Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 18
The Diagnostic and Assessment Value of Plasma Pentraxin 3 in Acute Exacerbation of Chronic Obstructive Pulmonary Disease
Authors Gu Y, Li P, Xiao Y, Zhang J, Su X
Received 23 December 2022
Accepted for publication 30 April 2023
Published 10 July 2023 Volume 2023:18 Pages 1391—1400
DOI https://doi.org/10.2147/COPD.S402463
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Richard Russell
Yu Gu,1,2,* Pei Li,3,* Yongying Xiao,2 Jiaojiao Zhang,2 Xin Su1,2
1Department of Respiratory and Critical Care Medicine, Nanjing Drum Tower Hospital Clinical College of Nanjing Medical University, Nanjing, People’s Republic of China; 2Department of Respiratory and Critical Care Medicine, Jinling Hospital, Nanjing, People’s Republic of China; 3Department of Respiratory and Critical Care Medicine, Taikang Xianlin Drum Tower Hospital, Nanjing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xin Su, Nanjing Drum Tower Hospital, 321 Zhongshan Road, Nanjing, 210008, People’s Republic of China, Email [email protected]
Background: Pentraxin 3 (PTX3) is an acute-phase protein and an important inflammatory mediator. We hypothesized plasma PTX3 could be a valuable diagnostic biomarker in acute exacerbation of chronic obstructive pulmonary disease (AECOPD).
Methods: In this prospective controlled study, 458 COPD patients and 71 healthy controls from May 2019 to December 2020 in two hospitals were enrolled. COPD patients were divided into AECOPD group (n = 173) and stable COPD group (n = 285). AECOPD patients were subdivided into mild or moderate group (n = 43) and severe group (n = 130) based on severity. Plasma PTX3 levels were detected by ELISA.
Results: Plasma PTX3 levels were significantly higher in AECOPD (2.8 ng/mL) compared to stable COPD (0.87 ng/mL) and healthy controls (0.83 ng/mL). In the analysis of AECOPD subgroups, plasma PTX3 level of severe group (4.51 ng/mL) was significantly higher than that of mild or moderate group (1.25 ng/mL). Patients with respiratory failure had higher PTX3 than those without respiratory failure. No difference was observed between stable COPD patients and healthy controls. ROC analysis showed that plasma PTX3 had a considerable ability to distinguish AECOPD from stable COPD [AUC: 0.85, 95% CI (0.81– 0.88), P < 0.0001; cut-off 1.25 ng/mL, sensitivity 77.5%, specificity 74%]. AUC of PTX3 was better than CRP regarding diagnosis of AECOPD. Combination of PTX3 and CRP was superior to either of them in diagnosing AECOPD.
Conclusion: Plasma PTX3 levels were significantly higher in AECOPD than stable COPD. The level was associated with the severity of exacerbation. Plasma PTX3 has potential value as a biomarker to diagnose and evaluate AECOPD.
Keywords: pentraxin 3, AECOPD, diagnosis, severity, C-reactive protein
Introduction
Chronic obstructive pulmonary disease (COPD) is a worldwide disease with increasing morbidity and mortality. Recent population-based survey in China estimated nearly 100 million people suffer from COPD.1 COPD in China accounts for 30% of global deaths.1,2 Of patients with COPD in the Asia-Pacific region population-based survey, 46% had at least one exacerbation in previous years, and 19% required hospitalization,3 which poses a major public health and economic burden. COPD is characterized by persistent and progressive airflow limitation with increased chronic inflammatory response, and the aggregation and activation of neutrophils play an important role in the pathogenesis of COPD.4 Moreover, it is well established that the systemic inflammation increased in COPD, especially in advanced stage or during exacerbations.5
Pentraxin 3 (PTX3) is an acute-phase protein and the member of pentraxin family, which includes C-reactive protein (CRP) and serum amyloid P. It has been reported to be produced by a variety of cells (such as white blood cells, endothelial cells, epithelial cells and mononuclear phagocytes) and released from neutrophil extracellular traps under condition of inflammation or tissue damage.6,7 Plasma PTX3 has been reported to increase in response to acute inflammation, such as acute myocardial infarction and sepsis.8,9 Moreover, increased circulation of PTX3 correlates with disease severity. Previous findings were reported that plasma PTX3 levels were significantly higher in stable COPD patients than healthy control.10,11 In contrast to this result, Beghé et al12 and Van Pottelberge et al13 found no such differences. In subgroup analysis of pulmonary aspergillosis in COPD, no statistical differences were observed of plasma PTX3 with patients in stable COPD and AECOPD group.14 Therefore, the value of PTX3 in COPD needs to be further determined.
The aim of our study was to determine plasma PTX3 levels in stable COPD and AECOPD patients and investigate the correlation between plasma PTX3 levels and disease severity.
Materials and Methods
Study Design and Patients Extraction
We undertook a prospective study in COPD patients and healthy volunteers from two hospitals. Stable COPD patients (n = 285) and AECOPD patients (n = 173) from two tertiary teaching hospitals in Nanjing (Jinling Hospital and Taikang Xianlin Drum Tower Hospital) were finally recruited from May 2019 to December 2020. COPD diagnosis was established according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines. Stable was defined as no exacerbation within previous 3 months before admitted into this study. Acute exacerbation was defined as acute worsening of respiratory symptoms (typical symptoms as aggravated cough, sputum or dyspnea) that required additional therapy. Mild AECOPD was defined as AECOPD requiring extra short-acting bronchodilators. Moderate AECOPD was defined as the requirement of antibiotics or oral corticosteroids on outpatient treatment. Severe AECOPD was defined as AECOPD requiring emergency visit or hospitalization, including admission to the general ward or the intensive care unit (ICU). Patients with any one of the following diseases were excluded: asthma, active pulmonary tuberculosis, pulmonary aspergillosis, acute myocardial infarction, acute coronary syndrome, active rheumatology diseases, heart failure, renal failure, hepatic failure, progressive malignancy, infectious diseases other than respiratory system, major trauma and surgery within 3 months prior to recruitment. Seventy-one healthy subjects without diseases mentioned above were enrolled as healthy control group. The study protocol was approved by the ethics committee of Taikang Xianlin Drum Tower Hospital and all subjects enrolled or their surrogates provided written informed consent.
Plasma Collection and PTX3 Detection
Peripheral venous blood samples were obtained from all subjects. For all enrolled patients and healthy controls, collection of blood samples was obtained and centrifuged (10 minutes at room temperature, 3000 rpm). Then, supernatants were extracted and stored at −80°C until PTX3 detection. Human PTX3 levels in plasma were measured using enzyme-linked immunosorbent assay (ELISA) kits via manufacturer’s instructions (DPTX30B, Human PTX3 Immunoassay, R&D Company, UK).
Demographic and Clinical Data
Demographic information and clinical data including age, gender, smoking history, acute exacerbations in previous years, pulmonary function, and laboratory parameters were obtained. Radiographic diagnosis was primarily obtained from radiologist’s reports.
Statistical Analysis
Quantitative variables were expressed as means ± standard deviation (SD) for normal distribution or median (interquartile range, IQR) for abnormal distribution. Categorical variables were presented as proportions. Quantitative variables between two groups were compared using the Mann–Whitney U-test or Student’s t-test. One-way analysis of variance (ANOVA) or Kruskal–Wallis test was used to analyze the difference in quantitative variables among three or more groups. Chi-squared or Fisher’s exact test was conducted to assess the difference in categorical variables. Spearman’s rank analysis was performed to evaluate correlations between two variables. A heat map was generated to describe Spearman correlation. Propensity Score Matching was used to match the baseline data of AECOPD group and stable COPD group in a ratio of 1:1. Diagnostic accuracy of plasma PTX3 was assessed using the area under curve (AUC) of receiver operating characteristic (ROC) curve. DeLong test was applied to compare the accuracy of the AUCs of plasma PTX3, CRP and combination of PTX3 and CRP. SPSS 25.0 and the GraphPad Prism 8 software were performed for data analysis. P values <0.05 were deemed statistically significant.
Results
Demographic and Laboratory Data
During the study period, 458 COPD patients from two hospitals and 71 healthy controls were enrolled, including 285 stable COPD cases and 173 AECOPD cases (Figure 1). The demographic data of all subjects are summarized in Table 1. There was no difference in gender distribution among three groups, while significant difference was observed in age and smoking history. The distribution of lung function differed between stable COPD and AECOPD (p< 0.0001). In terms of lung function, AECOPD patients had a higher proportion of GOLD 3 and 4 (67.6% vs 45.6%) compared to stable COPD patients.
 |
Table 1 Comparison of Clinical and Demographic Characteristics of Patients with COPD and Healthy Controls |
 |
Figure 1 Flow chart of COPD patient recruitment. |
Levels of Plasma PTX3 in Different Groups of COPD
The levels of plasma PTX3 of AECOPD group, stable COPD group and healthy group were 2.8 (1.28–6.22) ng/mL, 0.87 (0.55–1.29) ng/mL and 0.83 (0.66–1.1) ng/mL. Compared to patients with stable COPD and healthy controls, a significant increase of PTX3 was observed in patients with AECOPD (P < 0.0001). However, the difference between stable COPD group and control group had no significance (P = 0.607). The AECOPD patients were further classified as mid-to-moderate exacerbation (n = 43) and severe exacerbation (n = 130) subgroups. Subgroup analysis showed that plasma PTX3 in severe patients was significantly higher than that in mild or moderate patients [4.51 (2–8.2) vs 1.25 (1.09–1.57) ng/mL, P < 0.0001]. Meanwhile, mild or moderate AECOPD patients had a higher PTX3 level than stable COPD (P < 0.0001) (Figure 2). Among 130 patients with severe AECOPD, 38 cases (29.2%) complicated with respiratory failure. In the analysis of severe AECOPD subgroup, we found that PTX3 levels in patients with respiratory failure were significantly higher than those without respiratory failure [6.91 (3.91–13.62) vs 3.52 (1.36–5.59) ng/mL, P < 0.0001] (Figure 3).
 |
Figure 2 Plasma PTX3 levels of AECOPD, stable COPD and healthy controls. ****P < 0.0001; ns, P > 0.05. |
 |
Figure 3 Plasma PTX3 levels of patients with respiratory failure and without respiratory failure in severe AECOPD subgroup. ****P < 0.0001. |
Propensity score matching (PSM) was applied to balance the baseline data of AECOPD group and stable COPD group in a ratio of 1:1. After mating the age, gender, BMI, smoking history and lung function, the level of PTX3 in AECOPD group was still notably higher than that in stable COPD group (P<0.0001) (Table 2).
 |
Table 2 Baseline Characteristics and Plasma PTX3 Level of Patients with AECOPD and Stable COPD After PSM |
Correlation Between Clinical Parameters and Plasma PTX3 Level of Patients with COPD
To further explore the value of plasma PTX3 in COPD patients, we conducted correlation analysis between clinical parameters, plasma PTX3 (r1, P1) and GOLD stage (r2, P2) in patients with stable COPD (Table 3). There was no notable correlation between PTX3 and CAT scores, mMRC, FEV1%pred and GOLD stage. On the other hand, GOLD stage was positively correlated with CAT scores and mMRC (r2 = 0.28, P2 < 0.001; r2 = 0.34, P2 < 0.0001). Laboratory data of severe AECOPD patients is shown in Table 4. PTX3 levels in patients with severe AECOPD were positively correlated with PCT, NLR, AMO, ANC, WBC and CRP (r = 0.37, P < 0.0001; r = 0.36, P < 0.0001; r = 0.36, P < 0.0001; r = 0.34, P < 0.0001; r = 0.29, P < 0.001; r = 0.23, P < 0.1) and negatively correlated with AEC and ALC (r = −0.27, P < 0.01; r = −0.24, P < 0.01). No correlation was observed between PTX3 and Hb, or ALB (P = 0.3; P = 0.59) (Figure 4).
 |
Table 3 Spearman Correlation Analysis Between Plasma PTX3 (r1, P1), GOLD Stage (r2, P2) and CAT Scores, mMRC, FEV1%pred in Patients with Stable COPD |
 |
Table 4 Clinical and Laboratory Parameters of Severe AECOPD Patients |
Diagnostic Value of Plasma PTX3 for AECOPD
ROC curve analysis was applied to evaluate diagnostic value of plasma PTX3 for different types of AECOPD. The AUC for plasma PTX3 to diagnose all enrolled AECOPD was 0.85 (95% CI, 0.81–0.88). When taking the optimal cut-off of 1.25 ng/mL for PTX3, the sensitivity and specificity were, respectively, 77.5% and 74%. Stratifying AECOPD into mild or moderate AECOPD and severe AECOPD, significant AUCs were still observed for PTX3. The diagnostic cut-off value of plasma PTX3 was 1.05 ng/mL [AUC: 0.71, 95% CI (0.64–0.78); sensitivity: 81.4%, specificity: 63.2%; P < 0.0001] for mild or moderate AECOPD. The diagnostic cut-off value of plasma PTX3 was 1.94 ng/mL [AUC: 0.89, 95% CI (0.86–0.93); sensitivity: 76.2%, specificity: 89.8%; P < 0.0001] for severe AECOPD (Table 5, Figure 5).
 |
Table 5 Cut-Off Value, AUC, Sensitivity and Specificity of Plasma PTX3 for AECOPD |
 |
Figure 5 ROC curves of diagnostic value of plasma PTX3 for AECOPD, mild or moderate AECOPD and severe AECOPD. |
We further performed ROC curve analysis of PTX3 and CRP alone or combination in 124 patients with stable COPD and 137 patients with AECOPD. The difference in AUC between PTX3 and CRP was statistically significant [0.89, 95% CI (0.85–0.93) vs 0.63, 95% CI (0.57–0.69), P < 0.0001]. Combination of PTX3 and CRP improved the diagnostic value of PTX3 or CRP alone (comparing with PTX3: 0.93 vs 0.89, P = 0.0134; comparing with CRP: 0.93 vs 0.63, P < 0.0001) (Figure 6).
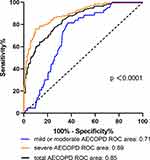 |
Figure 6 ROC curves of diagnostic value of plasma PTX3, CRP and combination of PTX3 with CRP for AECOPD. |
Discussion
AECOPD is an important event with high prevalence in COPD and a major determinant factor of prognosis in patients with COPD.15 Early identification of AECOPD and objective biomarkers of disease severity remained an unsolved issue. This prospective study demonstrated that AECOPD patients had a significantly elevated level of plasma PTX3 compared to patients with stable COPD. The AUC for PTX3 diagnosis of AECOPD was better than CRP. The combined diagnostic value was statistically superior to either of them. We further found that plasma PTX3 may correlate with the severity of acute exacerbations.
PTX3 is an acute-phase protein and has been reported as a new biomarker of systemic inflammatory conditions and disease severity.16–21 As a critical component of humoral innate immunity, PTX3 was involved in regulating inflammation. In our study, plasma PTX3 was increased in patients with AECOPD, and was better at distinguishing acute exacerbation from stable COPD than CRP according to the ROC analysis. Furthermore, subgroup analysis showed that PTX3 levels were increased with increasing severity from severe AECOPD to mild or moderate AECOPD. AECOPD patients with respiratory failure also had higher expression of PTX3. The PTX3 remarkably increased in severe AECOPD or patients with respiratory failure, which may contribute to assess disease severity. The possible function of PTX3 may increase the inflammation due to the increased activation of complement cascade.21
No difference was observed in plasma PTX3 levels between stable COPD and healthy controls, which was generally consistent with previous findings.13 In contrast, two other studies showed PTX3 was elevated in patients with stable COPD.10,11 In the present study, PTX3 levels had no correlation with mMRC, whereas Kurt et al10 found weak positive correlation. No correlation between pulmonary function, CAT score and plasma PTX3 was compatible with Kurt et al. The expression of PTX3 in stable COPD and its correlation with clinical parameters have not been reached. The findings in stable COPD and healthy controls contradicted. The previous study mentioned that the difference was minor and marginal due to the small number of study population, which may limit its statistical value.10 Noting that our study was prospective and enrolled more patients.
The PCT, CRP and NLR, as established inflammatory parameters, have been raised in AECOPD.22–24 Positive correlation between PTX3 and these clinical parameters suggests a role for PTX3 in assisting diagnosis and monitoring severity of AECOPD. Neutrophils have been shown to be involved in PTX3 storage.25 PTX3 is one of the component proteins of neutrophil extracellular traps (NETs).7 NETs play critically “double-edged” roles in host-protection.26 Neutrophilic inflammation plays a crucial role in the pathophysiology of COPD. NETs have also been found in the airways of stable and acute exacerbation of COPD.27 Thulborn et al demonstrated PTX3 levels positively correlated with ANC present in sputum.28 The positive correlation in acute exacerbations between WBC, AMO, ANC and PTX3 indicates neutrophils as a possible source for PTX3. We supposed whether NETs emerge in serum in patients with severe AECOPD due to host inflammation, which may be an interesting finding.
This study has some limitations. Although we matched basic clinical data between AECOPD and stable COPD by PSM, it is better to compare PTX3 levels of the same COPD group in acute exacerbation and stable state. Although patients recruited in the study had confirmed diagnosis of COPD, some of them were too severe to undertake pulmonary function during this recruitment. Given the subjective definition of exacerbation and its severity, the diagnostic value of plasma PTX3 combined with classical symptoms of acute exacerbation needs to be further explored.
In summary, we illustrated that plasma PTX3 was significantly higher in patients with AECOPD than those with stable COPD, as well as in severe AECOPD than mild or moderate AECOPD. Moreover, plasma PTX3 showed good discrimination to distinguish acute exacerbation from stable state. The findings suggest plasma PTX3 could be a biomarker for diagnosing AECOPD and evaluating severity of exacerbation.
Ethics Approval and Consent to Participate
The study protocol was approved by the Institute Ethics Committee of Taikang Xianlin Drum Tower Hospital (2020-CRP-002). The study protocol conformed to the ethical guidelines of the 1975 Helsinki Declaration. The patients were given oral and written information prior to inclusion and signed an informed consent. All data used in the current study were anonymous.
Funding
This work was supported by the Project of Natural Science Foundation of China (82270019, 82070011), the Key Project of Jiangsu Commission of Health (K2019004), and Beijing Bethune Charitable Foundation (Grant Number BJ-RW2020004J).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wang C, Xu J, Yang L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet. 2018;391(10131):1706–1717.
2. Zhou M, Wang H, Zhu J, et al. Cause-specific mortality for 240 causes in China during 1990-2013: a systematic subnational analysis for the Global Burden of Disease Study 2013. Lancet. 2016;387(10015):251–272.
3. Lim S, Lam DC, Muttalif AR, et al. Impact of chronic obstructive pulmonary disease (COPD) in the Asia-Pacific region: the EPIC Asia population-based survey. Asia Pac Fam Med. 2015;14(1):4.
4. Rabe KF, Hurd S, Anzueto A, et al. Global Initiative for Chronic Obstructive Lung D. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176(6):532–555.
5. Barnes PJ, Celli BR. Systemic manifestations and comorbidities of COPD. Eur Respir J. 2009;33(5):1165–1185.
6. Garlanda C, Jaillon S, Doni A, Bottazzi B, Mantovani A. PTX3, a humoral pattern recognition molecule at the interface between microbe and matrix recognition. Curr Opin Immunol. 2016;38:39–44.
7. Jaillon S, Peri G, Delneste Y, et al. The humoral pattern recognition receptor PTX3 is stored in neutrophil granules and localizes in extracellular traps. J Exp Med. 2007;204(4):793–804.
8. Lee YT, Gong M, Chau A, et al. International Heath Informatics Study N. Pentraxin-3 as a marker of sepsis severity and predictor of mortality outcomes: a systematic review and meta-analysis. J Infect. 2018;76(1):1–10.
9. Hansen CB, Bayarri-Olmos R, Kristensen MK, Pilely K, Hellemann D, Garred P. Complement related pattern recognition molecules as markers of short-term mortality in intensive care patients. J Infect. 2020;80(4):378–387.
10. Kurt OK, Tosun M, Kurt EB, Talay F. Pentraxin 3 as a novel biomarker of inflammation in chronic obstructive pulmonary disease. Inflammation. 2015;38(1):89–93.
11. Poznanski M, Brzezianska-Lasota E, Kiszalkiewicz J, et al. Serum levels and gene expression of pentraxin 3 are elevated in COPD. Adv Med Sci. 2019;64(1):85–89.
12. Beghe B, Verduri A, Bottazzi B, et al. Echocardiography, spirometry, and systemic acute-phase inflammatory proteins in smokers with COPD or CHF: an observational study. PLoS One. 2013;8(11):e80166.
13. Van Pottelberge GR, Bracke KR, Pauwels NS, Vermassen FE, Joos GF, Brusselle GG. COPD is associated with reduced pulmonary interstitial expression of pentraxin-3. Eur Respir J. 2012;39(4):830–838.
14. He Q, Li H, Rui Y, et al. Pentraxin 3 gene polymorphisms and pulmonary aspergillosis in chronic obstructive pulmonary disease patients. Clin Infect Dis. 2018;66(2):261–267.
15. Wedzicha JA, Singh R, Mackay AJ. Acute COPD exacerbations. Clin Chest Med. 2014;35(1):157–163.
16. Boutet MA, Nerviani A, Lliso-Ribera G, et al. Circulating and Synovial Pentraxin-3 (PTX3) Expression Levels Correlate With Rheumatoid Arthritis Severity and Tissue Infiltration Independently of Conventional Treatments Response. Front Immunol. 2021;12:686795.
17. Zhang Y, Tedrow J, Nouraie M, et al. Elevated plasma level of Pentraxin 3 is associated with emphysema and mortality in smokers. Thorax. 2021;76(4):335–342.
18. Ching LL, Nerurkar VR, Lim E, Shohet RV, Melish ME, Bratincsak A. Elevated Levels of Pentraxin 3 Correlate With Neutrophilia and Coronary Artery Dilation During Acute Kawasaki Disease. Front Pediatr. 2020;8:295.
19. Vanska M, Koivula I, Hamalainen S, et al. High pentraxin 3 level predicts septic shock and bacteremia at the onset of febrile neutropenia after intensive chemotherapy of hematologic patients. Haematologica. 2011;96(9):1385–1389.
20. Bastrup-Birk S, Skjoedt MO, Munthe-Fog L, Strom JJ, Ma YJ, Garred P. Pentraxin-3 serum levels are associated with disease severity and mortality in patients with systemic inflammatory response syndrome. PLoS One. 2013;8(9):e73119.
21. Moulana Z, Bagherzadeh M, Mirzakhani M, Rostami A, Mohammadnia-Afrouzi M, Shahbazi M. Increased Levels of serum Pentraxin 3 in Critical Coronavirus Disease-2019 Patients. Environ Sci Pollut Res Int. 2022;29(57):85569–85573.
22. Lin TL, Chen WW, Ding ZR, Wei SC, Huang ML, Li CH. Correlations between serum amyloid A, C-reactive protein and clinical indices of patients with acutely exacerbated chronic obstructive pulmonary disease. J Clin Lab Anal. 2019;33(4):e22831.
23. Taylan M, Demir M, Kaya H, et al. Alterations of the neutrophil-lymphocyte ratio during the period of stable and acute exacerbation of chronic obstructive pulmonary disease patients. Clin Respir J. 2017;11(3):311–317.
24. Koutsokera A, Stolz D, Loukides S, Kostikas K. Systemic biomarkers in exacerbations of COPD: the evolving clinical challenge. Chest. 2012;141(2):396–405.
25. Kunes P, Holubcova Z, Kolackova M, Krejsek J. Pentraxin 3(PTX 3): an endogenous modulator of the inflammatory response. Mediators Inflamm. 2012;2012:920517.
26. Liu J, Pang Z, Wang G, et al. Advanced role of neutrophils in common respiratory diseases. J Immunol Res. 2017;2017:6710278.
27. Grabcanovic-Musija F, Obermayer A, Stoiber W, et al. Neutrophil extracellular trap (NET) formation characterises stable and exacerbated COPD and correlates with airflow limitation. Respir Res. 2015;16:59.
28. Thulborn SJ, Dilpazir M, Haldar K, et al. Investigating the role of pentraxin 3 as a biomarker for bacterial infection in subjects with COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:1199–1205.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

