Back to Journals » Infection and Drug Resistance » Volume 7
The effect of a unique propolis compound (PropoelixTM) on clinical outcomes in patients with dengue hemorrhagic fever
Authors Soroy L, Bagus S, Yongkie I, Djoko W
Received 21 July 2014
Accepted for publication 27 August 2014
Published 9 December 2014 Volume 2014:7 Pages 323—329
DOI https://doi.org/10.2147/IDR.S71505
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Lardo Soroy,1 Sulistyo Bagus,2 Iswandi Purnama Yongkie,1 Wibisono Djoko2
1Division of Tropical Medicine and Infectious Disease, Department of Internal Medicine, 2Research Committee, Gatot Soebroto Central Army Hospital, Jakarta, Indonesia
Background: Dengue fever is a mosquito-borne virus belonging to the family Flaviviridae. It is an old virus that has re-emerged globally over the past 20 years and now causes a global burden of 50 million infections per year across approximately 100 countries. Despite this, there is no safe vaccine available, and therapy is largely supportive. Its pathogenesis is multifaceted and currently still poorly understood, leading to a lack of disease-specific therapy. Propolis is a natural antiviral and anti-inflammatory product derived from the saps of plants and mixed with the saliva of honeybees. Propoelix™ is a uniquely potent and water-soluble extract of propolis containing high concentrations of anti-inflammatory compounds like caffeic acid phenethyl ester.
Objective: The primary objective is to determine the effectiveness of a unique propolis extract (Propoelix™) on the clinical course of patients with dengue hemorrhagic fever (DHF). The secondary objective is to examine the effect of Propoelix™ on tumor necrosis factor-α (TNF-α) levels in patients with DHF.
Methods: A double-blind, randomized, placebo-controlled trial was conducted at the Department of Internal Medicine, Gatot Soebroto Central Army Hospital in Jakarta, Indonesia, from May 2012 to July 2013. Sixty-three patients who met the inclusion criteria were enrolled in the trial. Patients were randomized to receive either two capsules of Propoelix™ 200 mg three times a day or placebo daily for 7 days. Clinical and laboratory variables of both groups, including the anti-inflammatory marker TNF-α, were investigated. Patients were deemed technically fit for discharge if their platelet counts had recovered and exceeded 100,000/µL but were all observed as inpatients for 7 days.
Results: There were 31 patients in the Propoelix™ treatment group and 32 patients in the placebo group. Platelet counts in the Propoelix™-treated group showed a trend toward a faster recovery by day 3 of admission and became statistically significant by day 6 (101.42±48.79 vs 80.78±43.35 [103/mL], P=0.042) and day 7 (146.67±64.68 vs 107.84±57.22 [103/mL], P=0.006). Patients treated with Propoelix™ had a significantly greater decline in TNF-α levels on day 7 of therapy compared with patients in the placebo group (P=0.018). They also had a significantly shorter length of hospitalization compared with those in the placebo group (4.69±0.78 days vs 5.46±1.16 days, P=0.012).
Conclusion: Propoelix™ appears to hasten the improvement in platelet counts and TNF-α levels and shortens the duration of hospitalization in patients with DHF.
Keywords: effectiveness, Propoelix™, DHF
Introduction
Dengue is an acute febrile disease caused by the mosquito-borne dengue viruses (DENVs), consisting of four serotypes (DENV 1–4) that are members of the Flaviviridae family, genus flavivirus. The classical form of dengue fever (DF) is characterized by high fever, headache, stomach ache, rash, myalgia, and arthralgia. Severe dengue, dengue hemorrhagic fever (DHF), and dengue shock syndrome (DSS) are accompanied by thrombocytopenia, vascular leakage, and hypotension. DSS, which can be fatal, is characterized by systemic shock.
Dengue has become a major international health problem. In the last 3 years, the incidence of DF, DHF, and DSS has increased dramatically. The average annual number of DF/DHF cases reported to the World Health Organization (WHO) has grown every 10 years. From 1990 to 1999, there were 479,848 cases reported, and from 2000 to 2008, it grew to 1,656,870 cases, a 3.5-fold increase. It is currently estimated that 50–100 million cases are reported annually out of 2.5 billion people.1–4 In Indonesia, dengue has become a public health problem for the last 41 years. It was first described in Surabaya in 1968. From 1968 to 2009, there has been an increase in the number of dengue-endemic areas from two provinces and two cities to 32 provinces and 382 cities. In 2006, there were 113,640 cases of dengue resulting in 1,184 death cases. In 2007, Gatot Soebroto Central Army Hospital in Jakarta, Indonesia, treated 885 cases of DHF and 17 cases of DSS. The increase and spread of dengue cases are likely due to multiple factors, including urbanization, climate change, population distribution, and density change.
Despite intensive research, the underlying mechanisms causing severe dengue are still not well understood. The immunopathogenesis of DENV infection is likely to be a complex interplay of host-specific immune responses, including immune cell activation and the release of cytokines (interleukin [IL]-1β, IL-2, IL-6, IL-10, IL-13, and IL-18; macrophage migration inhibitory factor, tumor growth factor-β, tumor necrosis factor-α [TNF-α], and interferons [IFNs]) and chemokines (IL-8, monocyte chemoattractant protein-1).5–7 Of these, TNF-α has been shown to be associated with increased disease severity. Other important factors in the immunopathogenesis of DENV infection include complement activation, the production of inflammatory mediators, and autoimmunity.8,9
The cause of thrombocytopenia in dengue patients has also not been completely elucidated; its occurrence could be due to increased consumption, decreased production, or direct engagement of virus with platelets.8 To date, no specific therapy has been shown to alter the clinical course of DHF, and management has largely been supportive.
Propolis is a natural product derived from plant resins collected by honeybees. It is used as a sealant in the beehive, preventing diseases and parasites from entering the hive and to inhibit putrefaction and fungal and bacterial growth. It has been used since 300 BC by the Greeks, Romans, and Egyptians for its healing and anti-inflammatory properties. In temperate climates all over the world, poplar bud exudates (mainly of Populus Nigra L.) have been shown to be the main source of collected resin. Poplar propolis has approximately 50 constituents, primarily resins and vegetable balsams (50%), waxes (30%), essential oils (10%), and pollen (5%).10 The main biologically active compounds are found in the balsamic content and include polyphenolic compounds (caffeic acid phenethyl ester [CAPE]), flavonoids (chrysin, catechin, galangin), stilbene derivatives (resveratrol), and fatty acids. These compounds, in particular CAPE and flavonoids, have been shown to have potent anti-inflammatory and immunomodulating activity in vitro. CAPE significantly inhibits cytokine and lymphokine production, including TNF-α, IL-2, IL-10, IL-12, and IFN, and inhibits T-cell proliferation.11–13
Propoelix™ is a superextraction of poplar propolis performed using a unique extraction process that removes inert excipients (eg, resins), leaving behind the active ingredients in a uniquely water-soluble form. A previous pilot study that was single-blind, randomized, and placebo-controlled involving 92 patients with DHF (n=31 in placebo group, n=61 in treatment group) showed that Propoelix™ significantly improved platelet counts on day 4 of fever (platelets 95,533±39,841/μL in treatment group vs 76,302±58,812/μL in placebo group, P=0.027). Propoelix™ also shortened the mean length of stay (4.66±0.75 days in treatment group vs 5.52±1.34 days in placebo group, P<0.01).
The primary end point of this study was to confirm the clinical impact of Propoelix™ on the clinical course in patients diagnosed with DHF in the setting of a double-blind, randomized, placebo-controlled trial. The secondary end point of this study was to investigate TNF-α levels in the treatment and placebo groups on day 1 and day 7 of the study.
Materials and methods
Clinical trial study design
This study was a randomized, double-blind, placebo-controlled clinical trial. The inclusion criteria were a diagnosis of DHF based on WHO 1997 criteria in patients aged between 18 and 45 years who were admitted to the general ward of Gatot Soebroto Central Army Hospital in Jakarta between 30 May 2012 and 30 July 2013. Patients diagnosed with DSS or who had severe thrombocytopenia <20,000/μL requiring platelet transfusions were excluded from the study. Those with an allergy to bee products or who had other comorbid diseases such as diabetes, hypertension, renal failure, or cancer were also excluded from the study. Sixty-three consecutive patients who fulfilled the study criteria were enrolled. Patients were randomly allocated to the Propoelix™ group or placebo using a table of random numbers. Propoelix™ (200 mg per capsule) was given at a dose of two capsules three times a day and the placebo was administered as an externally identical tablet at the same frequency for 7 days. Managing physicians were blinded and were allowed to decide if patients were technically suitable for discharge based on their platelet counts (improving and exceeding 100,000/μL) and clinical status. However, all patients were observed for 7 days as inpatients.
The study was approved by the Ethics Committee Gatot Soebroto Central Army Hospital, Jakarta, Indonesia institutional human research committee (Human Ethical Committee) for research on human subjects, and written informed consent was obtained from all patients.
Investigations
All patients had baseline clinical parameters (including temperature, blood pressure, heart rate, body weight) and laboratory investigations (full blood count [FBC] including hematocrit, platelet count, and leukocyte count; liver function test and renal function test; and dengue serology [immunoglobulin {Ig}M and IgG]) performed upon inclusion into the study. FBC was repeated daily during hospitalization until day 7 of hospitalization. Liver function and renal function tests were repeated on day 7. Serum TNF-α concentrations were determined using the commercially available enzyme-linked immunosorbent assay kit supplied by R&D Systems Inc. (Minneapolis, MN, USA). The assay employs the quantitative sandwich enzyme immunoassay technique using recombinant human TNF-α, with antibodies raised against the recombinant protein. The minimum detectable level of the assay was 0.106 pg mL−1. Optical density was read with a microtiter plate reader by dual wavelength at 490 nm. All samples were assayed on day 1 of the study and repeated on day 7.
Statistical analysis
An earlier pilot study showed that Propoelix™ significantly improved platelet counts on day 4 of fever (platelets 95,533±39,841/μL in treatment group vs 76,302±58,812/μL in placebo group, P=0.027) and also shortened the mean length of stay (4.66±0.75 days in treatment group vs 5.52±1.34 days in placebo group, P<0.01). Given a type 1 error of 0.05 (two-sided) and a power of 0.9, we estimated the necessary sample size to be 27 in each group. Results were analyzed using chi-square exact test for proportions and the Student’s t-test for quantitative variables. Data are expressed as mean ± standard deviation.
Results
Pretreatment findings
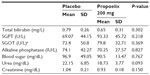
There were 32 patients in the placebo arm and 31 patients in the Propoelix™ arm who were enrolled into the study. All patients completed the study. There were no significant differences in the demographics, clinical characteristics, or baseline laboratory evaluations between both groups (Table 1).
Treatment findings
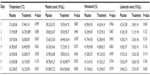
All patients had their renal and liver function tests repeated on day 7 of admission. There were no significant differences observed between the placebo and Propoelix™ arms (Table 2). Daily FBC results from day 1 to day 7 are charted in Table 3.
  | Table 3 Comparison of hematological indices from day 1 to day 7 of admission between patients on placebo and those treated with Propoelix™ |
Effect on platelets
There was no significant difference in platelet counts between the group treated with PropoelixTM and placebo on day 1 of admission. However, by day 3, platelet counts in the Propoelix™-treated group showed a trend toward a faster recovery (Figure 1). This became statistically significant by day 6 (101.42±48.79 vs 80.78±43.35 [103/mL], P=0.042) and day 7 (146.67±64.68 vs 107.84±57.22 [103/mL], P=0.006).
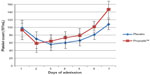  | Figure 1 Comparison of platelet counts in the Propoelix™-treated group versus placebo. |
Effect on hematocrit and leukocytes
There was no significant difference in hematocrit and leukocyte counts between patients in the group treated with Propoelix™ and placebo (Table 3).
Effect on length of hospitalization
Patients treated with Propoelix™ had a significantly shorter length of hospitalization compared with those in the placebo group (4.69±0.78 days vs 5.46±1.16 days, P=0.012) (Table 4).
  | Table 4 Mean difference in the length of hospitalization |
Effect on TNF-α levels on day 1 and day 7 of hospitalization
TNF-α levels were measured on day 1 and day 7 of hospitalization in all patients. Baseline TNF-α levels between both groups were similar on day 1 (7.11±5.85 pg/mL in the treated group vs 4.83±2.89 pg/mL with placebo, P=0.166). On day 7 of hospitalization, TNF-α levels in the treatment group had significantly declined compared with day 1 levels (7.11±5.85 pg/mL vs 3.67±1.69 pg/mL, P=0.019).
However, TNF-α levels in the placebo group on day 7 did not significantly change compared with day 1 levels (4.83± 2.89 pg/mL vs 3.32±1.70 pg/mL, P=0.082) (Figure 2).
  | Figure 2 Comparison of tumor necrosis factor-α (TNF-α) levels in the Propoelix™-treated group versus placebo group on day 1 and day 7 of hospitalization. |
Discussion
Dengue is the main cause of tropical fever among travelers (after malaria) and is the most important mosquito-borne viral disease in the world.2 Despite this, therapy has remained largely supportive, with a fatality rate of almost 20% if DHF progresses to DSS. One possible reason why there is a lack of disease-specific therapy may be our incomplete understanding of the disease pathogenesis and progression. However, recent studies have suggested a complex interplay of host-specific immune responses, including immune cell activation and the release of cytokines such as IL-2, IL-6, IL-10, IL-13, TNF-α, and IFN, with TNF-α possibly being associated with increased disease severity.13
Propolis is a natural product derived from plant resins collected by honeybees. It is a very rich source of polyphenolic compounds (CAPE), flavonoids (chrysin, catechin, galangin), stilbene derivatives (resveratrol), and fatty acids. These compounds, in particular CAPE and flavonoids, have been shown to have potent anti-inflammatory and immunomodulating activity in vitro. CAPE significantly inhibits cytokine and lymphokine production, including IL-2, IL-10, IL-12, TNF-α, and IFN, and inhibits T-cell proliferation.11–13
In this study, we examined the anti-inflammatory effect of Propoelix™, a uniquely potent and water-soluble form of propolis, on DHF. A previous pilot study that was single-blind, randomized, and placebo-controlled showed that Propoelix™ significantly improved platelet counts on day 4 of fever and also shortened the mean length of stay.
In this study, we have confirmed this finding in a rigorously performed, double-blinded, randomized, placebo-controlled trial. Platelet counts in the Propoelix™-treated group showed a trend toward faster recovery by day 3 of treatment and became statistically significant by day 6 (101.42±48.79 vs 80.78±43.35 [103/mL], P=0.042) and day 7 (146.67±64.68 vs 107.84±57.22 [103/mL], P=0.006).
Patients treated with Propoelix™ also had a significantly shorter length of hospitalization compared with those in the placebo group (4.69±0.78 days vs 5.46±1.16 days, P=0.012) (Table 4). Given the current lack of specific therapy for dengue, this may suggest a possible overall disease-modifying effect of Propoelix™. No significant difference, however, was seen in renal function, liver function, hematocrit, or leukocyte counts between both patient groups. Importantly, the treatment was well tolerated.
Since TNF-α levels have been associated with disease severity,13 levels were also measured on day 1 and day 7 of hospitalization. Patients treated with Propoelix™ were found to have a significant decline in TNF-α levels from day 1 to day 7 of hospitalization (7.11±5.85 pg/mL vs 3.67±1.69 pg/mL, P<0.05). This was compared with patients in the placebo group who did not have a significant decline in TNF-α levels. This observation is consistent with a possible anti-inflammatory effect of the compounds found in Propoelix™, although it is preliminary and should be confirmed in a larger study.
Patients with DSS and those with severe thrombocytopenia <20,000/μL requiring platelet transfusions were excluded from the study. Given the significant mortality and morbidity experienced by this group of patients, it would be important to see if Propoelix™ can similarly improve their clinical outcome or even prevent the progression of DHF to DSS.
Conclusion
Patients with DHF treated with Propoelix™ had a significantly faster recovery in platelet count and a shorter length of hospitalization. TNF-α levels were also significantly lower in the treated group. Propoelix™ may be a useful adjunctive therapy in patients with DHF.
Disclosure
The authors report no conflicts of interest in this work.
References
World Health Organization. Comprehensive Guidelines for Prevention and Control of Dengue and Dengue Haemorrhagic Fever: Revised and Expanded Edition. WHO Regional Office for South East Asia; 2010. Available from: http://www.searo.who.int/entity/vector_borne_tropical_diseases/documents/SEAROTPS60/en/. Accessed November 11, 2014. | |
Birnbaumer DM. Fever in the returning traveler. In: Slaven EM, Stone SC, Lopez FA, editors. Infectious Diseases Emergency Department Diagnosis and Management. New York, NY: McGraw-Hill; 2007:418–427. | |
Nathan MB, Drager RD, Guzman M. Epidemiology, burden of disease and transmission. In: Dengue Guidelines for Diagnosis, Treatment, Prevention and Control. New Edition. Geneva, Switzearland: World Health Organization; 2009:3–17. | |
Suharti C. Pathogenesis of dengue hemorrhagic fever and dengue shock syndrome. In: Dengue Hemorraghic Fever in Indonesia: The Role of Cytokines in Plasma Leakage, Coagulation and Fibrinolysis. Nijmegen University Press; 2001:15–23. | |
Villar LA, Centeno, Quijano FAD, Vega RAM. Biochemical alterations as markers of dengue hemorrhagic fever. Am J Trop Med Hyg. 2008;78(3):370–374. | |
Fenglin CF, Chiu SC, Hsiao YL, et al. Expression of cytokine, chemokine, and adhesion molecules endothelial cell activation induced by antibodies against dengue virus nonstructural protein. J Immunol. 2005;174:395–403. | |
Fenglin CF, Lei HY, Shiau AL, et al. Endothelial cell apoptosis induced by antibodies against dengue virus nonstructural protein 1 via production of nitric oxide. J Immunol. 2002;169:657–664. | |
Fenglin CF, Lei HY, Shiau AL, Liu HS, YehTM. Patient and mouse antibodies against dengue virus nonstructural protein 1 cross-react with platelet and cause their dysfunction or depletion. Am J Infect Dis. 2008;169(2):657–664. | |
Lei HY, Huang KJ, Lin YS, Yeh TM, LiuHS. Immunopathogenesis of dengue haemorrhagic fever. Am J Infect Dis. 2008;4(1):1–9. | |
Lotfy M. Biological activity of bee propolis in health and disease. Asian Pac J Cancer Prev. 2006;7:22–31. | |
Borelli F, Maffia P, Pinto L, et al. Phytochemical compounds involved in the anti-inflammatory effect of propolis extract. Fitoterapia. 2002; 73 Suppl 1:S53–S63. | |
Marquez N, Sancho R, Macho A, Calzado MA, Fiebich BL, Munoz E. Caffeicacid phenethyl ester inhibits T-cell activation by targeting both nuclear factor activated T-cell and NF-kB transcription factors. J Pharmacol Exp Ther. 2004;308:993–1001. | |
Chakravarti A, Kumaria R. Circulating levels of tumour necrosis factor-α and interferon-γ in patients with dengue and dengue haemorrhagic fever during an outbreak. Indian J Med Res. 2006; 123:25–30. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.