Back to Journals » International Journal of Nanomedicine » Volume 18
The Effect of Biomimetic Remineralization of Calcium Phosphate Ion Clusters-Treated Enamel Surfaces on Bracket Shear Bond Strength
Authors Paik Y, Kim MJ, Kim H , Kang SW, Choi YK, Kim YI
Received 23 May 2023
Accepted for publication 24 July 2023
Published 31 July 2023 Volume 2023:18 Pages 4365—4379
DOI https://doi.org/10.2147/IJN.S420462
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Youna Paik,1 Min Joo Kim,1 Hyeryeong Kim,1 Sun-Woo Kang,2 Youn-Kyung Choi,3,* Yong-Il Kim1,4,*
1Department of Orthodontics, Dental Research Institute, Pusan National University Dental Hospital, Yangsan, South Korea; 2Department of Prosthodontics, Dong-A University Hospital, Busan, South Korea; 3Department of Orthodontics, Biomedical Research Institute, Pusan National University Hospital, Busan, South Korea; 4Dental and Life Science Institute, School of Dentistry, Pusan National University, Yangsan, South Korea
*These authors contributed equally to this work
Correspondence: Youn-Kyung Choi; Yong-Il Kim, Tel +82-51-240-7430 ; +82-55-360-5163, Fax +82-51-240-7706 ; +82-55-360-5154, Email [email protected]; [email protected]
Purpose: To evaluate the remineralization effect of calcium phosphate ion clusters (CPICs) on demineralized enamel surfaces and their effects on bracket shear bond strength.
Patients and Methods: Extracted premolars were prepared in resin blocks. The samples in the form of resin blocks were divided into five experimental groups: control group, demineralized group, and groups of CPIC solution treatment for 30, 60, and 90s. The specimens were examined using scanning electron microscopy (SEM), energy-dispersive spectroscopy (EDX), microhardness testing, micro-computed tomography (micro-CT) assessment, shear bond strength (SBS) test, and adhesive remnant index (ARI) score.
Results: The SEM images revealed epitaxial growth of enamel and a decrease in the thickness of the demineralized enamel layer when treated with CPIC solution. The EDX analysis revealed an increase in the Ca/P ratio in the CPIC-treated groups. The microhardness value significantly increased when treated with CPICs; however, it showed a lower value than that of the sound enamel groups. As a result of the micro-CT test, radiolucency decreased gradually as the CPIC treatment time increased. The SBS test and ARI score results showed an improvement in bonding stability after treatment with CPICs.
Conclusion: We demonstrated an enamel biomodification approach using CPIC solution treatment, which is a promising strategy for enamel remineralization. Specifically, remineralization of demineralized enamel improves the orthodontic bracket SBS.
Keywords: calcium phosphate ion cluster, enamel remineralization, shear bond strength
Introduction
During orthodontic treatment, patients with fixed orthodontic appliances encounter difficulties in maintaining good oral hygiene and some develop early carious lesions.1 The early carious lesions in the enamel are clinically observed as milky-white opaque surfaces that are softer than the surrounding sound enamel, defined as “white spot lesions” (WSLs). It has been suggested that these spots are the primary sites of acid penetration during carious lesion formation of bacterial plaques. In conjunction with orthodontic treatment, enamel decalcification or white spot formation has been observed in patients who maintain fixed orthodontic appliances for prolonged periods.2 A localized dissolution of the superficial enamel was visualized in patients with WSLs using micro-radiographic images. When the demineralized space of the enamel reaches 400 µm of depth, which is adequate for light diffraction, the decalcified lesion is visibly detected as a white spot.1,3,4 Visible WSLs are known to clinically develop within 4 weeks, which is the period between one orthodontic appointment and the next.1
Dental practitioners are seeking effective methods to prevent the progression of WSLs to various lesions.3 Minimally invasive management of early carious lesions through enamel remineralization has been widely studied.5
Current remineralization systems can be classified into fluoride-mediated and non-fluoride-mediated treatments. Traditional fluoride-mediated remineralization, using an arginine-fluoride varnish6 or amelogenin with fluoride,7 is the most widely used method to date. Fluoride effectively interrupts demineralization and promotes remineralization by forming acid-resistant enamel fluorapatite (Ca5(PO4)3F) crystals.6,8 Remineralized fluorapatite is approximately 3–5 times harder than hydroxyapatite (HAP) and has antibacterial effects.9 However, overexposure to fluoride is associated with risk factors, such as the development of dental fluorosis10 and fluoride syndrome.11 A study reported that fluoride increased friction resistance and deteriorated the surface of the wire by attacking the titanium oxide (TiO2) layer, which protected orthodontic Ni-Ti wires.12 Therefore, there is a great need to develop remineralization systems that complement fluoride in its effectiveness and overcome its shortcomings.
Some approaches have been undertaken to develop new remineralization methods by replacing fluoride with other materials that can supply calcium and phosphate, which are key to remineralization. The non-fluoride enamel remineralization system can be divided into intrinsic (endogenous) and extrinsic (exogenous) methods. Calcium and phosphate are internally supplied through the saliva in the intrinsic system, whereas they are supplied externally to form enamel crystals in the extrinsic system.8
The use of amelogenin-based polypeptides is a representative method for intrinsic remineralization. Amelogenin-supported enamel mineralization significantly increases the microhardness of the enamel surface.13 However, this method was found to be unsuitable for clinical use because remineralization of the enamel layer required 7 days.14 For the extrinsic method, amorphous calcium phosphate (ACP) is discussed as a possible remineralization material. ACP (Ca3(PO4)2. nH2O) serves as a precursor for the epitaxial growth of the enamel surface.15 ACP is in unstable phases and can transform into HA. With little control, calcium and phosphate ions (free or from ACP) are available long enough to remineralize enamel surface. Therefore, the control of the reaction time is crucial for durable enamel remineralization using ACPs. If the reaction is not controlled, the unstable phase of ACP rapidly transforms into a stable HAP (Ca10(PO4)6(OH)2) state, which inhibits the start of the remineralization process.8
The use of calcium phosphate ion clusters as a way of non-fluoride mediated biomimetic remineralization system has been a challenge for a long period. The acellular nature of enamel has been a major challenge for its own regeneration. Also, a precise control of HAP, which is a fundamental element of enamel, has not been successfully solved yet.16 Therefore, a large number of recently published studies follow biomimetic mineralization to mimic the process of enamel growth.
Recently, enamel repair using a biomimetic mineralization frontier with calcium phosphate ion clusters (CPICs) has been suggested. The study proposed a new type of calcium phosphate ion clusters (CPICs) as a mineralization frontier to cause in situ remineralization. During the formation of the CPIC solution, a small highly volatile molecule in the form of triethylamine (TEA) effectively stabilizes CPIC solution and its controllable removal result in pure hydroxyapatite (HAP, Ca10(PO4)6(OH)2) formation.15 In a previous study of Shao et al, the stabilizing effect of TEA on CPIC solution was confirmed. CPIC solution was generated by mixing two ethanol solutions which resulted in small clusters with an average diameter of 1.5 ± 0.3nm, stable in ethanol for at least 2 days without any aggregation or size increase. TEA is volatile which can easily be removed through ethanol evaporation. With ethanol volatilization, the TEA in CPIC solution decreased resulting in ACP formation which could be explained by reduced stability.15 The experimental results showed that CPIC solution induced nanoparticles recovered mechanical property of enamel. The study presented a general pathway for epitaxial construction of enamel.15
Previously, the effect of CPIC solutions containing metastable calcium phosphate and flavonoids on the demineralized dentin matrix has been demonstrated.17 Treatment of dentin matrix with CPICs showed remineralization of the dentin, which ensured epitaxial growth and improved dentin bond strength and stability.17,18
Several studies have been conducted to address the storage and clinical application of ACP agents in products such as mouthwash,19,20 toothpaste,21 tooth desensitizer,22–24 and in resin products.25–27 However, studies on the clinical application of CPICs on decalcified enamel are still lacking. To further promote the translation of CPIC solution into clinical application, basic studies with adequate evaluation methods as well as relevant in vivo studies are still needed. In addition, whether CPIC solution has better remineralization effects compared to other remineralization agents remains to be further explored.
This study aimed to evaluate remineralization effect of CPICs on demineralized enamel surfaces and their effects on bracket shear bond strength (SBS). Specifically, we characterized CPIC-treated enamel surfaces to demonstrate the remineralization effect of CPICs using scanning electron microscopy (SEM), energy-dispersive spectroscopy (EDX), micro-computed tomography (micro-CT), and microhardness tests. In addition, the SBS and adhesive remnant index (ARI) scores of the sound, demineralized, and CPIC solution-treated enamels were compared.
Materials and Methods
Specimen Preparation
Freshly extracted premolars from patients aged 18–35 years were used. Informed consent was obtained from the patients. Teeth were collected from the Orthodontic Department of Pusan National University Dental Hospital (PNUDH), following the guidelines approved by the ethical committee (PNUDH-2022-03-003-002). The teeth were disinfected using 0.5% chloramine, stored in distilled water, and used within 3 months of extraction. Teeth with cracks, WSLs, soft-tissue attachments, or defects were excluded.
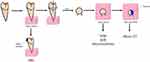
As shown in Figure 1, the specimens were prepared according to the evaluation method. Each tooth was embedded in acrylic resin and prepared as a block. For the SEM, microhardness, and micro-CT tests, the coronal enamel of the teeth was cut parallel to the occlusal plane using a low-speed diamond saw (Accutom-100; Struers, Clevek, OH, USA). For the micro-CT test, half of the buccal surfaces of the specimens were varnished. To test the SBS of the orthodontic bracket, the root of each specimen was embedded in an acrylic resin block with a metal bracket bonded to the buccal surface of each tooth.
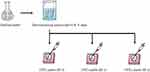
As shown in Figure 2, the specimens were randomly allocated to one of the following experimental groups: control group, demineralized group, and groups of CPIC treatment for 30, 60, and 90s.
Formulation of Experimental Solutions
Preparation of Demineralization Solution
Demineralization solution (2mM Ca(NO3)2, 2mM K3PO4, 75 mM CH3COOH; pH 4.4) was prepared according to the method proposed earlier.28,29 The following chemicals were used without further purification: calcium nitrate hydrate (Ca(NO3)2, Sigma-Aldrich), potassium phosphate tribasic (K3PO4, Sigma-Aldrich), and acetic acid (CH3COOH, Sigma-Aldrich).
Preparation of CPIC Solution
CPIC solutions were prepared according to the method specified by Shao et al.15
Two solutions were prepared for the synthesis of CPICs. For solution A, 40 mg of calcium chloride dihydrate (CaCl2·2H2O; 99.0%) was added to 16 mL of EtOH (C2H5OH; 99.9%) and vortexed until CaCl2·2H2O was completely dissolved. Following this, 760 µL of TEA ((C2H5)3N; 99.5%, Sigma-Aldrich, St. Louis, MO, USA) was added.
For solution B, 14 µL of phosphoric acid (H3PO4; 85% in H2O solution, Sigma-Aldrich) was added to 4 mL of EtOH and vortexed for 5s. The CPIC solution was generated by adding solution B to solution A with minimal agitation. The CPIC solution appeared as a white cloud-like agitated solution in a vial within a few minutes. The CPIC solution was centrifuged at 3000 rpm at 4℃ for 10 min. Therefore, gel-like CPIC solution was obtained.
Experimental Design
The specimens (n=10) stored in distilled water, in which no experimental solution was applied, served as the control group (CON). The specimens were immersed in a demineralizing solution (2 mM Ca(NO3)2, 2 mM K3PO4, 75 mM CH3COOH; pH 4.4) for 4 days (96 h).28,29 After demineralization, the demineralized specimens (n=40) were stored in distilled water. For CPIC-treated specimens, 100 µL of centrifuged CPIC pastes were applied on the buccal surface of demineralized specimens for 30, 60, and 90s. The surfaces were gently air-dried for 5s at room temperature.
SEM and Energy EDX Spectrum
Before demineralization process, all specimens (control, demineralized, and CPIC solution treated) were polished using a 4000-grit abrasive silicon carbide (SiC) paper (Buehler, Coventry, UK). After dehydration, the specimens were examined before and after demineralization process, and after CPIC solution treatment via SEM (SU-70, Hitachi, Tokyo, Japan). Images were obtained randomly on the surface at magnifications of 2000X and 100,000X. The vertical surface morphology of enamel layer was also examined via SEM to examine the change in demineralized layer according to CPIC solution application.
To examine the surface morphology and chemical composition of the surface, specimens were examined by scanning electron microscopy coupled with energy-dispersive X-ray spectroscopy (SEM/EDX, SU-70, Hitachi, Tokyo, Japan) under low vacuum conditions. The composition of Ca and P before and after demineralization and after CPIC solution application was analyzed to prove remineralization. SEM and SEM/EDX analysis were completed at the same time.
Specimens were mounted on an aluminum stage with a carbon tape. The surfaces were sputtered with Pt and observed under an accelerating voltage of 15kV. Both top-down and side views of the sectioned samples were observed using SEM and SEM/EDX.15
EDX analysis is a method to analyze the component of a sample by detecting specific X-rays with high-energy electron beams that react with samples with certain information on the structure or chemical composition. EDX can analyze component elements without damaging the specimen and is a suitable method for analyzing tooth hard tissue since it shows high sensitivity to the main elements of dental hard tissues.30,31 The SEM/EDX analyzed image may be distorted by the accumulation of charged particles on the surface of the non-conductive sample by the electron beam. To resolve the image distortion, the samples were coated with Pt and were measured in low vacuum since the electrons on the sample surface are removed by ions.
SBS Testing
Thirty specimens were prepared for each group to conduct the SBS test. The buccal surfaces of all specimens were etched with 35% H3PO4 (Ultra Etch; Ultradent, South Jordan, UT, USA) for 20s. The specimens were then rinsed with distilled water and air-dried for 10s. After complete drying with the chalky surface of the tooth, an adhesive (Adper Scotch Bond Multi-Purpose Plus, 3M; Monrovia, CA, USA) was applied to the enamel surface for 20s and slightly air-dried for 35s. Light-curing was performed for 10s using an LED curing unit (Valo Ultradent Products, South Jordan, UT, USA). Metal brackets (Archist; Daeseung Medical Co., Seoul, Korea) were bonded using Transbond XT (3M; Unitek, Monrovia, CA, USA). The excess resins were removed, and each increment was light cured for 10s. Samples were stored in distilled water for 24 h, and the shear bond strength (SBS) was measured using a universal testing machine (Instron, Norwood, MA, USA). The SBS value was calculated by measuring the maximum load (MPa) with the crosshead speed of 0.5 mm/min.32
The remaining resins were then evaluated using the adhesive remnant index (ARI) score using an endodontic microscope (SG/M320 F12, Leica Microsystems, Singapore). ARI scores were classified as shown in Table 1.
 |
Table 1 Definition of the Adhesive Remnant Index (ARI) Score |
Microhardness Test
Microhardness was measured using the Vickers hardness number on the buccal surface of all specimens at five random spots using a hardness testing machine (MVK-H1 Akashi, Kanagawa, Japan).33–35 Each specimen was subjected to five indentations and the average hardness value was calculated. The applied load was 100 gf, with a dwell time of 10s.
Micro-CT Assessment
To observe the surface changes of the enamel treated with the demineralization solution and demineralized enamel treated with CPIC solutions (for 30, 60, and 90s), the surface of the specimen was vertically divided into two sides. One side was covered with acid-resistant nail varnish as control. Owing to the varnish, the surface was not affected by the CPIC solution treatment. The other side of the surface was treated with CPIC solutions (for 30, 60, and 90s) without varnish to observe their effect on the demineralized enamel.
The specimens were scanned using a micro-CT machine (Skyscan 1173 ver. 1.6; Bruker-CT, Antwerp, Belgium) at a value of 100 kV, 100 µA, and 360° rotation with 0.15° steps. The enamel layer was examined with a resolution of 2016 × 1344 pixels with a voxel size of 8 µm. Two reference phantoms (0.25 gHAp/cm3 and 0.75 gHAp/cm3 hydroxyapatite disks) were obtained and scanned under same conditions as the samples for mineral density calibration. A 0.3 Al/0.25 Cu filter was used to reduce the beam hardening artifacts of micro-CT and to cut off the low energy x-rays. The density was calculated from the images using a three-dimensional imaging software (Ondemand3D, Irvine, CA, USA).36,37
Statistical Analysis
For estimating the sample size, power calculation was not separately used, and samples per group were set up with reference to Shao et al15 and Ibrahim et al.38 The values obtained following the SBS and microhardness tests were analyzed using the one-way analysis of variance, followed by the Bonferroni’s test for multiple comparisons. The ARI score was analyzed using the Kruskal–Wallis test. Data were analyzed using a statistical software (R language program, R Foundation for Statistical Computing, Vienna, Austria). The level of significance was set at p = 0.05.
Results
SEM
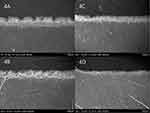
The surface morphologies of the enamel layers in each group are shown in Figure 3. The specimens of demineralized enamel presented significant fish scale-like patterns on the surface, which exhibited fewer mineral particles on the enamel core but retained the periphery (Figure 3B), compared to the specimens in the control group, which showed the sound enamel with a smooth surface (Figure 3A). Groups treated with CPICs (Figure 3C–E) showed an improvement in the surface, with the formation of rod-like crystallites in a certain direction of growth. As the application time of CPICs increased, the pattern on the enamel surface became more pronounced. The size of the “one fish scale” was similar among all groups (Figure 3Ai–Ei).
The vertical surface morphologies of the enamel layers were also examined. Figure 4 shows the SEM image of each group of specimens in the vertical direction. The specimens of the demineralized group (Figure 4A) showed a noticeable thickness of the demineralized layer compared with that in other groups. Groups treated with the CPIC solution (Figure 4B–D) demonstrated a decrease in the thickness of the demineralized layer. A noticeable change in the thickness of the demineralized surface was observed as the CPIC solution treatment time increased.
Figure 5 shows the SEM images at magnifications of 2000X and 100,000X for each group, along with the EDX analysis results. To simulate a carious lesion, the enamel was stored in a demineralizing solution. Compared with the sound enamel (Figure 5A), the specimens stored in the demineralizing solution (Figure 5B) showed a distinctive loss of enamel rods. Treatment with CPIC solution for 30, 60, and 90s showed the formation of rod-like crystallites in certain directions (Figure 5C–E). EDX analysis revealed that the enamel was composed of Ca and P before and after CPIC solution treatment. The Ca/P ratio was the lowest with demineralization solution treatment, whereas the highest ratio was observed in groups treated with CPIC solution for 60s. CPIC solution treatment for 30, 60, and 90s showed significant improvement in the Ca/P ratio compared with that in the control group.
Microhardness Test
The microhardness values of the Vickers hardness for each group are summarized in Table 2. The specimens in the control group showed the highest value (335.78 ± 14.38). The demineralized group showed the lowest value (41.24 ± 15.03), indicating that the demineralization process decreased the hardness of the enamel surface. Specimens treated with the CPIC solutions had values between those of the control and demineralized groups. The microhardness of CPIC-treated specimens for 30, 60, and 90s were (94.94 ± 18.29), (156.68 ± 13.24), and (172.80 ± 21.30), respectively. The hardness of the enamel improved as the application time of the CPIC solution increased.
 |
Table 2 The Vickers Hardness (Hv) Value of the Experimental Groups |
Micro-CT Test
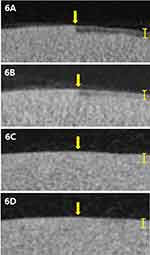
Figure 6 shows the micro-CT images of each experimental group. The control group, with the sound enamel covered with varnish, is on the left side of the yellow arrow (↓) shown in the image. Compared to the sound enamel, the right side of the yellow arrow shows the enamel layer of each experimental group. The surface of the demineralized enamel is more radiolucent than that of the sound enamel in image (A).36 As the CPIC solution treatment time increased, the radiolucency of the enamel decreased. When specimens were treated with CPICs for more than 60s, the enamel surface was as dense as the sound enamel.37,39
SBS Test
The results of the SBS tests are summarized in Table 3. According to the post hoc analysis, only the SBS value of the control group and the remaining groups (demineralized group, CPICs 30, 60, 90s) showed significance (p<0.001). The control group exhibited the highest bonding strength (18.41 ± 8.27 MPa, p = 0.02), while the demineralized group exhibited the lowest bonding strength (7.99 ± 3.73 MPa, p < 0.001). The specimens treated with CPICs for 90s showed an SBS value of 12.66 ± 7.39 MPa, which was higher than the value of the demineralized group but lower than that of the control. As the application time of the CPIC solution increased to 30, 60, and 90s, the SBS value also increased, as follows: 10.16 ± 6.03, 11.40 ± 4.99, and 12.66 ± 7.39 MPa, respectively. The comparison within CPIC solution treated groups revealed no significance statistically.
 |
Table 3 Shear Bond Strength (SBS) Values in MPa of the Experimental Groups |
ARI Score
The distribution of the ARI scores of the orthodontic brackets on the debonded surface is shown in Figure 7 and in Table 4. Each group had different debonding patterns; however, an ARI score of 3 was the most frequent. This indicated that all the adhesives remained on the enamel surface with a distinct impression of the bracket mesh.40 The frequency of the ARI score of 3 was the highest in the control group and lowest in the demineralized group. Application of the CPIC solution resulted in an increase in the frequency of the ARI score of 3. An ARI score of 0 was frequently observed in the demineralized group and the group treated with CPICs for 90s. Kruskal–Wallis test in Table 4 revealed p-value of 0.238 which revealed no significance in the distribution patterns between five groups. The ARI score distribution pattern in each group and in between groups showed no significance.
 |
Table 4 Adhesive Remnant Index (ARI) Score Analysis of the Experimental Groups |
 |
Figure 7 Percentage distribution of the adhesive remnant index (ARI) score of experimental groups. |
Discussion
In this study, we demonstrated an enamel remineralization approach with CPIC solution treatment. Treatment with CPICs showed remineralization on the enamel surface, which improved its structural and mechanical characteristics. This approach may be a promising strategy for enamel remineralization that may overcome the limitations of the current fluoride/non-fluoride-mediated treatment of WSLs.
In a previous study, CPIC solution treatment was effective on demineralized enamel surfaces. However, the application time of the CPIC solution to the enamel was only 48 h to allow calcium phosphate to crystallize on the enamel surface.41 This was not suitable for clinical use considering the length of time patients would spend in the dental chairs. In this study, we reduced the application time of the CPIC solution to 30, 60, and 90s. The remineralization effect was evaluated using SEM, microhardness, and micro-CT tests. The SBS test and ARI score assessments were used to investigate whether remineralization of the enamel with CPIC treatment improved the bond strength between the orthodontic bracket and enamel to a clinically acceptable level.
The enamel surface was observed as shown in the SEM images. The demineralized enamel exhibited well-aligned enamel rods with fish scale-shaped structures. Groups treated with CPICs (Figure 3C–E) showed improvement in the enamel surface. Enamel rods were epitaxially grown in all CPIC solution-treated groups, regenerating the hierarchical enamel microstructure. These results indicated remineralization of the enamel surface by the CPIC solution. The remineralization of the enamel was observed with the epitaxial growth of enamel rods in a certain direction, which is consistent with the results of previous studies.15,42 As the CPIC solution application time increased, the remineralization pattern of the enamel surface clarified. This indicates that the remineralization process of enamel proceeds in seconds, and that a period of 30s can show a significant difference.
A microhardness test was performed to measure the change in the enamel surface hardness after demineralization and remineralization. In this study, the test was used to evaluate the hardness of the sound, decalcified, and CPIC solution-treated enamel. The hardness of the demineralized enamel was significantly lower than that of the sound enamel. After applying the CPIC solution, the hardness value increased significantly; however, it was less than that of the sound enamel. These results indicate that the CPIC solution has great potential for repairing enamel erosion. In a previous study, the application of a CPIC solution to demineralized enamel improved the microhardness similar to that of the sound enamel.41 However, the study did not mention the duration of the CPIC solution treatment. Therefore, we can assume that an application time of more than 90s is needed for effective repair of the etched enamel. Based on the increase in microhardness with treatment time, longer application of CPICs may result in a harder enamel layer.
The micro-CT images showed a difference between the sound and demineralized enamel layers. The control group showed no radiolucency on the enamel surface with or without varnish. Radiolucency, which can be interpreted as demineralized enamel thickness, decreased gradually as the CPICs were applied for longer periods. This result corresponds with that of an earlier study, which reported that the remineralization period resulted in a lower radiolucent density of the enamel surface.36 Based on the results of 30, 60, and 90s of CPIC treatment, the application of CPIC solution for 60s is suggested for clinical use, because only the 60s treatment resulted in a hardness similar to that with 90s treatment with the consideration of SBS.
Remineralization of the enamel surface using CPICs significantly increased the SBS compared with that of the demineralized enamel. The bonding strength increased in proportion to the duration of the CPIC application. This can be interpreted as CPICs of a few nanometers acting as stable building blocks for ACP and HAP. An increase in stability through the removal of small organic molecules such as TEA induces ACP to assemble with HAP crystals, leading to the epitaxial growth of the enamel. This generates a precisely continuous enamel structure that promotes the ideal repair of demineralized enamel structures.15 The range of the bonding strength of metal brackets using Transbond XT was 15.49± 3.28 MPa,43 while it was 12.66 ± 7.39 MPa using CPIC solution for 90s. Therefore, applying a CPIC solution for more than 90s is recommended to achieve acceptable hardness and bond strength in clinical practice.
The ARI score was measured using an endodontic microscope. In all groups, a score of 3 was the most frequently obtained score, as all adhesives remained on the teeth with a distinct impression of the bracket mesh. As the application time increased, the frequency of the ARI score of 3 also increased. The debonding pattern based on the ARI score represents the vulnerability of the exposed enamel to demineralization. When the bracket is removed from the enamel for orthodontic treatment, the enamel surface is peeled off along with the bracket mesh, and its prism is exposed, reducing the resistance of the enamel to acids and demineralization.40 A higher ARI score indicates that the adhesives remain on the surface of the enamel, which is invulnerable to adverse environments. Compared with the demineralized group, treatment with the CPIC solution resulted in an increase in frequency of the score 3. Therefore, we can conclude that treatment with a CPIC solution may increase the bond strength and stability at the resin–enamel interface, which decreases the exposure of the enamel surface after bracket debonding.
The CPICs have an average size of 2 nm.41 Ultrasmall nanoclusters can easily aggregate; however, TEA prevents CPIC aggregation.15,41 In a previous FTIR spectrum analysis of CPICs, complete evaporation of ethanol and TEA resulted in pure calcium phosphate, which acts as a frontier to remineralize the enamel.41
EDX analysis indicated the composition ratio of the CPIC solution: Ca (35.18 wt%), P (23.16 wt%), and oxygen (41.66 wt%).41 Following repair with calcium phosphate nanoclusters, the elemental composition of the enamel included Ca (36.82 wt%), P (20.61 wt%), and oxygen (42.57%).44 In this study, the EDX analysis data coincided with those of previous studies, which showed that the enamel was mainly composed of Ca and P before and after CPIC treatment.42,44,45 The improvement in the Ca/P ratio supports the remineralization of demineralized enamel and structural enamel repair using a calcium phosphate solution. Because the Ca/P ratio improved with CPIC application, remineralization of enamel using CPICs is a promising approach for treating the early stages of tooth erosion.44
It has been discussed in a previous study that pH and the Ca/P ratio are important factors for the nucleation of calcium phosphate.44 CPICs have a Ca/P ratio of 1.52, which is similar to that of the Posner’s cluster (Ca9(PO4)6), with a Ca/P ratio of 1.5 in solution of pH 11–13.44 It can be assumed that a pH of 11–13 is appropriate for the formation of stable calcium phosphate clusters. TEA has a pKa value of 10.75, which forms a basic pH environment that assists remineralization by stabilizing calcium phosphate clusters. However, the use of excessive amounts may be detrimental. Owing to its toxicity, the permitted daily exposure to TEA is 62.5 mg/day, and it is classified as class 3 according to the International Council for Harmonisation, Q3C (R6).46 Although TEA is completely removed when CPICs induce ACP formation during ethanol evaporation, there are still concerns about its detrimental effects on human teeth. Therefore, further research is needed to evaluate alternative materials that are safe for enamel remineralization.
This study had some limitations. CPIC solution treatment was applied to the demineralized enamel for limited periods of 30–90s. Further research is required to determine the optimal time to achieve the greatest bonding integrity and stability of the enamel layer. In addition, the correlation between CPIC application time and enamel color change should be investigated.
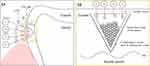
Figure 8 shows a schematic representation of remineralization on enamel surface induced by a CPIC solution. Early carious lesions, represented as WSLs, demineralize the enamel matrix, resulting in a decrease in the Ca/P ratio of the enamel. Application of the CPIC solution improves the Ca/P ratio of the enamel matrix and decreases the radiolucency of the enamel surface. These results coincide with the vertical SEM results presented in Figure 4, where the thickness of the demineralized enamel decreased with the application of the CPIC solution. The decalcified lesion is known to be invisible until it reaches a depth of approximately 400 µm when light diffraction is adequate to be visualized as a WSL.47 The CPIC solution likely infiltrates below the depth of the early carious lesions. The CPIC solution infiltrates the body of the carious lesion and supplies calcium phosphate ions that act as frontiers for epitaxial growth in the enamel rods. The increase in the Ca/P ratio of the enamel suggests that applying CPIC solution modifies the enamel matrix structure and improves the mechanical properties of the enamel. By applying the CPIC solution, we demonstrated an enamel remineralization approach that may be a promising strategy for biomimetic enamel remineralization.
Conclusion
This study demonstrated the effect of CPICs on demineralized enamel surfaces and their effects on bracket shear bond strength. The application of the CPIC solution improved the SBS, microhardness, micro-CT features, and ARI score. As the application time of the CPIC solution increased, the bonding ability and stability improved. This was visualized in SEM images as epitaxial growth in the demineralized enamel matrix and an increase in the Ca/P ratio after CPIC treatment. The application of CPIC solutions on demineralized enamel surfaces suggests a novel preclinical method that overcomes the limitations of the current fluoride/non-fluoride-mediated treatment of WSLs. We demonstrated an enamel remineralization approach through applying CPIC solution, which may be a promising strategy for biomimetic enamel remineralization.
Abbreviations
ACP, amorphous calcium phosphate; ARI, adhesive remnant index; CPICs, calcium phosphate ion clusters; EDX, energy-dispersive spectroscopy; HAP, hydroxyapatite; Micro-CT, micro-computed tomography; SBS, shear bond strength; SEM, scanning electron microscopy; TEA, triethylamine; WSLs, white spot lesions.
Ethics Approval and Informed Consent
Informed consent was obtained from the patients following the guidelines approved by the ethical committee. This study was reviewed and approved by Institutional Review Board of Pusan National University Dental Hospital (PNUDH-2022-03-003-002).
Funding
This study was supported by Biomedical Research Institute Grant (20230021), Pusan National University Hospital.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ogaard B, Rølla G, Arends J. Orthodontic appliances and enamel demineralization. Part 1. Lesion development. Am J Orthod Dentofacial Orthop. 1988;94(1):68–73. doi:10.1016/0889-5406(88)90453-2
2. Gorelick L, Geiger AM, Gwinnett AJ. Incidence of white spot formation after bonding and banding. Am J Orthod. 1982;81(2):93–98. doi:10.1016/0002-9416(82)90032-x
3. Al Mulla H, Al Kharsa S, Kjellberg H, et al. Caries risk profiles in orthodontic patients at follow-up using Cariogram. Angle Orthod. 2009;79(2):323–330. doi:10.2319/012708-47.1
4. Bishara SE, Ostby AW. White spot lesions: formation, prevention, and treatment, Semin. Orthod. 2008;14(3):174–182. doi:10.1053/j.sodo.2008.03.002
5. Hayashi M, Momoi Y, Fujitani M, et al. Evidence-based consensus for treating incipient enamel caries in adults by non-invasive methods: recommendations by GRADE guideline. Jpn Dent Sci Rev. 2020;56(1):155–163. doi:10.1016/j.jdsr.2020.09.005
6. Bijle MN, Abdalla MM, Ashraf U, et al. Enamel remineralization potential of arginine-fluoride varnish in a multi-species bacterial pH-cycling model. J Dent. 2021;104:103528. doi:10.1016/j.jdent.2020.103528
7. Fan Y, Sun Z, Moradian-Oldak J. Controlled remineralization of enamel in the presence of amelogenin and fluoride. Biomaterials. 2009;30:478–483. doi:10.1016/j.biomaterials
8. Grohe B, Mittler S. Advanced non-fluoride approaches to dental enamel remineralization: the next level in enamel repair management. Biomater Biosyst. 2021;4:100029. doi:10.1016/j.bbiosy.2021.100029
9. Koulourides T, Reed JL. Effects of calcium, phosphate and fluoride ions on the rate of softening and dissolution of tooth enamel. Arch Oral Biol. 1964;9:585–594. doi:10.1016/0003-9969(64)90022-6
10. Zohoori FV, Maguire A. Are there good reasons for fluoride labelling of food and drink. Br Dent J. 2018;224(4):215–217. doi:10.1038/sj.bdj.2018.123
11. Ball IA. The ‘fluoride syndrome’: occult caries? Br Dent J. 1986;160(3):75–76. doi:10.1038/sj.bdj.4805769
12. Abbassy MA. Fluoride influences nickel-titanium orthodontic wires’ surface texture and friction resistance. J Orthod Sci. 2016;5(4):121–126. doi:10.4103/2278-0203.192114
13. Fan Y, Wen ZT, Liao S, et al. Novel amelogenin-releasing hydrogel for remineralization of enamel artificial caries. J Bioact Compat Polym. 2012;27(6):585–603. doi:10.1177/0883911512458050
14. Mukherjee K, Ruan Q, Nutt S, et al. Peptide-based bioinspired approach to regrowing multilayered aprismatic enamel. ACS Omega. 2018;3(3):2546–2557. doi:10.1021/acsomega.7b02004
15. Shao C, Jin B, Mu Z, et al. Repair of tooth enamel by a biomimetic mineralization frontier ensuring epitaxial growth. Sci Adv. 2019;5(8):eaaw9569. doi:10.1126/sciadv.aaw9569
16. Yu HP, Zhu YJ, Lu BQ. Dental enamel-mimetic large-sized multi-scale ordered architecture built by a well controlled bottom-up strategy. Chem Eng J. 2019;360:1633–1645. doi:10.1016/j.cej.2018.11.025
17. Kim H, Choi A, Gong MK, et al. Effect of remineralized collagen on dentin bond strength through calcium phosphate ion clusters or metastable calcium phosphate solution. Nanomaterials. 2020;10(11):2203. doi:10.3390/nano10112203
18. Paik Y, Kim JH, Yoo KH, et al. Dentin biomodification with flavonoids and calcium phosphate ion clusters to improve dentin bonding stability. Materials. 2022;15(4):1494. doi:10.3390/ma15041494
19. Ruan Q, Zhang Y, Yang X, Nutt S. An amelogenin-chitosan matrix promotes assembly of an enamel-like layer with a dense interface. Acta Biomater. 2013;9:7289–7297. doi:10.1016/j.actbio.2013.04.004
20. Zhu Y, Yan J, Mujtaba BM, Li Y, Wei H, Huang S. The dual anti-caries effect of carboxymethyl chitosan nanogel loaded with chimeric lysin ClyR and amorphous calcium phosphate. Eur J Oral Sci. 2021;129:e12784. doi:10.1111/eos.12784
21. Hua F, Yan J, Zhao S, Yang H, He H. In vitro remineralization of enamel white spot lesions with a carrier-based amorphous calcium phosphate delivery system. Clin Oral Investig. 2020;24:2079–2089. doi:10.1007/s00784-019-03073-x
22. Pei D, Liu S, Huang C, Du X, Yang H, Wang Y. Effect of pretreatment with calcium-containing desensitizer on the dentine bonding of mild self-etch adhesives. Eur J Oral Sci. 2013;121:204–210. doi:10.1111/eos.12047
23. Chandavarkar SM, Ram SM. A comparative evaluation of the effect of dentin desensitizers on the retention of complete cast metal crowns. Contemp Clin Dent. 2015;6:S45–S50. doi:10.4103/0976-237x.152937
24. Yang H, Chen Z, Yan H, Huang C. Effects of calcium-containing desensitizers on the bonding stability of an etch-and-rinse adhesive against long-term water storage and pH cycling. Dent Mat J. 2018;37:122–129. doi:10.4012/dmj.2017-006
25. Wang Z, Ouyang Y, Wu Z, et al. A novel fluorescent adhesive-assisted biomimetic mineralization. Nanoscale. 2018;18:18980–18987. doi:10.1039/C8NR02078G
26. Utneja S, Talwar S, Nawal R, et al. Evaluation of remineralization potential and mechanical properties of pit and fissure sealants fortified with nano-hydroxyapatite and nano-amorphous calcium phosphate fillers: an in vitro study. J Conserv Dent. 2018;21:681–690. doi:10.4103/jcd.jcd_31_18
27. Schemehorn BR, Wood GD, McHale W, Winston AE. Comparison of fluoride uptake into tooth enamel from two fluoride varnishes containing different calcium phosphate sources. J Clin Dent. 2011;22(2):51–54.
28. Margolis HC, Moreno EC, Murphy BJ. Effect of low levels of fluoride in solution on enamel demineralization in vitro. J Dent Res. 1986;65(1):23–29. doi:10.1177/00220345860650010301
29. Theuns HM, van Dijk JW, Driessens FC, et al. Effect of time, degree of saturation, pH and acid concentration of buffer solutions on the rate of in-vitro demineralization of human enamel. Arch Oral Biol. 1985;30(1):37–42. doi:10.1016/0003-9969(85)90022-6
30. Poorni S, Kumar RA, Ramachandran S, et al. Effect of 10% sodium ascorbate on the calcium: phosphorus ratio of enamel bleached with 35% hydrogen peroxide: an in vitro quantitative energy-dispersive X-ray analysis. Contemp Clin Dent. 2010;1:223–226.
31. Das B, Muthu MS, Farzan JM. Comparison of the chemical composition of normal enamel from exfoliated primary teeth and teeth affected with early childhood caries: an in vitro study. Int J Paediatr Dent. 2016;26:20–25.
32. Linjawi AI, Abbassy MA. Comparison of shear bond strength to clinically simulated debonding of orthodontic brackets: an in vitro study. J Orthod Sci. 2016;5(1):25–29. doi:10.4103/2278-0203.176655
33. Farooq I, Ali S, Farooqi FA, et al. Enamel remineralization competence of a novel fluoride-incorporated bioactive glass toothpaste—a surface micro-hardness, profilometric, and micro-computed tomographic analysis. Tomography. 2021;7(4):752–766. doi:10.3390/tomography7040063
34. Song J, Li T, Gao J, et al. Building an aprismatic enamel-like layer on a demineralized enamel surface by using carboxymethyl chitosan and lysozyme-encapsulated amorphous calcium phosphate nanogels. J Dent. 2021;107:103599. doi:10.1016/j.jdent.2021.103599
35. Cui FZ, Ge J. New observations of the hierarchical structure of human enamel, from nanoscale to microscale. J Tissue Eng Regen Med. 2007;1(3):185–191. doi:10.1002/term.21
36. Nakata K, Nikaido T, Nakashima S, et al. An approach to normalizing micro-CT depth profiles of mineral density for monitoring enamel remineralization progress. Dent Mater J. 2012;31(4):533–540. doi:10.4012/dmj.2011-228
37. Hong SC, Lee DY, Kim YJ. Micro-computed tomographic evaluation of the effect of fluoride agents on white spot lesions: an in vitro study. Korean J Orthod. 2022;52(1):75–79. doi:10.4041/kjod.2022.52.1.75
38. Ibrahim AI, Thompson VP, Deb S. A novel etchant system for orthodontic bracket bonding. Sci Rep. 2019;9:9579. doi:10.1038/s41598-019-45980-9
39. Zhang X, Li Y, Sun X, et al. Biomimetic remineralization of demineralized enamel with nano-complexes of phosphorylated chitosan and amorphous calcium phosphate. J Mater Sci Mater Med. 2014;25(12):2619–2628. doi:10.1007/s10856-014-5285-2
40. Pont HB, Özcan M, Bagis B, et al. Loss of surface enamel after bracket debonding: an in-vivo and ex-vivo evaluation. Am J Orthod Dentofacial Orthop. 2010;138(4):387e1–387e9. doi:10.1016/j.ajodo.2010.01.028
41. Wang CH, Mutalik C, Yougbaré S, et al. Calcium phosphate nanoclusters for the repair of tooth enamel erosion. Nanomaterials. 2022;12:1997. doi:10.3390/nano12121997
42. White SN, Luo W, Paine ML, et al. Biological organization of hydroxyapatite crystallites into a fibrous continuum toughens and controls anisotropy in human enamel. J Dent Res. 2001;80:321–326. doi:10.1177/00220345010800010501
43. Hellak A, Rusdea P, Schauseil M, et al. Enamel shear bond strength of two orthodontic self-etching bonding systems compared to Transbond™ XT. J Orofac Orthop. 2016;77:391–399. doi:10.1007/s00056-016-0046-0
44. Chen H, Lv C, Guo L, et al. Surface stability and morphology of calcium phosphate tuned by pH values and lactic acid additives: theoretical and experimental study. ACS Appl Mater Interfaces. 2022;14(4):4836–4851. doi:10.1021/acsami.1c18727
45. Kim H, Yoo KH, Yoon SY, et al. A remineralizing orthodontic etchant that utilizes calcium phosphate ion clusters. Front Bioeng Biotechnol. 2022;10:944869. doi:10.3389/fbioe.2022.944869
46. ICH. Impurities: guideline for residual solvents Q3C (R6). In:
47. Roberts WE, Mangum JE, Schneider PM. Pathophysiology of demineralization, Part II: enamel white spots, cavitated caries, and bone infection. Curr Osteoporos Rep. 2022;20:106–119. doi:10.1007/s11914-022-00723-0
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.