Back to Journals » Clinical Epidemiology » Volume 8
The Danish Fetal Medicine Database
Authors Ekelund CK, Kopp TI, Tabor A, Petersen OB
Received 24 November 2015
Accepted for publication 12 January 2016
Published 25 October 2016 Volume 2016:8 Pages 479—483
DOI https://doi.org/10.2147/CLEP.S99477
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Henrik Sørensen
Charlotte Kvist Ekelund,1 Tine Iskov Kopp,2 Ann Tabor,1 Olav Bjørn Petersen3
1Department of Obstetrics, Center of Fetal Medicine, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark; 2Registry Support Centre (East) – Epidemiology and Biostatistics, Research Centre for Prevention and Health, Glostrup, Denmark; 3Fetal Medicine Unit, Aarhus University Hospital, Aarhus Nord, Denmark
Aim: The aim of this study is to set up a database in order to monitor the detection rates and false-positive rates of first-trimester screening for chromosomal abnormalities and prenatal detection rates of fetal malformations in Denmark.
Study population: Pregnant women with a first or second trimester ultrasound scan performed at all public hospitals in Denmark are registered in the database.
Main variables/descriptive data: Data on maternal characteristics, ultrasonic, and biochemical variables are continuously sent from the fetal medicine units' Astraia databases to the central database via web service. Information about outcome of pregnancy (miscarriage, termination, live birth, or stillbirth) is received from the National Patient Register and National Birth Register and linked via the Danish unique personal registration number. Furthermore, results of all pre- and postnatal chromosome analyses are sent to the database.
Conclusion: It has been possible to establish a fetal medicine database, which monitors first-trimester screening for chromosomal abnormalities and second-trimester screening for major fetal malformations with the input from already collected data. The database is valuable to assess the performance at a regional level and to compare Danish performance with international results at a national level.
Keywords: prenatal screening, nuchal translucency, fetal malformations, chromosomal abnormalities
Aim of the database
The Danish National Board of Health issued a new guideline on prenatal screening in 2004.1 This guideline recommends that all pregnant women should be offered a first-trimester scan, including risk assessment for trisomy 21, based on a combination of maternal factors, ultrasound, and biochemical screening, and a second-trimester scan for fetal malformations. Since June 2006, all obstetric departments in Denmark have offered these two ultrasound screenings, and >90% of Danish women choose to have both first-trimester and second-trimester screening performed.2 The Danish Fetal Medicine Database was initiated by the Danish fetal medicine specialists and established in 2008–2010 through collaboration between all obstetric departments in Denmark. It has been fully operational since 2011.
The aim of the Danish Fetal Medicine Database is to provide a tool for local and national quality assessment and research within prenatal screening in Denmark, and to ensure uniform high screening quality by providing relevant and useful feedback on screening performance to all departments and regions on a regular basis.
Study population
The Danish Fetal Medicine Database contains data from all pregnant women with prenatal screening results dating back to January 1, 2008, from all hospital departments of obstetrics and gynecology in Denmark (Bornholm Hospital from January 1, 2011). In 2014, the database contained data about >362,000 pregnancies, of which 353,049 are singleton pregnancies. Of them, 359,058 have had first-trimester screening for trisomy 21 and/or second-trimester screening for fetal malformations.
Main variables
The Danish Fetal Medicine Database consists of data from the following four sources: The local Astraia fetal medicine databases (Astraia GMBH; www.Astraia.com) used in all departments of obstetrics and gynecology in Denmark, the Danish Cytogenetic Central Register, the Danish National Patient Register, and the Danish National Birth Register (Figure 1).
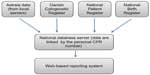  | Figure 1 Data sources of the Danish Fetal Medicine Database. |
The primary data source for the Danish Fetal Medicine Database is the local Astraia databases, from where the following data are retrieved: data on maternal characteristics, first-trimester screening data, including risk assessments, fetal biometries, and registered prenatal malformations at any gestational age. These data have been recorded as part of routine obstetric practice at all departments in Astraia in accordance with national standards since January 1, 2008, and the national database includes data on singleton and twin pregnancies. On a daily basis, data from all local Astraia servers are automatically sent to the national database after encryption.
Before the national database was initiated, national standards on how the pregnancies were dated, how the first-trimester risk assessment was performed and handled, and use of specified biometric reference curves had been issued.3 The International Classification of Diseases, 10th revision code system (ICD-10) is used to code malformations in the fetus and in the infant.
Pregnancy outcome data are collected from the Danish National Patient Register (including spontaneous and induced abortions and information on congenital malformations), the Danish National Birth Register (information about pregnancy complications, delivery, and the newborn), and the Danish Cytogenetic Central Register (results of pre- and postnatal chromosome analyses). Information from these data sources is linked to the Danish Fetal Medicine Database using the unique personal identification number (CPR number), which everyone is given at birth or on immigration to Denmark. Algorithms have been developed to ensure that linking of data from different registries is pregnancy specific. For each pregnancy ultrasound scanning, information on one or more fetuses is linked to a karyotype result if performed during the pregnancy or just after birth of the fetus/infant. In addition, information on the outcome of pregnancy is available for all pregnancies, whether it is miscarriage, termination, stillbirth, or live birth. More than 95% of the pregnancies have an outcome registered. In some of the cases with unknown outcome, migration to other countries can explain the missing data. The high completeness of all variables and the outcome data is unique and internationally highly acknowledged. A list of variables is shown in Table 1.
The clinicians have access to their locally collected data and selected quality indicators in comparison with national data through the web-based reporting system (in Danish: Analyseportalen) (Figure 1). Data related to the quality indicators are reported yearly in the annual database report. The quality indicators provide clinicians and administrators with information about the quality of the first-trimester screening for chromosomal abnormalities and the second-trimester screening for anomalies (Table 2).
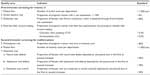  | Table 2 Quality indicators used to measure the quality of the prenatal screening examinations |
A Danish Fetal Medicine Study group was established during implementation of the national database with one representative from all Danish Obstetric/Fetal Medicine Departments joining the study group. This has proven to be essential in the process of cleaning up data, obtaining missing data, and maintenance of the local data collection system.
A major upgrade of the Fetal Medicine Database and the data collection system will be implemented in 2016. It includes an additional number of variables on prenatal ultrasound scanning data and a new function, which enables update of the national database when corrections in the local source data (Astraia) are made.
Follow-up
The database is updated annually with information on congenital malformations and postnatal karyotypes on all live born babies.
Audit of the data used to calculate the detection rates of trisomy 21, neural tube defects, and abdominal wall defects is performed yearly. The audit has recognized that data on fetal malformations retrieved from the Danish Fetal Medicine Database are less complete, especially for the years 2008–2010. In the planned upgrade of the database in 2016, data on fetal malformations will be entered by organ-specific tick boxes in addition to the recorded ICD-10 codes, which is expected to improve the quality of the data substantially.
Examples of research
The database serves as an important data source and has in total provided data for 44 research projects that have been presented at international conferences and/or published in peer-reviewed journals. The first-trimester screening results in Denmark in 2008–2013 have been published in a paper which also provides more detailed information about the database establishment and organization and thus serves as a reference paper for future research based on the database data.4 Due to the large amount of population-based data in the database, it has enabled us to study rare outcomes, such as rare chromosomal abnormalities and other adverse outcomes in both singleton and twin pregnancies.5–8 A recent editorial article in the Scandinavian Journal of Obstetrics and Gynecology complimented the Danish Fetal Medicine Collaboration and their efforts in the establishment of the Danish Fetal Medicine Database.9 The author hopes that the Danish Fetal Medicine Database can be used to “identify associations between early fetal development, obstetric pathologies, and morbidities that are recognized in infancy, and then to use these data prospectively to improve perinatal and infant outcomes” in the future.
Administrative issues and funding
The Danish Fetal Medicine Database has an interdisciplinary steering committee with fetal medicine experts and sonographers from all five regions in Denmark, as well as a clinical geneticist and a representative from the Registry Support Centre of Clinical Quality and Health Informatics (East). This unit has supported the establishment of the database by hosting the servers and developing the software system that provides local and national access to data. After initial establishment of the database, the Danish Fetal Medicine Database was included as one of >60 clinical databases funded, hosted, and supported by the Danish Clinical Registries (RKKP) and financed and owned by the Danish Regions.
The establishment of the Danish Fetal Medicine Database has had an important impact on the national fetal medicine collaboration. The local and national data are discussed at annual meetings and provided the information needed to discuss local differences and possible changes necessary to optimize the national screening program.
Conclusion
Within a few years, it has been possible to establish a national clinical database, including data regarding fetal screening, prenatal diagnostics, and pregnancy outcome. The primary data source is the Astraia system, which is the local fetal medicine database and electronic health care record used at all obstetric/fetal medicine units in Denmark. The quality and completeness of the entered data are extremely high due to the use of data entry validation and decision-aid support. Furthermore, since all data are transferred electronically to the national database, no additional registration or data entry is necessary, thus, there is no extra workload for the clinicians and administrative staff when collecting data.
The Danish Fetal Medicine Study group, with representatives from all departments, has proven to be advantageous in terms of management and maintenance of the data collection system, as well as solving practical and legal issues in the process of cleaning up and obtaining missing data.
Acknowledgments
We thank all sonographers and fetal medicine specialists who daily record the prenatal ultrasound data and the Danish Cytogenetic Central Register for collecting, organizing, and sharing national cytogenetic data with the Danish Fetal Medicine Database. This paper was funded by the Program for Clinical Research Infrastructure (PROCRIN), established by the Lundbeck Foundation and the Novo Nordisk Foundation, and administered by the Danish Regions.
Disclosure
The authors report no conflicts of interest in this work.
References
Sundhedsstyrelsens [webpage on the Internet]. Sundhedsstyrelsens Retningslinjer for Prænatal Diagnostik, 2004 (National Board of Health’s Guideline for Prenatal Diagnostics). Available from: http://www.sst.dk/~/media/413C46E891DC4F3AA94D65FE7C53B70B.ashx. Accessed April 1, 2014. | |
Ekelund CK, Jørgensen FS, Petersen OB, Sundberg K, Tabor A; Danish Fetal Medicine Research Group. Impact of a new national screening policy for Down’s syndrome in Denmark: population based cohort study. BMJ. 2008;337:a2547. | |
Dansk Føtalmedicinsk Selskab [webpage on the Internet]. National Fetal Medicine Guidelines. Available from: http://www.dfms.dk/cms/index.php/2012-06-30-01-20-53/guidelines. Accessed January 14, 2016. | |
Ekelund CK, Petersen OB, Jørgensen FS, et al; Danish Fetal Medicine Research Group. The Danish fetal medicine database: establishment, organization and quality assessment of the first trimester screening program for trisomy 21 in Denmark 2008–2012. Acta Obstet Gynecol Scand. 2015;94(6):577–583. | |
Mathiesen JM, Aksglaede L, Skibsted L, Petersen OB, Tabor A; Danish Fetal Medicine Study Group. Outcome of fetuses with short femur length detected at second-trimester anomaly scan: a national survey. Ultrasound Obstet Gynecol. 2014;44(2):160–165. | |
Petersen OB, Vogel I, Ekelund C, et al; Danish Fetal Medicine Study Group; Danish Clinical Genetics Study Group. Potential diagnostic consequences of applying non-invasive prenatal testing: population-based study from a country with existing first-trimester screening. Ultrasound Obstet Gynecol. 2014;43(3):265–271. | |
Kristiansen M, Joensen B, Ekelund C, Petersen OB, Sandager P; Danish Fetal Medicine Study Group. Perinatal outcome after first-trimester risk assessment in monochorionic and dichorionic twin pregnancies: a population-based register study. BJOG. 2015;122(10):1362–1369. | |
Engelbrechtsen L, Brøndum-Nielsen K, Ekelund C, Tabor A, Skibsted L; Danish Fetal Medicine Study Group. Detection of triploidy at 11–14 weeks’ gestation: a cohort study of 198 000 pregnant women. Ultrasound Obstet Gynecol. 2013;42(5):530–535. | |
Hyett JA. The Danish fetal medicine database: revealing the fruits of collaborative research. Acta Obstet Gynecol Scand. 2015;94(6):561–562. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

