Back to Journals » Journal of Inflammation Research » Volume 16
The Correlation Between Aldosterone and Leukocyte-Related Inflammation: A Comparison Between Patients with Primary Aldosteronism and Essential Hypertension
Authors Rao KR , Bao RY , Ming H, Liu JW, Dong YF
Received 18 February 2023
Accepted for publication 23 May 2023
Published 5 June 2023 Volume 2023:16 Pages 2401—2413
DOI https://doi.org/10.2147/JIR.S409146
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tara Strutt
Kun-Rui Rao,1,* Ru-Yi Bao,1,* Hu Ming,1 Jian-Wei Liu,1 Yi-Fei Dong1,2
1Department of Cardiovascular Medicine, The Second Affiliated Hospital of Nanchang University, Nanchang, People’s Republic of China; 2Key Laboratory of Molecular Biology in Jiangxi Province, Nanchang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yi-Fei Dong, Department of Cardiovascular Medicine, the Second Affiliated Hospital of Nanchang University, Nanchang, People’s Republic of China, Email [email protected]
Background: Hypertension patients with primary aldosteronism (PA) have a higher risk of cardiovascular complications than blood pressure-matched essential hypertension (EH) patients. The cause may be closely related to inflammation. We explored the correlations between leukocyte-related inflammation parameters and plasma aldosterone concentration (PAC) in PA patients and clinical characteristics-matched EH patients.
Methods: A total of 346 PA and 346 sex, age and 24-h blood pressure-matched EH patients at the 2nd Affiliated Hospital of Nanchang University from January 2020 to June 2021 were enrolled in this study. The differences and correlations of aldosterone and leukocyte parameters between the two groups were analyzed.
Results: Compared with EH patients, the lymphocyte count was significantly lower (P = 0.004), the neutrophil–lymphocyte ratio (NLR) (P = 0.023) and the monocyte–lymphocyte ratio (MLR) (P = 0.037) were significantly higher in PA patients. Linear regression analysis and multivariate regression analysis identified that lymphocyte count, NLR and MLR were significantly and independently correlated with PAC in PA patients, and the correlations were stronger with increasing levels of aldosterone. However, in EH patients, only NLR maintained an independent correlation with PAC.
Conclusion: Leukocyte-related inflammation parameters, including lymphocyte count, NLR, and MLR, were significantly and independently correlated with PAC in PA patients. The correlations were stronger with increasing levels of aldosterone. However, the above correlations were not always present in patients with EH matched for clinical characteristics.
Keywords: aldosterone, inflammation, leukocytes, primary aldosteronism, essential hypertension
Introduction
Primary aldosteronism (PA) is a common secondary hypertension characterized by elevated plasma aldosterone concentrations and inhibition of renin activity1. PA accounted for 5.9% of cases in the general hypertensive population2 and more than 4.0% of patients with newly diagnosed hypertension in a Chinese study.3 The prevalence of PA in refractory hypertension was as high as 17 to 23%.4
Compared with patients with essential hypertension (EH) matched by age, sex, and blood pressure level, patients with PA have a higher incidence of adverse cardiovascular and cerebrovascular events and more severe target organ damage.5,6 PA patients also had a higher prevalence of chronic kidney disease, and the degree of renal impairment increased during the development of PA, independent of the blood pressure (BP) control.7 The above data suggest that excess aldosterone may induce adverse events by influencing mechanisms partially independent of blood pressure.5 Previous studies showed that aldosterone increased the expression of inflammatory markers8. The aldosterone receptor antagonist eplerenone was found to attenuate the expression of pro-inflammatory markers in rat hearts, accompanied by attenuated vascular and myocardial damage in rats.9 Another study showed the association between aldosterone and inflammation in the rat heart, indicating a role of aldosterone-related inflammation in the development of cardiovascular disease.10 Excess aldosterone-related low-grade inflammation was suggested to contribute to a high risk of cardiovascular events in PA patients.11,12
Leukocytes are widely used markers of inflammation and include granulocytes (mainly neutrophils), monocytes, and lymphocytes. Compared to that of many other inflammatory markers, the assessment of leukocytes is cheaper, more convenient, and more widely used. Previous study showed that the white blood cell count, neutrophil count, neutrophil ratio, and neutrophil–lymphocyte ratio (NLR) in hypertensive patients were significantly higher than those in normotensive patients.13 Leukocyte-related inflammation markers were also shown to be closely related to an increased risk of developing hypertension13–15 and the severity of hypertension.16
The above studies have suggested a relationship between leukocyte-related inflammatory markers and BP, as well as between aldosterone and inflammation; however, to date, few studies have compared the similarities and differences in leukocyte-related inflammatory markers between PA patients and EH patients.17,18 More importantly, the association between leukocyte-related inflammatory markers and aldosterone in hypertensive populations remains unclear as aldosterone levels change. In this study, we aimed to explore the relationship between leukocyte-related inflammatory markers and aldosterone in EH and PA patients.
Materials and Methods
Subject Source and Grouping
One thousand three hundred and fourteen hypertensive patients received PA screening in the Department of Cardiovascular Medicine at the Second Affiliated Hospital of Nanchang University from January 2020 to June 2021. The diagnostic procedure for PA was based on current guidelines.19 The details were briefly described before admission, patients taking medications that affected the aldosterone/renin ratio (ARR) testing were required to stop or switch to verapamil and/or terazosin for 2–4 weeks. Patients with an ARR larger than 30 (ng/dL)/(ng/mL/h) had their PA diagnosis confirmed using the captopril suppression test or saline infusion test. In diagnosing PA, we require patients to have an upright aldosterone greater than 15 ng/dl, unless the patient has very typical clinical signs of PA, such as persistent hypokalemia, refractory hypertension and abnormal adrenal morphology. The diagnostic procedure for EH was based on current guidelines in China.20 Three hundred and sixty-five patients confirmed PA and six hundred and twenty patients confirmed EH were enrolled for 1:1 propensity score matching with the set caliper value as 0.02 by age, sex, 24 h mean systolic blood pressure (SBP) and diastolic blood pressure (DBP) as covariates. Three hundred and twenty-nine patients were excluded from the study because they met one or more of the following exclusion criteria: (1) age <18 years or pregnancy; (2) immune system, blood system, or acute infectious diseases; (3) interfering drugs (antibiotics, immunosuppressants, corticosteroids, etc.); (4) abnormal baseline total leukocyte count (lower than the lower limit and/or greater than the upper limit of the leukocyte count in the Clinical Laboratory at the Second Affiliated Hospital of Nanchang University); (5) malignant tumor, clinically obvious cerebral infarction, cerebral hemorrhage, heart failure (NYHA grades III or IV), myocardial infarction and endovascular stent placement in the past 3 months. After propensity score matching, 346 PA patients and 346 EH patients were finally entered into this study (Figure 1). The study complies with the Declaration of Helsinki and was approved by the ethics committee of the Second Affiliated Hospital of Nanchang University (IIT-O-2021-032) and was part of the study of the Nanchang Primary Aldosteronism Study which was registered on chictr.org (ChiCTR2200057297). A signed informed consent was obtained from each patient before participation.
General Clinical Data
Age, sex, 24-hour ambulatory blood pressure (Schiller Br-102 Plus Ambulatory BP Monitor), smoking, alcohol consumption, body mass index (BMI), drug use, history of cardiovascular and cerebrovascular diseases (including heart failure, coronary atherosclerotic heart disease, myocardial infarction, atrial infarction, cerebral infarction, cerebral hemorrhage), diabetes, and chronic kidney disease data were collected.
Biochemistry Test
Routine blood tests (total white blood cell count, neutrophil count, lymphocyte count, monocyte count), serum creatinine (Scr), estimated glomerular filtration rate (eGFR), serum potassium, total cholesterol (TC), triglycerides (TG), low-density lipoprotein (LDL), and high-density lipoprotein (HDL) were assessed in the hospital’s central testing laboratory. A fully automated chemiluminescence immunoassay system (Automatic Chemiluminescence Analyzer MAGLUMI 4000 Plus; Shenzhen New Industries Biomedical Engineering Co., Ltd, Shenzhen, China) was used to measure the plasma aldosterone concentration and plasma renin activity (PRA). The NLR, monocyte–lymphocyte ratio (MLR), and aldosterone–renin ratio (ARR) were calculated.
Statistical Analysis
Measurement data following a normal distribution are represented by the mean (standard deviation), and comparisons between two groups were made by independent sample T-tests; those following a nonnormal distribution are represented by the median (interquartile range) and were analyzed by the Mann–Whitney U rank sum test. Categorical variables were expressed as percentages and compared using the chi-square test. The correlations between the lymphocytes and aldosterone were tested by Spearman correlation analysis, and further by multivariate regression. In multivariate regression, model 1 was adjusted by the confounding factors of age, sex and the 24-h mean SBP/DBP, and model 2 was adjusted by the confounding factors of age, sex, 24-h mean SBP/DBP, BMI, eGFR, diabetes, cardiovascular disease, carotid plaque, smoking history, alcohol consumption, antiplatelet drugs and lipid-lowering drugs. Normal distribution transformation of lymphocyte, NLR and MLR were performed by using Box-Cox transform before the multivariate regression. A difference with P <0.05 was considered statistically significant. Statistical analyses were performed using SPSS 25.0 and EmpowerStats 4.1.
Results
Basic Clinical Characteristics of the Patients
This study eventually enrolled 346 patients with EH and 346 patients with PA. Age (P = 0.077), sex (P = 0.589), 24-h mean SBP (P = 0.511), 24-h mean DBP (P = 0.735), BMI (P = 0.121), cigarette smoking (P = 0.759), and alcohol consumption (P = 0.905) were not significantly different between the PA and EH patients. Compared to the EH patients, PA patients had significantly higher rates of combined diabetes (P = 0.015) and chronic kidney disease (P = 0.027), took more lipid-lowering drugs (P = 0.007) and anti-platelet drugs (P = 0.008), had lower levels of TC (P < 0.001), LDL (P < 0.001), eGFR (P < 0.001), serum potassium (P < 0.001) and renin activity (P < 0.001), and had higher levels of plasma aldosterone (P < 0.001) and ARR (P < 0.001) (Table 1).
 |
Table 1 Comparison of the General Clinical Data Between the Two Patient Groups |
Comparison of Leukocytes Between the Two Patient Groups
There were no significant differences in total white blood cell counts (P = 0.472), neutrophil counts (P = 0.529), or monocyte counts (P = 0.913) between PA and EH patients. However, the lymphocyte count was significantly lower in PA patients than that in EH patients (P = 0.004). The NLR (P = 0.023) and the MLR (P = 0.037) in PA patients were significantly higher than those in EH patients (Table 2).
 |
Table 2 Comparison of White Blood Cells Between the Two Patient Groups |
Comparison of White Blood Cells and Aldosterone Among the Morphological and Functional Grouping of PA
Of the 346 patients with PA enrolled in this study, 343 had adrenal CT data. Of these, 118 (34.4%) showed no abnormalities on CT, 99 (28.9%) suggested adenomas, and the remaining 126 (36.7%) suggested changes such as adrenal nodules and/or hyperplasia. As shown in the Supplement Table 1, we did not find differences in total white blood cell counts (P = 0.895), neutrophil counts (P = 0.600), lymphocyte counts (P = 0.566), monocyte counts (P = 0.806), NLR (P = 0.240), and MLR (P = 0.539) among the three groups. However, there were significant differences in upright aldosterone (P < 0.001) among the three groups. We further grouped the PA patients functionally. Three hundred and thirty-six of 343 PA patients underwent adrenal vein sampling (AVS), of which 269 were successful bilaterally. The 269 patients with successful AVS were divided into unilateral PA (n = 154, 58%) and bilateral PA (n = 115, 42%) according to their results. As shown in the Supplement Table 2, there were no significant differences in total white blood cell counts (P = 0.694), neutrophil counts (P = 0.690), lymphocyte counts (P = 0.054), monocyte counts (P = 0.561), NLR (P = 0.099) and upright aldosterone (p = 0.464) between unilateral PA patients and bilateral PA patients. However, the unilateral PA patients had a significantly higher MLR than the bilateral PA patients (p = 0.035).
Correlation Analysis of the Lymphocyte, NLR, and MLR with Aldosterone Levels
Table 3 shows the correlation analysis of the lymphocyte count, NLR, MLR and aldosterone levels in the overall hypertensive patients. The lymphocyte counts were significantly and negatively correlated with the aldosterone levels in PA patients (r = −0.226, P < 0.001) (Figure 2a) and the total population (PA and EH patients) (r = −0.155, P < 0.001). In contrast, such a correlation was not found in EH patients. The NLR was significantly and positively correlated with the aldosterone levels in PA patients (r = 0.221, P < 0.001) (Figure 2b), EH patients (r = 0.116, P = 0.032) and the total population (r = 0.187, P < 0.001). The MLR was significantly and positively correlated with the aldosterone levels in PA patients (r = 0.113, P = 0.035) (Figure 2c) and the total population (r = 0.079, P = 0.038). Such a correlation was not found in EH patients.
 |
Table 3 Correlation Analysis of the Lymphocyte Count, NLR, MLR and Aldosterone Levels in Hypertensive Patients |
Multivariate Regression Analysis of the Lymphocyte, NLR, MLR, and Aldosterone Levels
Table 4 shows the correlations of the lymphocyte, NLR, and MLR with aldosterone levels in total population by multivariate regression analysis. Lymphocyte counts were significantly and negatively correlated with aldosterone levels in unadjusted model and adjusted models 1 and 2, indicating an independent correlation between lymphocyte and aldosterone levels in total population. Subjects were ranked by aldosterone level and then divided equally into 3 subgroups based on the number of subjects. The mean aldosterone levels in each group (13.43, 21.40, and 33.27 ng/dl in T1, T2, and T3 groups, respectively) are shown in Supplement Table 3. The P for trends were maintained significant not only in unadjusted model (P < 0.001) but also in adjusted model 1 (P < 0.001) and 2 (P = 0.001), indicating a stronger correlation between lymphocyte and aldosterone with the increasing of aldosterone level. A significant correlation between NLR/MLR and aldosterone levels was present in unadjusted model and adjusted models 1 and 2, indicating an independent correlation between NLR/MLR and aldosterone levels in total population. After trisecting the group according to aldosterone levels, p for trends of NLR was maintained significant not only in unadjusted model (P < 0.001) but also in adjusted model 1 (P < 0.001) and 2 (P < 0.001), and p for trends of MLR were significant in adjusted model 1 (P = 0.003) and 2 (P = 0.014), indicating a stronger correlation between NLR/MLR and aldosterone with the increasing of aldosterone level.
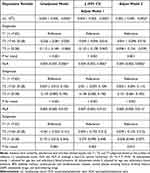 |
Table 4 Multivariate Regression Analysis of the Lymphocyte Count, NLR, MLR, and Aldosterone Levels in Hypertensive Patients |
Table 5 shows the correlations of the lymphocyte, NLR, and MLR with aldosterone levels in PA and EH patients by multivariate regression analysis. Subjects were ranked by aldosterone level and then divided equally into 3 subgroups based on the number of subjects. The average level of each tertile in EH group was 11.79, 18.00, and 26.56 ng/dl, respectively (Supplement Table 3). The average level of each tertile in PA group was 16.65, 24.48, and 39.33 ng/dl, respectively (Supplement Table 3). Lymphocyte counts were significantly and negatively correlated with aldosterone levels in the unadjusted model and adjusted models 1 and 2 in PA patients. However, such a correlation was not found in EH patients, indicating the negative correlation of lymphocyte and aldosterone levels present independently only in PA patients but not in EH patients. After trisecting the group according to aldosterone levels, the P for trend remained significant not only in the unadjusted model (P < 0.001) but also in the adjusted models 1 (P < 0.001) and 2 (P = 0.004), indicating a stronger correlation between lymphocytes and aldosterone as aldosterone levels increased in PA patients. In contrast, p for trend was not significant in EH patients. NLR was significantly and positively correlated with aldosterone levels in both PA and EH patients. After trisecting the group according to aldosterone levels, p for trends remained significant not only in the unadjusted model but also in the adjusted models 1 and 2 for both PA and EH patients, indicating that NLR and aldosterone levels were correlated independently, and such a correlation increased as aldosterone increased in PA and EH patients. Compared to NLR, a significant correlation between MLR and aldosterone was only found in the adjusted model 1 of PA patients suggesting that the association between MLR and aldosterone levels was unstable in PA patients. In contrast, for EH patients, no significant correlation between MLR and aldosterone levels was found in either model. Supplement Table 4 shows the white blood cell count, neutrophil count, lymphocyte count, monocyte count, NLR, and MLR within each tertile range in different groups.
 |
Table 5 Multivariate Regression Analysis of the Lymphocyte Count, NLR, MLR, and Aldosterone Levels in PA and EH Patients |
Discussion
In this study, we found that lymphocyte counts were significantly lower and NLR and MLR were significantly higher in PA patients compared with EH patients. We also found that lymphocyte count, NLR, and MLR, were significantly and independently correlated with plasma aldosterone levels in the whole hypertensive population. These correlations remained significant in PA patients and were more robust as the aldosterone increased in the whole hypertensive population and PA patients. However, the above correlations were not always present in patients with EH matched for clinical characteristics. With these results, our study supported the role of aldosterone in inflammation in hypertensive patients.
Previously, few studies had investigated aldosterone-related inflammation in human beings. One study found that the NLR did not differ between PA patients and EH patients, and NLR was correlated with plasma aldosterone levels only in PA patients.17,18 Another study found that PA patients had a higher MLR than EH patients, and neutrophil counts were positively correlated with plasma aldosterone levels, and lymphocyte counts were negatively correlated with plasma aldosterone levels in all patients.17,18 However, the previous study did not match BP levels between PA patients and EH patients, and the office BP levels were significantly higher in PA patients than in EH patients. The effect of BP levels on leukocyte-related inflammatory markers has been confirmed by extensive studies. The latter study had a very limited number of subjects and included a total of 15 PA patients and 15 EH patients.
The NLR is a cheap, easily obtained and widely available inflammatory marker. On the one hand, it reflects the role of neutrophils (responsible for the nonspecific immune response in inflammation); on the other hand, it reflects the role of lymphocytes as a key player in a specific immune response, so the NLR represents the ratio of two opposite but complementary immune pathways. Compared to leukocytes, the NLR is much less affected by physiological conditions.21,22 Data from an international study showed that a healthy adult NLR had values between 0.78 and 3.53, with an average NLR of 1.65.23 The average NLRs for healthy men and women were 1.550 and 1.587, respectively.24 In hypertension studies, an increased NLR (especially an NLR > 2) was significantly associated with hypertensive events, especially in the older male population.14,15 An elevated NLR was also independently associated with the severity of hypertension in untreated EH patients.16 The mean NLR of EH patients in this study was 2.01, which was higher than that of the healthy population, indicating the presence of systemic low-grade inflammation in patients with hypertension. This study also showed that the mean NLR of PA patients was 2.21, which was significantly higher than that of EH patients. Combined with the significant positive association between NLR and aldosterone levels, these results suggested that PA patients had higher levels of inflammation than EH patients and that this inflammation was positively correlated with aldosterone levels. Persistent systemic inflammation may be harmful and will lead to progressive tissue damage, organ dysfunction, and fibrosis. An elevated NLR had been found to be associated with the presence and amount of carotid atherosclerotic plaque25 and the severity of coronary artery disease.26 The disease-related NLR was independently associated with left ventricle (LV) hypertrophy in hypertensive patients and could be used as a simple indicator to judge LV hypertrophy.27 Other studies have found a significantly higher NLR in nondipper hypertension than in dipper hypertension.28 Patients with resistant hypertension also had a higher NLR than those with BP control.29 Therefore, an elevated NLR positively associated with aldosterone levels may be one of the reasons for the significantly higher risk of cardiovascular and cerebrovascular adverse events in PA patients than in EH patients, and the NLR is expected to be a biomarker indicating PA severity.
Monocytes play a role in innate immunity; they remain in a steady state in blood vessels and cross the endothelium when stimulated with inflammatory cytokines into the vascular intima to differentiate into macrophages that absorb lipids to form foam cells that account for a large proportion of atherosclerotic plaques.30 The results of a cross-sectional community study showed that the monocyte count was an independent predictor of subclinical carotid atherosclerosis.31 The percentage and number of monocytes may be used to predict cardiovascular events.32 Lymphocytes are an important part of the body’s immune system, and lymphopenia was found to be an independent risk factor for cardiovascular complications in kidney transplant recipients.33 The MLR combines risk factor monocytes with protective factor lymphocytes, and this increase may be associated with poorer outcomes of various diseases. A cohort study found that the MLR was associated with an increased risk of chronic kidney disease and that the MLR remained an independent risk factor for chronic kidney disease after adjustment for factors such as age, sex, and systolic blood pressure.34 Other studies have indicated that the MLR can be used as an independent predictor of the severity of carotid artery stenosis in ischemic stroke patients and can more effectively reflect the severity of coronary artery disease than the NLR.35,36 In the present study, we found that MLR was significantly higher in PA patients than in EH patients. At the same time, the correlation between MLR and aldosterone levels persisted in PA patients but not in EH patients. These results suggested that as excess aldosterone increased, more inflammation-related indicators occurred. It indicated the presence of higher levels of inflammation in PA patients, and further, it supported that higher inflammation levels in PA patients were associated with higher aldosterone levels.
The low lymphocyte count was regarded as a marker of inflammation and immunosuppression, and a low lymphocyte percentage was associated with a higher risk of frailty, suggesting that lymphocytes might help in assessing patient health status.37 On the other hand, observational and genetic analyses demonstrated a positive and potentially causal relationship between lymphocytes with a slight increase in SBP and DBP.38 These studies suggest a complex role of immune cells in diseases. This study also found that the lymphocyte count in PA patients was lower than that in EH patients and that the lymphocyte count was negatively correlated with aldosterone levels in PA patients, suggesting that aldosterone may have an inhibitory effect on lymphocytes.
Our study has limitations. First, we could not observe changes in leukocyte-related inflammatory markers in PA patients after the drug or interventional/surgical treatment, which is a significant limitation of the study. Comparing changes in leukocyte-related inflammatory markers before and after treatment in PA patients would provide valuable information for exploring aldosterone-related inflammation. Second, including non-hypertensive subjects matched for clinical characteristics would be more helpful in investigating the association between leukocyte-related inflammatory markers and high blood pressure. Third, the use of antihypertensive drugs (verapamil and/or terazosin), lipid-lowering drugs and antiplatelet agents might affect leukocyte-related inflammatory markers, and we could not match these the drug types and doses in the study. Fourth, limited by cross-sectional studies, our results could not yield a causal association between PA and leukocyte-associated inflammatory markers. Fifth, future analysis between other important inflammatory markers such as CRP, IL-6, TNF-α, and aldosterone levels would provide valuable information for the study of the role of inflammation in PA. Sixth, prospective observation of blood pressure levels and analysis of the correlation between blood pressure changes and leukocytes may provide important information on the correlation between aldosterone and leukocyte-related inflammation. Seventh, some EH patients who did not meet the diagnostic criteria for a confirmatory test but had high absolute values of aldosterone and an ARR >30 might develop typical PA after several years. It thus needed to be considered when interpreting the differences between the two groups of patients in the present study. Finally, in our study, cases were enrolled from one medical center, and despite the relatively large number of cases, there might still be a selection bias. In the present study, neither morphological nor functional subgroups provided data to support a causal association between lymphocyte count and PA. Therefore, the design of relevant prospective cohort studies may provide more research data for the causal association between the two. Moreover, in the future, a longitudinal cohort study of EH/PA populations and typing lymphocytes may provide more precise data for studies between aldosterone and lymphocyte-associated inflammation.
In summary, lymphocyte count significantly decreased, and NLR and MLR significantly increased in PA patients compared to EH patients. Further, these leukocyte-associated inflammatory markers were weakly but significantly correlated with aldosterone levels in PA patients. However, such a correlation was not always present in EH patients.
Data Sharing Statement
Data of this study are available upon request from the corresponding author Yi-Fei Dong.
Funding
The present study was funded by the National Natural Science Foundation of China (32260214).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Unger T, Borghi C, Charchar F, et al. 2020 international society of hypertension global hypertension practice guidelines. Hypertension. 2020;75(6):1334–1357. doi:10.1161/HYPERTENSIONAHA.120.15026
2. Monticone S, Burrello J, Tizzani D, et al. Prevalence and clinical manifestations of primary aldosteronism encountered in primary care practice. J Am Coll Cardiol. 2017;69(14):1811–1820. doi:10.1016/j.jacc.2017.01.052
3. Xu Z, Yang J, Hu J, et al. Primary aldosteronism in patients in China with recently detected hypertension. J Am Coll Cardiol. 2020;75(16):1913–1922. doi:10.1016/j.jacc.2020.02.052
4. Calhoun DA. Is there an unrecognized epidemic of primary aldosteronism? Pro. Hypertension. 2007;50(3):447–453. doi:10.1161/HYPERTENSIONAHA.106.086116
5. Milliez P, Girerd X, Plouin PF, Blacher J, Safar ME, Mourad JJ. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol. 2005;45(8):1243–1248. doi:10.1016/j.jacc.2005.01.015
6. Monticone S, Moretti C, Ascenzo F, et al. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2018;6(1):41–50. doi:10.1016/S2213-8587(17)30319-4
7. Fernández-Argüeso M, Pascual-Corrales E, Bengoa Rojano N, García Cano A, Jiménez Mendiguchía L, Araujo-Castro M. Higher risk of chronic kidney disease and progressive kidney function impairment in primary aldosteronism than in essential hypertension. Case-control study. Endocrine. 2021;73(2):439–446.
8. Muñoz-Durango N, Vecchiola A, Gonzalez-Gomez LM, et al. Modulation of immunity and inflammation by the mineralocorticoid receptor and aldosterone. Biomed Res Int. 2015;2015:652738. doi:10.1155/2015/652738
9. Rocha R, Rudolph AE, Frierdich GE, et al. Aldosterone induces a vascular inflammatory phenotype in the rat heart. Am J Physiol Heart Circ Physiol. 2002;283(5):H1802–10. doi:10.1152/ajpheart.01096.2001
10. Sun Y, Zhang J, Lu L, Chen SS, Quinn MT, Weber KT. Aldosterone-induced inflammation in the rat heart: role of oxidative stress. Am J Pathol. 2002;161(5):1773–1781. doi:10.1016/S0002-9440(10)64454-9
11. Rickard AJ, Morgan J, Tesch G, Funder JW, Fuller PJ, Young MJ. Deletion of mineralocorticoid receptors from macrophages protects against deoxycorticosterone/salt-induced cardiac fibrosis and increased blood pressure. Hypertension. 2009;54(3):537–543. doi:10.1161/HYPERTENSIONAHA.109.131110
12. Ferreira NS, Tostes RC, Paradis P, Schiffrin EL. Aldosterone, Inflammation, Immune System, and Hypertension. Am J Hypertens. 2021;34(1):15–27. doi:10.1093/ajh/hpaa137
13. Pusuroglu H, Akgul O, Erturk M, et al. A comparative analysis of leukocyte and leukocyte subtype counts among isolated systolic hypertensive, systo-diastolic hypertensive, and non-hypertensive patients. Kardiol Pol. 2014;72(8):748–754. doi:10.5603/KP.a2014.0044
14. Liu X, Zhang Q, Wu H, et al. Blood neutrophil to lymphocyte ratio as a predictor of hypertension. Am J Hypertens. 2015;28(11):1339–1346. doi:10.1093/ajh/hpv034
15. Jhuang YH, Kao TW, Peng TC, et al. Neutrophil to lymphocyte ratio as predictor for incident hypertension: a 9-year cohort study in Taiwan. Hypertens Res. 2019;42(8):1209–1214. doi:10.1038/s41440-019-0245-3
16. Çimen T, Sunman H, Efe TH, et al. The relationship between 24-hour ambulatory blood pressure load and neutrophil-to-lymphocyte ratio. Rev Port Cardiol. 2017;36(2):97–105. doi:10.1016/j.repc.2016.07.009
17. Renata L, Jinbo H, Chee Min R, et al. A multicenter study of neutrophil-to-lymphocyte ratio in primary aldosteronism. J Endocr Soc. 2020;4(12). doi:10.1210/jendso/bvaa153
18. van der Heijden C, Smeets E, Aarntzen E, et al. Arterial wall inflammation and increased hematopoietic activity in patients with primary aldosteronism. J Clin Endocrinol Metab. 2020;105(5):e1967–80. doi:10.1210/clinem/dgz306
19. Funder JW, Carey RM, Mantero F, et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(5):1889–1916. doi:10.1210/jc.2015-4061
20. Liu LS. Chinese guidelines for the prevention and treatment of hypertension. Chin Cardiovasc J. 2019;24(1):24–56. doi:10.3969/j.issn.1007-5410.2019.01.002
21. Azab B, Zaher M, Weiserbs KF, et al. Usefulness of neutrophil to lymphocyte ratio in predicting short- and long-term mortality after non-ST-elevation myocardial infarction. Am J Cardiol. 2010;106(4):470–476. doi:10.1016/j.amjcard.2010.03.062
22. Bhat T, Teli S, Rijal J, et al. Neutrophil to lymphocyte ratio and cardiovascular diseases: a review. Expert Rev Cardiovasc Ther. 2013;11(1):55–59. doi:10.1586/erc.12.159
23. Forget P, Khalifa C, Defour JP, Latinne D, Van Pel MC, De Kock M. What is the normal value of the neutrophil-to-lymphocyte ratio. BMC Res Notes. 2017;10(1):12. doi:10.1186/s13104-016-2335-5
24. Wang J, Zhang F, Jiang F, Hu L, Chen J, Wang Y. Distribution and reference interval establishment of neutral-to-lymphocyte ratio (NLR), lymphocyte-to-monocyte ratio (LMR), and platelet-to-lymphocyte ratio (PLR) in Chinese healthy adults. J Clin Lab Anal. 2021;35(9):e23935. doi:10.1002/jcla.23935
25. Corriere T, Di marca S, Cataudella E, et al. Neutrophil-to-Lymphocyte Ratio is a strong predictor of atherosclerotic carotid plaques in older adults. Nutr Metab Cardiovasc Dis. 2018;28(1):23–27. doi:10.1016/j.numecd.2017.10.022
26. Kaya H, Ertaş F, Soydinç MS. Association between neutrophil to lymphocyte ratio and severity of coronary artery disease. Clin Appl Thromb Hemost. 2014;20(2):221. doi:10.1177/1076029613499821
27. Yu X, Xue Y, Bian B, et al. NLR-A simple indicator of inflammation for the diagnosis of left ventricular hypertrophy in patients with hypertension. Int Heart J. 2020;61(2):373–379. doi:10.1536/ihj.19-138
28. Sunbul M, Gerin F, Durmus E, et al. Neutrophil to lymphocyte and platelet to lymphocyte ratio in patients with dipper versus non-dipper hypertension. Clin Exp Hypertens. 2014;36(4):217–221. doi:10.3109/10641963.2013.804547
29. Belen E, Sungur A, Sungur MA, Erdoğan G. Increased neutrophil to lymphocyte ratio in patients with resistant hypertension. J Clin Hypertens. 2015;17(7):532–537. doi:10.1111/jch.12533
30. Auffray C, Fogg D, Garfa M, et al. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317(5838):666–670. doi:10.1126/science.1142883
31. Chapman CM, Beilby JP, McQuillan BM, Thompson PL, Hung J. Monocyte count, but not C-reactive protein or interleukin-6, is an independent risk marker for subclinical carotid atherosclerosis. Stroke. 2004;35(7):1619–1624. doi:10.1161/01.STR.0000130857.19423.ad
32. Berg KE, Ljungcrantz I, Andersson L, et al. Elevated CD14++CD16- monocytes predict cardiovascular events. Circ Cardiovasc Genet. 2012;5(1):122–131. doi:10.1161/CIRCGENETICS.111.960385
33. Ducloux D, Challier B, Saas P, Tiberghien P, Chalopin JM. CD4 cell lymphopenia and atherosclerosis in renal transplant recipients. J Am Soc Nephrol. 2003;14(3):767–772. doi:10.1097/01.ASN.0000048718.43419.44
34. Zhang M, Wang K, Zheng H, Zhao X, Xie S, Liu C. Monocyte lymphocyte ratio predicts the new-onset of chronic kidney disease: a cohort study. Clin Chim Acta. 2020;503:181–189. doi:10.1016/j.cca.2019.11.021
35. Chen H, Li M, Liu L, Dang X, Zhu D, Tian G. Monocyte/lymphocyte ratio is related to the severity of coronary artery disease and clinical outcome in patients with non-ST-elevation myocardial infarction. Medicine. 2019;98(26):e16267. doi:10.1097/MD.0000000000016267
36. Zuo B, Zhu S, Meng X, Zhao D, Zhang J. Monocyte/lymphocyte ratio is associated with carotid stenosis in ischemic stroke: a retrospective analysis. Brain Behav. 2019;9(10):e01429. doi:10.1002/brb3.1429
37. Núñez J, Sastre C, D’Ascoli G, et al. Relation of low lymphocyte count to frailty and its usefulness as a prognostic biomarker in patients >65 years of age with acute coronary syndrome. Am J Cardiol. 2020;125(7):1033–1038. doi:10.1016/j.amjcard.2020.01.006
38. Siedlinski M, Jozefczuk E, Xu X, et al. White blood cells and blood pressure: a Mendelian randomization study. Circulation. 2020;141(16):1307–1317. doi:10.1161/CIRCULATIONAHA.119.045102
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


