observed in the baseline serum urate ≥9 mg/dL group, followed by the 8–8.9 mg/dL group, and the smallest in the 7–7.9 mg/dL group during 0–4 weeks, 0–8 weeks, and 0–12 weeks (Figure 3C). The univariate linear regression analysis showed that Δ serum urate 0–12 weeks=0.8318*baseline serum urate-4.492 (R2=0.37) (Supplemental Material). During 0–4 weeks, when the same 20mg febuxostat was used, Δ serum urate was significantly highest in the baseline serum urate ≥9 mg/dL group, followed by the 8–8.9 mg/dL group and significantly smallest in the 7–7.9 mg/dL group. When 40mg febuxostat was administered to patients whose serum urate was >6 mg/dL on the fourth weekend, Δ serum urate was significantly higher in the baseline serum urate ≥9 mg/dL group compared to the 7–7.9 mg/dL group during 4–8 weeks. Interestingly, there were no significant differences in Δ serum urate among the three groups during 8–12 weeks (Figure 3D).
The Proportion of Participants Achieving Target Serum Urate
After 12 weeks of ULT, 60.4% of participants achieved serum urate levels less than 6mg/dL. There were no significant differences in the proportion of participants achieving serum urate levels ≤6.0 mg/dL among the three groups after 12 weeks of ULT (Figure 3A). After 12 weeks of ULT, serum urate decreased to less than 5mg/dL in 17.3% of participants. The proportion of participants achieving serum urate levels ≤5.0 mg/dL was significantly lower in the baseline serum urate ≥9 mg/dL group (9%) compared to the 7–7.9 mg/dL group (25%) (Figure 3B).
Poisson Regression Model for the Number of Gout Flares During Follow-Up of 12 Weeks
In univariable Poisson regression analysis, gout duration, the number of gout flares before treatment 1 year, tophus, BMI, baseline serum urate and Δ serum urate 0–12 weeks was significantly associated with the number of gout flares (P<0.05, Table 2). We focused on the following two variables: Δ serum urate 0–12 weeks and baseline serum urate. In multivariate Poisson regression analysis 1, after adjusting for confounding variables in which baseline serum urate was not included, Δ serum urate 0–12 weeks (IRR 1.184, 95% CI, 1.062–1.320; P=0.002), the number of gout flares before treatment 1 year (1.017, 1.010–1.024; P<0.001) and tophus (1.580, 1.023–2.440; P=0.039) remained significantly associated with the number of gout flares (Table 2). In multivariate Poisson regression analyses 2, after adjusting for confounding variables in which baseline serum urate and Δ serum urate 0–12 weeks were included, baseline serum urate (IRR 1.256, 95% CI, 1.050–1.503; P=0.013) and the number of gout flares before treatment 1 year (1.014, 1.007–1.022; P<0.001) remained significantly associated with the number of gout flares, but Δ serum urate 0–12 weeks (1.055, 0.923–1.207; P=0.433) was no longer a risk factor (Table 2). Hence, it appeared that effect of Δ serum urate was affected by baseline serum urate.
 |
Table 2 Poisson Regression Model for the Number of Gout Flares During Follow-Up of 12 Weeks |
Discussion
This is the first study, to the best of our knowledge, that examines the relationship between decrease in serum urate and the number of gout flares by a prospective cohort design during the initial 12 weeks of ULT, a critical period for gout flare occurrence. We adjusted for some potential confounding factors that may influence the risk of gout flares by Poisson regression analysis, and reached a more convincing conclusion: gout flares induced by ULT depend on the degree of decrease in serum urate. The effect of decrease in serum urate for the risk of gout flares was affected by baseline serum urate.
In multivariate Poisson regression analyses 1, without baseline serum urate, Δ serum urate 0–12 weeks was independently associated with the number of gout flares, which strongly indicates that gout flares have been associated with a decrease in serum urate in response to ULT. Previous studies have also found similar phenomena. The results from the NOR‑Gout study reported that in the first year of ULT, decrease in serum urate was higher among patients with flares, and decrease in serum urate was correlated with flare occurrences.8 A post-hoc analysis conducted by Mandell also indicated that absolute change in serum urate before flares was associated with flares in a multivariate linear regression analysis.9 In addition, a recent change in serum urate was associated with a decline in HRQOL/function or condition of health in the first six months following ULT initiation or ULT change due primarily to a reduction in serum urate in spite of anti-inflammatory prophylaxis.17
Decreases in serum urate can initiate an inflammatory process that manifests as gout flares. ULT may alter the solubility of tissue MSU crystals, which may increase the flare rate due to changes in the chemical or physical state of preexisting MSU crystals when ULT causes rapid changes in serum urate.18 MSU crystals are thus solubilized, exposing uncoated MSU crystals to monocytes and synoviocytes, which activate the NALP-3 inflammasome and increase the expression of proinflammatory cytokines, such as interleukin (IL)-1 and IL-18.19 This view is also confirmed by the article of Mandell et al from the opposite side: fluctuations in serum urate were also found in patients without ULT; however, when flares and non-flare group were compared, there was no difference in serum urate change in patients without ULT.9 Because in the absence of ULT, despite fluctuations in serum urate, MSU did not dissolve.9
Poisson regression analysis 2 suggested that baseline serum urate was independently associated with the number of gout flares. Previous studies have shown similar results that graded increases in serum urate were associated with an increased risk of incident and recurrent gout flares. Using administrative claims data from US, a retrospective cohort study reported that gout flares are associated with higher serum urate in a dose-dependent manner.20 In a case–control study, higher serum urate is independently associated with more than two gout flares.21 Moreover, a Chinese cross-sectional study found that serum urate is an independent risk factor of frequent gout flares (>20 times).22 Higher serum urate was also correlated with reduced physical and mental health-related quality of life, as well as an increase in pain during ULT initiation.17 In addition, Dalbeth et al23 reported that gout incidence is strongly predicted by serum urate in a nonlinear concentration-dependent manner among participants with gout-free at baseline.
In this study, the univariate linear regression analysis showed that Δ serum urate 0–12 weeks=0.8318*baseline serum urate-4.492 (R2=0.37). During the first four weeks of using 20 mg febuxostat, higher baseline serum urate was associated with a greater decrease in serum urate. Consistent with previous studies, baseline serum urate was independently associated with the decrease in serum urate.24,25 Chino et al26 also reported that patients with higher baseline serum urate experienced greater reductions in serum urate after taking an SGLT2 inhibitor. In multivariate Poisson regression analyses 2 considering baseline serum urate, it was found that baseline serum urate was independently associated with the number of gout flares, while Δ serum urate 0–12 weeks was no longer a significant risk factor. This confirms the effect of Δ serum urate for gout flares was affected by baseline serum urate.
Hisashi Yamanaka et al7 found that a stepwise dose escalation of febuxostat can be an effective alternative to prophylaxis with low-dose colchicine. Moreover, gout flares may be masked by prophylaxis. In addition, low-dose febuxostat is commonly administered in Asian countries.27 Therefore, anti-inflammatory prophylaxis was not administered in this study. Our choice of ULT is a stepwise dose escalation of febuxostat to 40mg. However, although a stepwise dose escalation of febuxostat was administered in this study, gout patients with higher baseline serum urate experienced more gout flares. Prophylaxis with low-dose colchicine or lower starting dosage of febuxostat may be helpful to reduce gout flares for gout patients with higher serum urate during ULT initiation.
Meanwhile, this study had some limitations. First, this is a single-center, single-ethnic study, these results may not be generalizable to non-Chinese gout patients (including female patients). We recommend conducting multicenter, multiethnic studies that include gout patients from different regions, races, and genders. Second, we instructed the patients to limit their purine intake in the follow-up. However, the detailed information on the dietary intake of the participants, such as the frequency and quantity of purine-rich foods, were not collected. In the future, we suggest collecting more detailed information on the dietary intake of the participants, such as the frequency and quantity of purine-rich foods, using validated food frequency questionnaires or dietary records. This would enable us to adjust for the dietary factors in the statistical analysis.
Conclusion
This study found that gout flares induced by ULT depend on the degree of decrease in serum urate. The effect of decrease in serum urate for the risk of gout flares was affected by baseline serum urate. A higher baseline serum urate and a greater decrease in serum urate lead to a higher risk of gout flares.
Therefore, for gout patients, we recommend that they follow the prescribed ULT regimen, monitor their serum urate levels regularly, and carry anti-inflammatory prophylaxis medication in pocket when starting ULT. For clinics, we suggest that they develop individualized ULT plans for gout patients based on their baseline serum urate levels. For patients with higher serum urate, physicians would start with a lower dose of urate-lowering drugs to minimize the risk of gout flares due to a rapid fall of serum urate during ULT initiation. For related stakeholders, such as the government, we urge them to increase awareness, so that gout patients can learn more about the knowledge that rapid fall of serum urate can increase the risk of gout flares.
Data Sharing Statement
The author can provide the data supporting the study’s results upon reasonable request. Related data and materials are available upon request to the first author Lei Pang. Before sharing data with any third party, we will remove any personal identifiers such as name, date of birth, address, phone number, and others from the patient data and assign a unique code to each patient. We will also encrypt the patient data and store it securely.
Ethics Statement
This study was conducted in accordance with the Declaration of Helsinki. The study protocol was approved by the Ethics Committee of the Affiliated Hospital of Qingdao University (Permit Number: QYFYWZLL26144) and registered at the China Clinical Trial Registration Center (Registration number: ChiCTR2100043573). All participants provided written informed consent and enrolled voluntarily.
Acknowledgments
We sincerely thank all participants.
Funding
This work was sponsored by Taishan Scholar Programme of Shandong Province (#tsqn202211377), Shandong Provincial Science Foundation for Outstanding Youth Scholars (#ZR2021YQ56), National Key R&D Program of China (#2022YFC2503300, #2022YFE0107600), National Natural Science Foundation of China (#82220108015) and Natural Science Foundation of Shandong Province (#ZR2022QH065).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Song J, Jin C, Shan Z, Teng W, Li J. Prevalence and risk factors of hyperuricemia and gout: a cross-sectional survey from 31 provinces in Mainland China. J Transl Intern Med. 2022;10(2):134–145. doi:10.2478/jtim-2022-0031
2. Shoji A, Yamanaka H, Kamatani N. A retrospective study of the relationship between serum urate level and recurrent attacks of gouty arthritis: evidence for reduction of recurrent gouty arthritis with antihyperuricemic therapy. Arthritis Rheum. 2004;51(3):321–325. doi:10.1002/art.20405
3. Singh JA, Bharat A, Khanna D, et al. Health care utilization in patients with gout: a prospective multicenter cohort study. BMC Musculoskelet Disord. 2017;18(1):233. doi:10.1186/s12891-017-1573-6
4. Uhlig T, Karoliussen LF, Sexton J, et al. One- and 2-year flare rates after treat-to-target and tight-control therapy of gout: results from the NOR-Gout study. Arthritis Res Ther. 2022;24(1):88. doi:10.1186/s13075-022-02772-3
5. Wertheimer A, Morlock R, Becker MA. A revised estimate of the burden of illness of gout. Curr Ther Res Clin Exp. 2013;75:1–4. doi:10.1016/j.curtheres.2013.04.003
6. Cipolletta E, Tata LJ, Nakafero G, Avery AJ, Mamas MA, Abhishek A. Association between gout flare and subsequent cardiovascular events among patients with gout. JAMA. 2022;328(5):440–450. doi:10.1001/jama.2022.11390
7. Yamanaka H, Tamaki S, Ide Y, et al. Stepwise dose increase of febuxostat is comparable with colchicine prophylaxis for the prevention of gout flares during the initial phase of urate-lowering therapy: results from FORTUNE-1, a prospective, multicentre randomised study. Ann Rheum Dis. 2018;77(2):270–276. doi:10.1136/annrheumdis-2017-211574
8. Uhlig T, Karoliussen LF, Sexton J, et al. Fluctuation and change of serum urate levels and flares in gout: results from the NOR-Gout study. Clin Rheumatol. 2022;41(12):3817–3823. doi:10.1007/s10067-022-06416-4
9. Mandell BF, Fields TR, Edwards NL, Yeo AE, Lipsky PE. Post-hoc analysis of pegloticase pivotal trials in chronic refractory gout: relationship between fluctuations in plasma urate levels and acute flares. Clin Exp Rheumatol. 2021;39(5):1085–1092. doi:10.55563/clinexprheumatol/b7jjnb
10. Neogi T, Jansen TL, Dalbeth N, et al. 2015 Gout classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2015;74(10):1789–1798. doi:10.1136/annrheumdis-2015-208237
11. Sundy JS, Schumacher HR, Kivitz A, et al. Rilonacept for gout flare prevention in patients receiving uric acid-lowering therapy: results of RESURGE, a Phase III, international safety study. J Rheumatol. 2014;41(8):1703–1711. doi:10.3899/jrheum.131226
12. Bursill D, Taylor WJ, Terkeltaub R, et al. Gout, Hyperuricaemia and Crystal-Associated Disease Network (G-CAN) consensus statement regarding labels and definitions of disease states of gout. Ann Rheum Dis. 2019;78(11):1592–1600. doi:10.1136/annrheumdis-2019-215933
13. Liu J. Highlights of the 2018 Chinese hypertension guidelines. Clin Hypertens. 2020;26(1):8. doi:10.1186/s40885-020-00141-3
14. American Diabetes Association. Standards of medical care in diabetes-2018 abridged for primary care providers. Clin Diabetes. 2018;36(1):14–37. doi:10.2337/cd17-0119
15. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi:10.7326/0003-4819-150-9-200905050-00006
16. Shiozawa A, Szabo SM, Bolzani A, Cheung A, Choi HK. Serum uric acid and the risk of incident and recurrent gout: a systematic review. J Rheumatol. 2017;44(3):388–396. doi:10.3899/jrheum.160452
17. Topless R, Noorbaloochi S, Merriman TR, Singh JA. Change in serum urate level with urate-lowering therapy initiation associates in the immediate term with patient-reported outcomes in people with gout. Semin Arthritis Rheum. 2022;56:152057. doi:10.1016/j.semarthrit.2022.152057
18. Becker MA, MacDonald PA, Hunt BJ, Lademacher C, Joseph-Ridge N. Determinants of the clinical outcomes of gout during the first year of urate-lowering therapy. Nucleosides Nucleotides Nucleic Acids. 2008;27(6):585–591. doi:10.1080/15257770802136032
19. Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440(7081):237–241. doi:10.1038/nature04516
20. Shiozawa A, Buysman EK, Korrer S. Serum uric acid levels and the risk of flares among gout patients in a US managed care setting. Curr Med Res Opin. 2017;33(1):117–124. doi:10.1080/03007995.2016.1239193
21. Abhishek A, Valdes AM, Zhang W, Doherty M. Association of serum uric acid and disease duration with frequent gout attacks: a case-control study. Arthritis Care Res. 2016;68(10):1573–1577. doi:10.1002/acr.22855
22. Liang J, Jiang Y, Huang Y, et al. Comorbidities and factors influencing frequent gout attacks in patients with gout: a cross-sectional study. Clin Rheumatol. 2021;40(7):2873–2880. doi:10.1007/s10067-021-05595-w
23. Dalbeth N, Phipps-Green A, Frampton C, Neogi T, Taylor WJ, Merriman TR. Relationship between serum urate concentration and clinically evident incident gout: an individual participant data analysis. Ann Rheum Dis. 2018;77(7):1048–1052. doi:10.1136/annrheumdis-2017-212288
24. Koide H, Hira D, Tsujimoto M, et al. Previous dosage of allopurinol is a strong determinant of febuxostat efficacy. Biol Pharm Bull. 2017;40(5):681–686.
25. Milionis HJ, Kakafika AI, Tsouli SG, et al. Effects of statin treatment on uric acid homeostasis in patients with primary hyperlipidemia. Am Heart J. 2004;148(4):635–640. doi:10.1016/j.ahj.2004.04.005
26. Chino Y, Kuwabara M, Hisatome I. Factors influencing change in serum uric acid after administration of the sodium-glucose cotransporter 2 inhibitor luseogliflozin in patients with type 2 diabetes mellitus. J Clin Pharmacol. 2022;62(3):366–375. doi:10.1002/jcph.1970
27. Wu X, Li C. Fresh perspectives on the CARES trial and the use of febuxostat in an asian population: comment on the Article by Choi et al. Arthritis Rheumatol. 2019;71(3):479–481. doi:10.1002/art.40741
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.