Back to Journals » International Journal of General Medicine » Volume 16
The Diagnostic Value of Kinetics of NLR to Identify Secondary Pulmonary Bacterial Infection Among COVID-19 Patients at Single Tertiary Hospital in Indonesia
Authors Sumardi U , Valentino B, Prasetya D, Debora J , Sugianli AK
Received 17 April 2023
Accepted for publication 25 July 2023
Published 1 August 2023 Volume 2023:16 Pages 3281—3289
DOI https://doi.org/10.2147/IJGM.S417569
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Uun Sumardi,1,* Bima Valentino,1 Dimmy Prasetya,1 Josephine Debora,1 Adhi Kristianto Sugianli2,*
1Department of Internal Medicine, Hasan Sadikin General Hospital, Faculty of Medicine Universitas Padjadjaran, Bandung, Indonesia; 2Department of Clinical Pathology, Hasan Sadikin General Hospital, Faculty of Medicine Universitas Padjadjaran, Bandung, Indonesia
*These authors contributed equally to this work
Correspondence: Adhi Kristianto Sugianli, Department of Clinical Pathology, Hasan Sadikin General Hospital, Faculty of Medicine Universitas Padjadjaran, Jl. Pasteur No. 38, Bandung, West Java, 40161, Indonesia, Tel +62-22-2033307, Email [email protected]
Purpose: Coronavirus disease 2019 (COVID-19) is a new respiratory tract infection caused by severe acute respiratory syndrome coronavirus-2. The presence of secondary pulmonary bacterial infection (SPBI) made COVID-19 difficult to treat. Neutrophil-lymphocyte count ratio (NLR) is a systemic inflammatory marker used in the diagnosis and prognosis of viral or bacterial infection. At the first 3– 5 days after hyperinflammation, it occurs in relation to clinical outcome. Therefore, this study aimed to evaluate the diagnostic value of NLR based on leukocyte kinetics upon admission and after 72 hours among COVID-19 patients with or without SPBI.
Patients and Methods: This retrospective cross-sectional study analyzed medical records data of admitted patients with COVID-19 according to the International Classification of Disease 10th Revision (ICD-10) between January and December 2021. The list of patients was extracted and followed by a hand search to identify the inclusion or exclusion criteria and stratified into proven and non-proven SPBI based on clinical data. The study distinguished between SPBI by means of a cut-off value (COV) on the first (D1) and third day (D3), assessed using receiver operating characteristics (ROC), as well as determined the magnitude of sensitivity, specificity, and prevalence ratio.
Results: A screening process was conducted on 2902 COVID-19 patients, of which 236 were included, accounting for 8.1%. Among these patients, 87 (36.9%) were found to have proven SPBI. A considerable difference in NLR value between proven and non-proven SPBI was observed on both D1 (11.1 vs 4.2) and D3 (15.3 vs 5.2), with optimal COV of NLR on D1, D3 was found to be 5.29, 9.47, respectively (p < 0.001).
Conclusion: NLR on the D1 and D3 distinguished the occurrence of SPBI among COVID-19 patients. The application of NLR assisted in the early determination of bacterial infection and helped in controlling the empirical use of antibiotics.
Keywords: COVID-19, neutrophil–lymphocyte ratio, NLR, secondary pulmonary bacterial infection
Introduction
Coronavirus disease 2019 (COVID-19) is a new respiratory tract infection caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). It results in a rapid increase in the number of cases and spread throughout the world in a short period.1 As of August 25, 2021, data from the World Health Organization (WHO) recorded approximately 212,357,898 confirmed cases and a total of 4,439,843 deaths. Among these cases, Indonesia reported 4,026,837 confirmed cases with a mortality of 129,293.2
The clinical manifestations of patients ranged from asymptomatic to severe symptoms. The most common symptoms were fever, dry cough, shortness of breath, fatigue, myalgia, nausea, vomiting, or diarrhoea.1 However, patients with SARS-CoV-2 infection were also susceptible to bacterial coinfection.3–5 Previous studies reported increased morbidity and mortality due to secondary bacterial infection.6 The occurrence of secondary pulmonary bacterial infection (SPBI) was underpinned by three mechanisms, namely dysregulation of the immune response, dysbiosis of the respiratory tract microbiota, and damage to respiratory tract epithelial cells.7,8
The management of COVID-19 has been impacted by issues related to SPBI, such as the high rate of hospitalization and prolonged hospital stays, which increase the risk of hospital-acquired infection.9 Difficulties in differentiating between COVID-19 and pulmonary bacterial infection have resulted in antibiotic overuse.10,11 “However, there was an information gap regarding treatment guidelines for COVID-19 with SPBI, which led to poor clinical outcomes. The hyperinflammatory response and rapid progression of COVID-19 were crucial and similar to that of septic patients. Previous studies had shown that the initial 72 hours of critical care in septic patients were crucial for physician decision-making and clinical outcomes.12,13 Therefore, this critical period should also be applied for COVID-19, including the use of inflammatory markers as the diagnostic and prognostic outcome.
Neutrophil–lymphocyte ratio (NLR) is a systemic inflammatory marker used as a determinant of the diagnosis as well as prognosis of patients with a viral or bacterial infection.13–15 Previous study has shown the importance of leukocyte kinetics, which is represented as NLR, in the first 3–5 days after hyperinflammation occurred.13 “As a result, when compared to other inflammatory markers such as Procalcitonin and C-Reactive Protein, NLR proved to be a more rapid, feasible, cost-effective, and affordable marker that could be easily performed in any healthcare facility setting.
Previous study has shown the utility of NLR in the context of COVID-19.16–18 However, the observation of dynamic changes in NLR among COVID-19 patients is still inadequate, including detection time and serial observation, particularly concerning the onset of bacterial infection. Therefore, this study assesses the diagnostic efficacy of NLR based on leukocyte kinetics upon hospital admission and after 72 hours among patients to identify the presence of secondary bacterial infection.
Materials and Methods
Study Design and Population
This retrospective cross-sectional study presents an examination of medical records data on admitted patients who were diagnosed with COVID-19 under the International Classification of Disease 10th Revision (ICD-10) Code U07.1. The study encompasses the period spanning from January to December 2021 and was conducted at tertiary teaching hospital, Hasan Sadikin General Hospital Bandung, Indonesia. The list of patients within a certain period was extracted from hospital information system (Sistem Informasi Rumah Sakit Hasan Sadikin, Bandung Indonesia) and followed by a hand search to identify the inclusion or exclusion criteria, based on clinical information available. The inclusion criteria were (1) adult patients that are 18 years or above, (2) admitted to hospital with any degree of COVID-19 severity,19 (3) submitted sputum specimen for culture, and (4) performed complete blood count and inflammatory marker during hospitalization. Meanwhile, the exclusion criteria were (1) patients with HIV/AIDS, malignancy, autoimmune disease, long-term use of steroids, and urinary tract infection, (2) patients readmitted to hospital within the same period, (3) poor sputum quality, based on Gram stain result which indicated with more than 10 epithelial cells per large visual field, regardless of the number of leukocytes,20 and (4) inaccessible or missing information in the medical record. Finally, laboratory information systems (HCLAB, Sysmex, Asia Pacific) were accessed when information about laboratory results was not available in the medical records of patients.
Data Collection
Baseline demographics, including age, gender, presence of comorbidities, disease severity, NLR value, sputum culture result, and clinical outcome were extracted from the medical records. NLR value was performed using the automatic hematology analyzer with flow cytometry method (XN series, Sysmex, Japan) and was calculated by dividing the absolute value of neutrophil-lymphocytes.21,22 This study collected NLR value on the first day when patients were admitted to hospital/ward (D1), and the third day after hospital admission (D3). The sputum culture was performed for microorganism identification and antimicrobial susceptibility testing, using an automatic microbiology analyzer (Vitek2Compact, Biomerieux, France). The protocol for microorganism identification and antimicrobial susceptibility testing follows the Clinical and Laboratory Standards Institute (CLSI) guidelines.20,23 In cases where multiple sputum culture data were available for the study population, only the initial sputum cultures obtained during hospitalization were collected and included in the data analysis.
Study Variable Definition
SPBI can be characterized as a respiratory infection that manifested in COVID-19 patients, with the following criteria: (1) identification of bacterial growth through sputum cultures, (2) procalcitonin levels exceeding 0.25 ng/mL, and (3) the presence of infiltrates on chest imaging.12,24,25 The study population was stratified into those with and without proven SPBI. Chronic Obstructive Pulmonary Disease (COPD) was characterized as a condition marked by progressive airway limitation that cannot be reversed and was often accompanied by an abnormal inflammatory response of the lungs to harmful gas particles. This was confirmed by spirometry examination, where the results showed a Forced Expiratory Volume 1/Forced Vital Capacity <0.70, after administering a bronchodilator.26 Chronic Kidney Disease (CKD) includes damage or a decreased glomerular filtration rate of <60 mL/min/1.73 m2 for at least 3 months.27 Type 2 Diabetes Mellitus (DM) was defined as an increase in plasma glucose level of more than 126 mg/dL (7.0 mm/L) or HbA1C ≥6.5.28 Meanwhile, hypertension was defined as a blood pressure of ≥140/90 mmHg.29 Multi-Drug Resistance (MDR) pathogens were defined as pathogens that were not susceptible to at least one agent in three or more antimicrobial categories.30
Statistical Analysis
Data were entered into Microsoft Excel 2013 (Microsoft Corp.) and analyzed using the Statistical Product and Service Solutions (SPSS) Windows version 25.0 (IBM, United States). Descriptive statistics, including the mean-standard deviation (SD), median-interquartile range (IQR), and number with percentage, were used as appropriate according to the data distribution of variables. Mean-SD and median-IQR were used for normally and non-normally distributed quantitative data, respectively. The normality of data distribution of variables was determine using Kolmogorov–Smirnov test. Normally and non-normally distributed quantitative variables were compared using the t-test and Mann–Whitney tests, respectively. Meanwhile, the X2 test or Fisher's exact test was applied as appropriate for categorical variables with a p-value less than 0.05.
ROC analysis was performed to determine the cut-off value (COV) of NLR between COVID-10 with and without proven SPBI on D1 and D3. The magnitude of sensitivity, specificity, and prevalence ratio31 based on the COV of NLR was obtained and evaluated. ROC analysis was performed using MedCalc version 5.2 (MedCalc Software Ltd, Belgium).
Results
Population Characteristics
A total of 2902 COVID-19 patients were screened and 236 at 8.1% were included for analysis. Among the population included in the study, 36.9% (n = 87) demonstrated proven SPBI. This finding highlights the utility of culture positivity as the standard diagnostic measure for bacterial infection. Figure 1 illustrates the practical application of inflammatory markers, including Procalcitonin <0.25 ng/mL, to effectively identify individuals within the non-proven SPBI category, with a success rate of up to 63.1%. This strategy involves the integration of chest imaging and culture in conjunction with the aforementioned markers.
 |
Figure 1 Study flow chart. Abbreviations: COVID-19, coronavirus disease 2019; ICD-10, International Classification of Disease version 10; n, number of patients. |
Based on the data presented in Figure 1 and Table 1, 8.1% (236/2902) of adult patients, with an average age of 50 and at least one comorbidity, were admitted to hospital. The most frequently occurring comorbidities in proven and non-proven SPBI groups were hypertension and Type 2 DM. However, the disease severity was significantly higher in the proven-SPBI group, as compared to the non-proven group, with a statistically significant difference (p=<.001) observed in the incidence of severe (55.2% vs 51.0%) and critical (23.0% vs 4.7%) cases. Furthermore, the survival rates were comparatively higher among patients without SPBI (non-proven group) as compared to those with proven-SPBI (87.2% vs 63.2%, p =< 0.001).
 |
Table 1 Baseline Characteristics of Study Population |
Gram-negative bacteria (GNB) (89/114) were dominantly observed among the sputum culture of the proven-SPBI group. Pathogens, ie Acinetobacter baumannii (34/114, 29.8%), Klebsiella pneumoniae (25/114, 21.9%), Pseudomonas aeruginosa (8/114, 7.0%), and Stenotrophomonas maltophilia (8/114, 7.0%) were commonly identified and well known as the etiology of bacterial pneumonia.32 The presence of MDR pathogens was 36.8% among GNB and Gram-Positive Bacterial (GPB). However, the presence of fungal infection was low (7.0%), compared with overall identified bacterial (GNB, GPB), as shown in Table 2.
 |
Table 2 Distribution of Identified Pathogens Among COVID-19 Patients with (Proven) Secondary Pulmonary Bacterial Infection Group |
The Diagnostic Value of NLR on Admission and 72 Hours Thereafter in Proven and Non-Proven SPBI Group
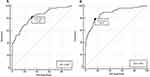
NLR value between proven and non-proven SPBI was compared and showed distinguishable between groups, as shown in Figure 2. Moreover, the high difference between the two groups was also shown in D1 (11.1 vs 4.2, p < 0.001) and D3 (15.3 vs 5.2, p < 0.001), as shown in Table S1. In the ROC curve analysis, NLR in D1 (AUC 0.836) and D3 (AUC 0.880) presented a good ability to differentiate SPBI among COVID-19 population (p < 0.001). An optimal COV was calculated based on the ROC analysis and showed COV in D1 and D3 of 5.29 and 9.47, as shown in Figure 3. Based on the COV of NLR, the prevalence ratio of SPBI on the first (D1) and third day (D3) was 4.7 (95% CI 2.9%–7.7%) and 5.9 (95% CI 3.8%–9.2%), as shown in Table S2. Therefore, COV of NLR for D1 and D3 was significantly differentiated between COVID-19 patients with and without SPBI.
Discussion
SPBI has become a problematic situation during COVID-19 but was reported that the occurrence was approximately less than 20% of cases.3,32 Differentiating between COVID-19 with or without the presence of bacterial infection was challenging. Value of NLR was evaluated according to the leukocyte kinetics among COVID-19 patients, to identify the presence of SPBI, in two different time observations. NLR value can differentiate the occurrence of SPBI, in the first 72 hours of hospital admission, with the onset of illness between 3 and 11 days. Similar to other forms of severe inflammation, such as sepsis, leukocytes play a critical role in the immune response to COVID-19.13 In general, an immune response to viral infection involves the activation and proliferation of lymphocytes such as cytotoxic T-cells and Natural Killer (NK) cells. This is accompanied by the secretion of antibodies or cytokines/lymphokines, such as interferon (IFN), to eliminate infected cells and clear the virus. However, in the case of SARS-CoV-2 infection, there is dysregulation of these immune responses, leading to a hyperinflammatory state characterized by a decrease in lymphocytes, including cytotoxic T-cells and NK cells, as well as decreased IFN levels and increased cytokine levels.7 This state also affects the respiratory microbiota, resulting in high levels of dysbiosis and poor microbial structural complexity in the respiratory microbiota of patients.
Immune response in SARS-CoV-2 and bacterial infection also induce the role of neutrophil recruitment and degranulation.7,13 Therefore, the increasing number of neutrophils and decreased number of lymphocytes resulted in an increasing NLR value. Previous studies showed the importance of increasing NLR value in the first 72 hours of bacteremia, which corresponds with the development of leukocyte kinetics ranging from 1 to 3 days. A similar finding was also observed in the sepsis situation, which highlighted the critical role of leukocyte kinetics in the first 3 days after sepsis.12,13 Despite the limited reports on leukocyte kinetics, a previous study indicated that COVID-19 patients with bacteremia showed an increase in NLR between the first and third day.22,33–36 Therefore, this finding supports the kinetics of NLR on the first and third days of COVID-19, particularly differentiating the occurrence of bacterial infection.
There is a high number of isolates with MDR GNB identified among patients with SPBI. Several factors may contribute to this situation: First, during COVID-19, the increase of antibiotic use as initial empirical treatment was unavoidable. Previous systematic reviews show the development of antibiotic resistance mainly occurs between 0 and 6 months after the use of related antibiotics.37 Second, human factors and equipment contribute to the increase of MDR organisms. The use of Personal Protective Equipment (PPE), which was meant to protect healthcare staff and reduce infection, led to suboptimal utilization and disease transmission.6,38 Third, host factors, including older ages, and comorbidities, facilitate the risk of MDR, as reported previously.39
In this study, the optimal COV of NLR was demonstrated to differentiate between COVID-19 with and without SPBI. A previous study defined certain COV of NLR in COVID-19 patients but was dedicated to prognostic, disease severity, and mortality markers.14,22,40 Therefore, the COV of NLR showed the other value to determine the occurrence of SPBI. A high prevalence ratio of NLR was also observed, indicating that a high NLR above the COV during the first three days is likely to suggest the presence of bacterial infection. Moreover, it is a predictor of successful culture and positive radiologic findings, with a 4–5 times greater chance of occurrence compared to those with an NLR below the COV, as shown in Table S2.31,41 Furthermore, the utility of NLR in the first 3 days also contributes to controlling the use of antibiotic and support the antimicrobial stewardship, which is interrupted during COVID-19.11,42,43 There are several limitations to this study; first, the atypical bacterial was not performed for the sputum culture, resulting in unavoidable bias for COVID-19 without SPBI group. Second, this study was not designed to measure further analysis, such as NLR value to differentiate GNB and GPB related to clinical outcomes or disease severity in COVID-19. This is due to the rapid progression of the virus and limited information among the included patient. Third, since it was a retrospective data collection study, the potential of subject selection bias was unavoidable, particularly in culture examination. The study population was defined by identifying the list of all hospitalized COVID-19 patients and manually searching their medical records. However, it is important to note that previous reports have indicated the potential bias of cultural results.44,45
Bacterial infection has become a priority concern globally, especially concerning the use of antibiotics and the emergence of antibiotic resistance. During COVID-19 pandemic, the presence of bacterial infection among the population has been shown to result in poor clinical outcomes and increased mortality rates. The employment of a simple, feasible, ease to access and cost-effective marker, such as NLR, can significantly aid in the early detection of bacterial infection.46,47 This method can be useful in COVID-19 and other hyperinflammatory states, including sepsis. Therefore, the implementation of NLR can serve two primary purposes: (1) promoting the regulation of antibiotic use, specifically empirical treatment, and (2) contributing to the reinforcement of the antimicrobial stewardship program by guiding the empirical treatment protocol. Considering COVID-19 pandemic, it is imperative to prevent the long-term consequences of this disease, particularly the surge of antibiotic resistance.
Conclusion
In conclusion, the utilization of NLR on admission and the third day after 72 hours proved effective in distinguishing the emergence of SPBI among COVID-19 patients, with COV value of 5.29 and 9.47, respectively. Employing a straightforward and economical marker, such as NLR facilitates the prompt identification of bacterial infection and enables regulation of the use of antibiotics as empirical treatment, which is a frequent occurrence in healthcare facilities.
Ethical Considerations
This study was conducted under the Declaration of Helsinki, and the study protocol was approved by the Ethics Committee of Hasan Sadikin Hospital (LB.02.01/X.6.5/147/2022). Meanwhile, informed consent was not required to obtain the data from hospital and laboratory information system. Therefore, the ethics committee waived the need for written patient consent.
Acknowledgments
The authors are grateful to the health workers at Dr. Hasan Sadikin General Hospital for the service and sacrifice provided during COVID-19 pandemic. The authors also thank Mr. Evan Susandi for his assistance in statistical analysis, and the Universitas Padjadjaran for the financial support.
Disclosure
The authors declared no conflicts of interest in this work.
References
1. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513.
2. Pusat Informasi & Koordinasi Provinsi Jawa Barat. Sebaran Kasus Covid-19 di Jawa Barat. Pusat Informasi & Koordinasi Provinsi Jawa Barat; 2022. Available from: https://pikobar.jabarprov.go.id/distribution-case.
3. Langford BJ, So M, Raybardhan S, et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect. 2020;26:1622–1629.
4. Lansbury L, Lim B, Baskaran V, Lim WS. Co-infections in people with COVID-19: a systematic review and meta-analysis. J Infection. 2020;81:266–275.
5. Lee S, Koh JS, Kim YJ, et al. Secondary infection among hospitalized COVID‐19 patients: a retrospective cohort study in a tertiary care setting. Respirology. 2021;26:277–278.
6. Witt LS, Howard-Anderson JR, Jacob JT, Gottlieb LB. The impact of COVID-19 on multidrug-resistant organisms causing healthcare-associated infections: a narrative review. JAC Antimicrobial Resistance. 2022;5:dlac130.
7. Borczuk AC, Yantiss RK. The pathogenesis of coronavirus-19 disease. J Biomed Sci. 2022;29:87.
8. Hernández-Terán A, Mejía-Nepomuceno F, Herrera MT, et al. Dysbiosis and structural disruption of the respiratory microbiota in COVID-19 patients with severe and fatal outcomes. Sci Rep. 2021;11:21297.
9. Garcia-Vidal C, Sanjuan G, Moreno-García E, et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: a retrospective cohort study. Clin Microbiol Infect. 2021;27:83–88.
10. Nori P, Cowman K, Chen V, et al. Bacterial and fungal coinfections in COVID-19 patients hospitalized during the New York City pandemic surge. Infect Control Hosp Epidemiol. 2021;42:84–88.
11. Owoicho O, Tapela K, Djomkam Zune AL, Nghochuzie NN, Isawumi A, Mosi L. Suboptimal antimicrobial stewardship in the COVID-19 era: is humanity staring at a postantibiotic future? Future Microbiol. 2021;16:919–925.
12. Schuetz P, Maurer P, Punjabi V, Desai A, Amin DN, Gluck E. Procalcitonin decrease over 72 hours in US critical care units predicts fatal outcome in sepsis patients. Crit Care. 2013;17:R115.
13. Li Q, Xie J, Huang Y, et al. Leukocyte kinetics during the early stage acts as a prognostic marker in patients with septic shock in intensive care unit. Medicine. 2021;100:e26288.
14. Önal U, Gülhan M, Demirci N, et al. Prognostic value of neutrophile-to-lymphocyte ratio (NLR) and lactate dehydrogenase (LDH) levels for geriatric patients with COVID-19. BMC Geriatr. 2022;22:362.
15. Westerdijk K, Simons KS, Zegers M, Wever PC, Pickkers P, de Jager CPC. The value of the neutrophil-lymphocyte count ratio in the diagnosis of sepsis in patients admitted to the Intensive Care Unit: a retrospective cohort study. PLoS One. 2019;14:e0212861.
16. Sayed AA, Allam AA, Sayed AI, Alraey MA, Joseph MV. The use of neutrophil-to-lymphocyte ratio (NLR) as a marker for COVID-19 infection in Saudi Arabia: a case-control retrospective multicenter study. SMJ. 2021;42:370–376.
17. Parthasarathi A, Padukudru S, Arunachal S, et al. The Role of Neutrophil-to-Lymphocyte Ratio in Risk Stratification and Prognostication of COVID-19: a Systematic Review and Meta-Analysis. Vaccines. 2022;10:1233.
18. Wang Y, Zhao J, Yang L, Hu J, Yao Y. Value of the Neutrophil-Lymphocyte Ratio in Predicting COVID-19 Severity: a Meta-analysis. Dis Markers. 2021;2021:1–10.
19. Kementerian Kesehatan RI. Pedoman Pencegahan dan Pengendalian Coronavirus Disease (COVID-19) [Guidelines for Prevention and Control of Coronavirus Disease (COVID-19)]. Available from: https://covid19.kemkes.go.id/protokol-covid-19/kmk-no-hk-01-07-menkes-413-2020-ttg-pedoman-pencegahan-dan-pengendalian-covid-19.
20. Vandepitte J, eds.; World Health Organization. Basic Laboratory Procedures in Clinical Bacteriology.
21. Liu -C-C, H-J K, Liu W-S, et al. Neutrophil-to-lymphocyte ratio as a predictive marker of metabolic syndrome. Medicine. 2019;98:e17537.
22. Liu J, Liu Y, Xiang P, et al. Neutrophil-to-lymphocyte ratio predicts critical illness patients with 2019 coronavirus disease in the early stage. J Transl Med. 2020;18:206.
23. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Supplement M100.
24. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and Treatment of Adults with Community-acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200:e45–e67.
25. Hughes S, Troise O, Donaldson H, Mughal N, Moore LSP. Bacterial and fungal coinfection among hospitalized patients with COVID-19: a retrospective cohort study in a UK secondary-care setting. Clin Microbiol Infect. 2020;26:1395–1399.
26. Singh D, Agusti A, Anzueto A, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease: the GOLD science committee report 2019. Eur Respir J. 2019;53:1900164.
27. Liu P, Quinn RR, Lam NN, et al. Accounting for Age in the Definition of Chronic Kidney Disease. JAMA Intern Med. 2021;181:1359.
28. American Diabetes Association. Standards of Medical Care in Diabetes—2022 Abridged for Primary Care Providers. Clin Diabetes. 2022;40:10–38.
29. Unger T, Borghi C, Charchar F, et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension. 2020;75:1334–1357.
30. Magiorakos A-P, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–281.
31. Deddens JA, Petersen MR. Approaches for estimating prevalence ratios. Occup Environ Med. 2008;65:501–506.
32. Subagdja MFM, Sugianli AK, Prodjosoewojo S, Hartantri Y, Parwati I. Antibiotic Resistance in COVID-19 with Bacterial Infection: laboratory-Based Surveillance Study at Single Tertiary Hospital in Indonesia. IDR. 2022;15:5849–5856.
33. de Jager CP, van Wijk PT, Mathoera RB, de Jongh-Leuvenink J, van der Poll T, Wever PC. Lymphocytopenia and neutrophil-lymphocyte count ratio predict bacteremia better than conventional infection markers in an emergency care unit. Crit Care Med. 2010;14:1–8.
34. Nasir N, Rehman F, Omair SF. Risk factors for bacterial infections in patients with moderate to severe COVID‐19: a case‐control study. J Med Virol. 2021:56.
35. Sun S, Cai X, Wang H, et al. Abnormalities of peripheral blood system in patients with COVID-19 in Wenzhou, China. Clin Chimica Acta. 2020;507:174–180.
36. Singh Y, Singh A, Rudravaram S, et al. Neutrophil-to-lymphocyte Ratio and Platelet-to-lymphocyte Ratio as Markers for predicting the Severity in COVID-19 Patients: a Prospective Observational Study. Indian J Critical Care Med. 2021;25:847.
37. Poku E, Cooper K, Cantrell A, et al. Systematic review of time lag between antibiotic use and rise of resistant pathogens among hospitalized adults in Europe. JAC Antimicrobial Resistance. 2022;5:dlad001.
38. Sayed AA, Allam AA, Alruwaili AK, Alraey MA, Elsayed EM, Aloraini GS. The Use of COVID-19 Surveillance Measures in Detecting Cases of Tuberculosis (TB). Hygiene. 2023;3:1–11.
39. Santoso P, Sung M, Hartantri Y, et al. MDR Pathogens Organisms as Risk Factor of Mortality in Secondary Pulmonary Bacterial Infections Among COVID-19 Patients: observational Studies in Two Referral Hospitals in West Java, Indonesia. IJGM. 2022;15:4741–4751.
40. Tadesse Z, Bekele Bayissa A, Diriba T, Chernet N, Tsegaye S, Tsega M. Neutrophil-to-Lymphocyte Ratio and Cut-off Values as Predictor of Severity and Mortality in COVID-19 Patients in Millennium COVID-19 Care Center, Addis Ababa, Ethiopia. IJGM. 2022;15:6739–6755.
41. Santoso D, Asfia SKBM, Mello MB, et al. HIV prevalence ratio of international migrants compared to their native-born counterparts: a systematic review and meta-analysis. eClinicalMedicine. 2022;53:101661.
42. Ghosh S, Bornman C, Zafer MM. Antimicrobial Resistance Threats in the emerging COVID-19 pandemic: where do we stand? J Infect Public Health. 2021;14:555–560.
43. Pierce J, Stevens MP. COVID-19 and antimicrobial stewardship: lessons learned, best practices, and future implications. Int J Infectious Dis. 2021;113:103–108.
44. Rempel OR, Laupland KB. Surveillance for antimicrobial resistant organisms: potential sources and magnitude of bias. Epidemiol Infect. 2009;137:1665–1673.
45. Laupland KB, Ross T, Pitout JDD, Church DL, Gregson DB. Investigation of sources of potential bias in laboratory surveillance for anti-microbial resistance. Clin Invest Med. 2007;30:E159–66.
46. Bayraktar M, Tekin E, Kocak MN. How to diagnose COVID-19 in family practice? Usability of complete blood count as a COVID-19 diagnostic tool: a cross-sectional study in Turkey. BMJ Open. 2023;13:e069493.
47. Sayed AA. The Cost-Effectiveness of Requesting a Complete Blood Count (CBC) in the Management of COVID-19 in Saudi Arabia. Healthcare. 2022;10:1780.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.