Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 18
The COPD Assessment Test (CAT) and Depression: A Longitudinal Analysis During the COVID-19 Pandemic
Received 16 January 2023
Accepted for publication 28 April 2023
Published 13 June 2023 Volume 2023:18 Pages 1187—1195
DOI https://doi.org/10.2147/COPD.S405050
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Augusta Beech,1,2 Dave Singh1,2
1Division of Infection, Immunity and Respiratory Medicine, School of Biological Sciences, Faculty of Biology, Medicine and Health, Manchester Academic Health Science Centre, The University of Manchester, Manchester, UK; 2Medicines Evaluation Unit, Manchester University NHS Foundation Trust, Manchester, UK
Correspondence: Augusta Beech, Department of Medicine and Health, University of Manchester, Education and Research Centre, Manchester, M23 9LT, UK, Tel +44 161 946 4050, Fax +44 161 946 1459, Email [email protected]
Purpose: Chronic obstructive pulmonary disease (COPD) is multifaceted, with some patients experiencing anxiety and depression. Depression in COPD has been associated with worse total scores for the COPD assessment test (CAT). Also, CAT score worsening has been observed during the COVID-19 pandemic. The relationship between the Center for Epidemiologic Studies Depression Scale (CES-D) score and CAT sub-component scores has not been evaluated. We investigated the relationship between CES-D score and CAT component scores during the COVD-19 pandemic.
Patients and Methods: Sixty-five patients were recruited. Pre-pandemic (baseline) was defined as 23rd March 2019– 23rd March 2020, CAT scores and information related to exacerbations were collected via telephone at 8-week intervals between 23rd March 2020– 23rd March 2021.
Results: There were no differences in CAT scores pre- compared to during the pandemic (ANOVA p = 0.97). Total CAT scores were higher in patients with symptoms of depression compared to those without both pre- (p < 0.001) and during-pandemic (eg, at 12 months 21.2 versus 12.9, mean difference = 8.3 (95% CI = 2.3– 14.2), p = 0.02). Individual CAT component scores showed significantly higher chest tightness, breathlessness, activity limitation, confidence, sleep and energy scores in patients with symptoms of depression at most time points (p < 0.05). Significantly fewer exacerbations were observed during- compared to pre-pandemic (p = 0.04). We observed that COPD patients with symptoms of depression had higher CAT scores both pre- and during the COVID-19 pandemic.
Conclusion: Presence of depressive symptoms was selectively associated with individual component scores. Symptoms of depression may potentially influence total CAT scores.
Keywords: COPD, depression, COPD assessment test, CAT, center for epidemiological studies depression score, CES-D
Introduction
Patients with chronic obstructive pulmonary disease (COPD) suffer with dyspnoea, reduced exercise capacity, cough and sputum production, reduced quality of life and exacerbations.1 Some individuals also experience anxiety and depression, with prevalence estimates ranging from 10% to 42%.2 Risks of healthcare resource utilisation are reportedly higher in COPD patients with anxiety and depression compared to those without.3,4 Furthermore, depression is associated with a worse quality of life in COPD patients.5
The COPD assessment test (CAT) is commonly used to measure the impact of COPD, providing a short and simple measure of COPD-related health status.6 This tool is used for measuring patient reported outcomes in both clinical practice and research. CAT is composed of 8 questions which assess different symptomatic and psychometric components, on a semantic differential scale, from which a composite score is calculated.6 Total CAT score may be affected by multiple comorbidities in COPD; arrhythmias, gastroesophageal reflux disease (GERD), and anxiety and/or depression,7 with depressive symptoms found to be associated with higher total CAT scores using various definitions of depression.7–12 Furthermore, the presence of depression and relationship with individual CAT items has been investigated using Hospital Anxiety and Depression Scale (HADS), Beck’s depression inventory (BDI) and current use of anti-depressant medication to define depression.7,9,13 The results of these studies have differed, partly due to differences in the instruments used to define depression. The Center for Epidemiological Studies Depression (CES-D) score is a well-established tool for assessing symptoms of depression.14 The relationship between CES-D scores and CAT sub-component scores has not been evaluated.
COPD patients have increased susceptibility to viral infection including coronaviruses,15 and appear to have worse outcomes from coronavirus 19 (COVID-19).16 During the COVID-19 pandemic, mitigating measures were enforced such as national and local “lock-downs” thereby reducing human mobility and promoting self-isolation and shielding of clinically vulnerable individuals to prevent infection. COPD patients were identified as an at-risk group during the pandemic due to several potentially negative interrelationships between COPD and COVID-19,17 including increased susceptibility to viral infection,15,18,19 impaired pulmonary function and presence of extra-pulmonary comorbidities.16 Self-isolation was a contributing factor to the reduction in COPD exacerbation rates reported during the pandemic. Despite this reduction in exacerbations, the CAT has shown worsening of the impact of COPD during the pandemic.20 Furthermore, the prevalence of depression and anxiety has increased in both the general population21 and in COPD patients during the pandemic.20,22,23 These observations suggest a significant interaction between depression and COPD-related health status during the pandemic, although this has not been directly investigated.
We performed a longitudinal cohort study to evaluate the relationship between symptoms of depression, defined using the CES-D questionnaire, and CAT scores, including individual items. As a secondary objective, we also studied changes in total CAT score and exacerbation rates, and the relationship between these clinical outcomes.
Materials and Methods
Study Cohort
Sixty-five patients with physician diagnosed COPD were recruited from the Medicines Evaluation Unit (Manchester University NHS Foundation Trust). The following inclusion criteria were met by patients recruited in this study: spirometrically confirmed airway obstruction at baseline (FEV1/FVC <70%), no previous asthma diagnosis and a pack-year history of >10 and were aged ≥40 years old. Patients provided written informed consent using protocols approved by local Ethics Committees (Tameside & Glossop, reference: 05/Q1402/41 and North West – Preston, reference: 16/NW/0836) and the study was conducted in accordance with the Declaration of Helsinki.
Study Design
The present study utilised both prospective data collection and retrospective data collected from an internal database at the Medicines Evaluation Unit, a schematic representation of data collection is presented in Figure 1. The pre-pandemic period (baseline) consisted of retrospective data collected between 23rd March 2019–23rd March 2020; demographics, including baseline CAT scores and spirometry, were taken from the most recent measurement during the stable state; 30 patients also had a pre-pandemic CES-D score. Spirometry data were available at baseline only. Data from the pandemic period were prospectively collected between 23rd March 2020–23rd March 2021; CAT scores (during stable state, not during exacerbations) and information related to exacerbations were collected at 8-week intervals (6 time points) via telephone call. Exacerbations were defined by patient recall, additional therapy and health-care utilisation was recorded. A baseline CES-D score obtained from the retrospective data collection (available for n = 30 patients) ≥16 defined the presence of depression symptoms.6
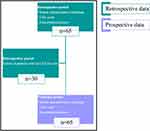 |
Figure 1 Schematic representation of data collection. Notes: Turquoise and purple colours represent retrospective and prospective data collection, respectively. |
CES-D and CAT Scores
The impact of COPD was assessed at baseline using the CAT questionnaire, 8 questions assessed individual components including: cough, phlegm, chest tightness, breathlessness after walking up a hill or one flight of stairs, activity limitation at home, confidence leaving the home, sleep quality and energy. Meanwhile, the presence of depression symptoms was assessed using the CES-D questionnaire, which assessed components including: depressed mood, feelings of guilt and worthlessness, feelings of helplessness and hopelessness, psychomotor retardation, loss of appetite, and sleep disturbance.14 Several questions are used to assess each individual component for the CES-D scale, with a total of 20 questions.
Statistical Analysis
No formal power calculations were undertaken, as the nature of patient recruitment at the start of the pandemic was opportunistic and we attempted to include as many patients as possible. Comparisons between parametric data were analysed using a Student’s t-test or a repeated measures ANOVA, with the Geisser-Greenhouse correction and post-hoc assessment using a Dunnetts’ multiple comparisons test. Exacerbation rates prior to and during the pandemic were compared using a Student’s t-test. Associations between parametric variables were assessed using a Pearson correlation coefficient. Analyses were performed using GraphPad Prism version 9.00 (San Diego, USA). p < 0.05 was considered statistically significant.
Results
Sixty-five COPD patients were recruited. The cohort mean pack-year history was 41.5; 25 (38.5%) were current smokers. The baseline demography is presented in Table 1; mean (SD) post-bronchodilator FEV1 was 67.2% (16.6) predicted, with most patients being GOLD stage 2 (n = 43; 66.2%). The mean post-bronchodilator FEV1/FVC ratio was 53.1%, while the baseline exacerbation rate was 0.89/year. The mean total CAT score was 16.9 and CES-D score (n = 30) was 12.8.
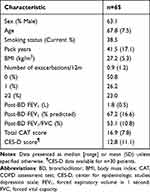 |
Table 1 Baseline Characteristics for COPD Patients (n = 65) |
CAT Scores Pre- versus During Pandemic
Most patients completed a high number of CAT assessments; 39 patients provided data for all 6 time-points, with 20 providing 5 time points, and 6 provided 3 or 4 time points. Mean total CAT scores were similar pre- versus all time points during the pandemic (ANOVA p = 0.88); baseline score (SD) = 16.9 (7.8); pandemic scores = 17.1 (8.1), 17.4 (8.3), 17.2 (7.4), 17.0 (8.1), 16.1 (7.5) and 16.4 (7.8) at 8-week intervals up to 1 year.
Relationship of Depression Symptoms with CAT Scores
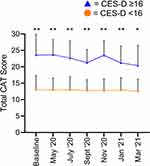
Within the subset of patients with baseline CES-D scores available, a strong correlation was observed between baseline CES-D and total CAT scores, both at baseline (rho = 0.70, p < 0.0001, Figure 2A) and during the pandemic period at 12 months (rho = 0.61, p = 0.001, Figure 2B). At baseline, 12 patients (40%) showed clinically significant levels of depression symptoms (CES-D score ≥16). Total CAT scores were higher in patients with symptoms of depression compared to those without pre-pandemic (23.6 versus 13.1, mean difference = 10.5 (95% CI = 4.9 to 16.1), p < 0.001, Figure 3) and at all time-points during the pandemic (eg, at 12 months 21.2 versus 12.9, mean difference = 8.3 (95% CI = 2.3–14.2), p = 0.02, Figure 3). The individual component scores showed significantly higher chest tightness, breathlessness, activity limitation, confidence, sleep and energy scores in patients with symptoms of depression at most time points (p < 0.05), while cough and phlegm scores showed no consistent differences (Figure 4).
Exacerbation Rates During Pandemic
Significantly fewer exacerbations were reported during- compared to pre-pandemic (means: 0.60 versus 0.89 exacerbations/year respectively, p = 0.04). No patients had PCR-confirmed COVID-19 infection during the study period.
To assess the association between total CAT score and exacerbations, the cohort was grouped based on the number of exacerbations in the year before the pandemic (0 exacerbations; n = 33 and ≥1 exacerbation; n = 32) and during the pandemic period (0 exacerbations; n = 44 and ≥1 exacerbation; n = 21). No difference in baseline total CAT score was observed between exacerbation defined groups (total CAT = 15.52 and 18.28 for patients with 0 versus ≥1 exacerbation, respectively, p = 0.15). Similar observations were observed during the pandemic, with no differences in total CAT score observed between exacerbation defined groups at any time point (p > 0.05 for all comparisons).
Discussion
In a well-characterised cohort of COPD patients, we observed that COPD patients with symptoms of depression suffered a higher impact of COPD, measured using the CAT score, both before and during the COVID-19 pandemic. Furthermore, specific CAT component scores were higher in patients with symptoms of depression, namely chest tightness, breathlessness, activity limitation, confidence, sleep and energy scores. CAT scores were unchanged during compared to pre-pandemic, while there was a reduction in COPD exacerbations during the pandemic.
CAT provides an overall composite score, which does not inform a clinician which disease symptoms or characteristics require further assessment or management. We identified 6 CAT components which were higher in patients with symptoms of depression (chest tightness, dyspnoea, activity limitation, confidence, sleep and energy scores). Treatable traits are disease components,24 within a complex condition such as COPD, requiring further management. Sub-analysis of the CAT score presented here shows how symptoms of depression (a treatable trait) are associated with specific components of the CAT score (other treatable traits), highlighting how treatable traits often “cluster”.25
Psychological disturbances are underreported and undertreated in COPD.26 Depression in COPD is associated with a worse quality of life,5 and the present analysis extends such findings to reveal an association between depression (as assessed by CES-D score) and the impact of COPD. Using DSM IV criteria to define depression, a previous study reported that a total CAT score >20 was associated with major depression,11 which is in concordance with the present analysis where patients with symptoms of depression had a mean CAT score >20. Our findings suggest that symptoms of depression may strongly influence total CAT score, which has potential implications for its use in both research and clinical practice.
Here, total CAT and CES-D scores showed a strong correlation both at baseline and during the pandemic period. These results indicate that in COPD patients with an elevated CAT score, the possibility of concomitant depression being present is higher and should therefore be assessed clinically. We have evaluated associations, so we are unable to elucidate “cause and effect”, as it is possible that patients with depression may self-report higher CAT scores or conversely patients who experience a greater impact on life due to COPD (higher CAT score) may be more likely to suffer depression symptoms. Of the 20 questions included in the CES-D score, only one question is similar to the components measured in the CAT, namely sleep quality (questions 11 and 7 of the CES-D and CAT scales, respectively). The correlation between these scores is therefore unlikely to be driven by similarity of the questions used.
A previous COPD study20 reported that CAT scores worsened during the pandemic, while we observed no overall change in CAT scores, although our smaller sample size (n = 65 versus 375) likely affected the ability to detect a change. In this previous study, component score analysis showed that chest tightness, dyspnoea, confidence in leaving the home and energy clearly worsened during the pandemic, there was a borderline worsening of activity limitation, and cough, sputum production and sleep habits were unchanged. In the present analysis, 4 CAT components associated with symptoms of depression clearly overlapped with the components previously shown to worsen during the pandemic. Taken together, these findings show that CAT items measuring psychosomatic (confidence and energy) and some symptom (dyspnoea and chest tightness) components, but not all (cough and phlegm), are related to symptoms of depression and in a previous study worsened during the pandemic.
Our findings relating to the association between some individual CAT items and symptoms of depression are mostly consistent with previous observations; using HADS to define depression in a large Japanese cohort, authors found that depression was associated with chest tightness, activity limitation and a reduction in confidence, sleep and energy.9 Elsewhere, depression defined on the basis of prescriptions for depression was associated with activity limitation and a reduction in confidence and energy.7 BDI-defined depression has also been associated with lack of energy and sleep.13 In support of our findings, depression was not associated with cough or phlegm scores in other studies.7,9,13 The association between dyspnoea and presence of symptoms of depression in the present analysis has not been previously reported. It is likely that differences between studies are due at least partly to the instrument used to define depression, in addition to cohort clinical characteristics. For example, the contents of HADS are biased towards identifying symptoms of anhedonia (the inability to experience pleasure),27 whereas the contents of CES-D contain a higher number of somatic elements.28
Previous studies have shown a reduction in severe (27–78%) and mild/moderate (39–55%)29 COPD exacerbations compared to pre-COVID-19 pandemic rates, although not all studies show this.23 Our results concur with the majority of analyses showing reduced COPD exacerbations during the pandemic.29 These findings may reflect reduced community circulation of viruses during lock-downs, a reduction in air pollution or reduced accessibility of health-care services resulting in lower exacerbation reporting. However, a greater observed reduction in hospitalisation of COPD patients for exacerbations compared to myocardial infarction during the pandemic30 argues against reduced accessibility of health-care services. Furthermore, increased adherence to inhaled treatment may have contributed to reduced exacerbations.23
Total CAT score has been shown to be associated with both time to first exacerbation and exacerbation risk, with elevated CAT scores observed in frequent versus infrequent exacerbators when recorded during stable disease state. More specifically, a previous report described a significant association between exacerbation risk and a CAT score ≥13.5. We observed no association between total CAT score and exacerbations prior to or during the pandemic, suggesting a dissociation between total CAT score and exacerbation rate. These findings may be attributed to the high overall baseline CAT score of the cohort (mean = 16.2 (7.8)), and limited sample size for this subgroup analysis.
There are limitations to this study, including small sample size. Symptoms of depression were analysed in a subgroup with historic CES-D scores, due to difficulties in collecting CES-D remotely during the pandemic. For other questionnaires, administration via telephone was performed as opposed to in person, as there appears to be no evidence of systematic bias between these methods.31
Conclusion
In summary, albeit from a small sample size, we observed that CAT scores were higher in COPD patients with symptoms of depression compared to those without, both before and during the COVID-19 pandemic. This finding was particularly relevant for individual items such as confidence, energy, dyspnoea and chest tightness but not cough and phlegm production. Our findings further highlight how symptoms of depression can potentially influence total CAT scores. This may be relevant in both research and clinical practice. Higher CAT scores could act as a trigger to assess for the presence of symptoms of depression in clinical practice.
Abbreviations
ANOVA, analysis of variance analysis; BD, bronchodilator; BDI, Beck’s depression inventory; BMI, body mass index; CAT, COPD assessment test; CES-D, Center for epidemiologic studies depression scale; CI, confidence interval; COPD, Chronic obstructive pulmonary disease; COVID-19, Coronavirus-19; FEV1, Forced expiratory volume in one second; FVC, Forced vital capacity; GOLD, Global initiative for chronic obstructive pulmonary disease; HADS, Hospital anxiety and depression scale; NHS, National health service.
Data Sharing Statement
Data for this study are not publicly available.
Ethics Approval and Informed Consent
Patients provided written informed consent using protocols approved by local Ethics Committees (Tameside and Glossop, reference: 05/Q1402/41 and North West – Preston, reference: 16/NW/0836).
Acknowledgments
Dave Singh and Augusta Beech are supported by the National Institute for Health Research (NIHR) Manchester Biomedical Research Centre (BRC).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
DS has received sponsorship to attend and speak at international meetings, honoraria for lecturing or attending advisory boards from the following companies: Aerogen, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, CSL Behring, Epiendo, Genentech, GlaxoSmithKline, Glenmark, Gossamerbio, Kinaset, Menarini, Novartis, Orion, Pulmatrix, Sanofi, Synairgen, Teva, Theravance and Verona. AB has no conflicts of interest to declare.
References
1. Park HY, Chang Y, Kang D, et al. Blood eosinophil counts and the development of obstructive lung disease: the Kangbuk Samsung Health Study. Eur Respir J. 2021;58(4):2003823. doi:10.1183/13993003.03823-2020
2. Maurer J, Rebbapragada V, Borson S, et al. Anxiety and depression in COPD: current understanding, unanswered questions, and research needs. Chest. 2008;134(4 Suppl):43S–56S. doi:10.1378/chest.08-0342
3. Dalal AA, Shah M, Lunacsek O, Hanania NA. Clinical and economic burden of depression/anxiety in chronic obstructive pulmonary disease patients within a managed care population. COPD. 2011;8(4):293–299. doi:10.3109/15412555.2011.586659
4. Atlantis E, Fahey P, Cochrane B, Smith S. Bidirectional associations between clinically relevant depression or anxiety and COPD: a systematic review and meta-analysis. Chest. 2013;144(3):766–777. doi:10.1378/chest.12-1911
5. Quint JK, Baghai-Ravary R, Donaldson GC, Wedzicha JA. Relationship between depression and exacerbations in COPD. Eur Respir J. 2008;32(1):53–60. doi:10.1183/09031936.00120107
6. Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(3):648–654. doi:10.1183/09031936.00102509
7. Miyazaki M, Nakamura H, Chubachi S, et al. Analysis of comorbid factors that increase the COPD assessment test scores. Respir Res. 2014;15:13. doi:10.1186/1465-9921-15-13
8. Miravitlles M, Molina J, Quintano JA, et al. Depressive status explains a significant amount of the variance in COPD assessment test (CAT) scores. Int J Chron Obstruct Pulmon Dis. 2018;13:823–831. doi:10.2147/COPD.S154791
9. Marietta von Siemens S, Alter P, Lutter JI, et al. CAT score single item analysis in patients with COPD: results from COSYCONET. Respir Med. 2019;159:105810. doi:10.1016/j.rmed.2019.105810
10. Hilmarsen CW, Wilke S, Engan H, et al. Impact of symptoms of anxiety and depression on COPD Assessment Test scores. Eur Respir J. 2014;43(3):898–900. doi:10.1183/09031936.00163913
11. Silva Junior JL, Conde MB, de Sousa Correa K, da Silva C, da Silva Prestes L, Rabahi MF. COPD Assessment Test (CAT) score as a predictor of major depression among subjects with chronic obstructive pulmonary disease and mild hypoxemia: a case-control study. BMC Pulm Med. 2014;14:186. doi:10.1186/1471-2466-14-186
12. Lee YS, Park S, Oh YM, et al. Chronic obstructive pulmonary disease assessment test can predict depression: a prospective multi-center study. J Korean Med Sci. 2013;28(7):1048–1054. doi:10.3346/jkms.2013.28.7.1048
13. Lim JU, Park CK, Kim T-H, et al. The difficulty of improving quality of life in COPD patients with depression and associated factors. Int J Chron Obstruct Pulmon Dis. 2019;14:2331–2341. doi:10.2147/COPD.S216746
14. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. doi:10.1177/014662167700100306
15. Higham A, Mathioudakis A, Vestbo J, Singh D. COVID-19 and COPD: a narrative review of the basic science and clinical outcomes. Eur Respir Rev. 2020;29(158):200199. doi:10.1183/16000617.0199-2020
16. Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195(5):557–582. doi:10.1164/rccm.201701-0218PP
17. Singh D, Mathioudakis AG, Higham A. Chronic obstructive pulmonary disease and COVID-19: interrelationships. Curr Opin Pulm Med. 2022;28(2):76–83. doi:10.1097/MCP.0000000000000834
18. Sajjan US. Susceptibility to viral infections in chronic obstructive pulmonary disease: role of epithelial cells. Curr Opin Pulm Med. 2013;19(2):125–132. doi:10.1097/MCP.0b013e32835cef10
19. Wilkinson TMA, Aris E, Bourne S, et al. A prospective, observational cohort study of the seasonal dynamics of airway pathogens in the aetiology of exacerbations in COPD. Thorax. 2017;72(10):919–927. doi:10.1136/thoraxjnl-2016-209023
20. Kahnert K, Lutter JI, Welte T, et al. Impact of the COVID-19 pandemic on the behaviour and health status of patients with COPD: results from the German COPD cohort COSYCONET. ERJ Open Res. 2021;7(3):00242–2021. doi:10.1183/23120541.00242-2021
21. Santomauro DF, Mantilla Herrera AM, Shadid J; Collaborators C-MD. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet. 2021;398(10312):1700–1712. doi:10.1016/S0140-6736(21)02143-7
22. Wei L, Islam JY, Mascareno EA, Rivera A, Vidot DC, Camacho-Rivera M. Physical and mental health impacts of the COVID-19 pandemic among US adults with chronic respiratory conditions. J Clin Med. 2021;10(17):3981. doi:10.3390/jcm10173981
23. McAuley H, Hadley K, Elneima O, et al. COPD in the time of COVID-19: an analysis of acute exacerbations and reported behavioural changes in patients with COPD. ERJ Open Res. 2021;7(1):00718–2020. doi:10.1183/23120541.00718-2020
24. Agusti A, Bel E, Thomas M, et al. Treatable traits: toward precision medicine of chronic airway diseases. Eur Respir J. 2016;47(2):410–419. doi:10.1183/13993003.01359-2015
25. Rennard SI, Locantore N, Delafont B, et al. Identification of five chronic obstructive pulmonary disease subgroups with different prognoses in the ECLIPSE cohort using cluster analysis. Ann Am Thorac Soc. 2015;12(3):303–312. doi:10.1513/AnnalsATS.201403-125OC
26. Kunik ME, Roundy K, Veazey C, et al. Surprisingly high prevalence of anxiety and depression in chronic breathing disorders. Chest. 2005;127(4):1205–1211. doi:10.1378/chest.127.4.1205
27. Langvik E, Hjemdal O, Nordahl HM. Personality traits, gender differences and symptoms of anhedonia: what does the Hospital Anxiety and Depression Scale (Hads) measure in nonclinical settings? Scand J Psychol. 2016;57(2):144–151. doi:10.1111/sjop.12272
28. Bock K, Bendstrup E, Hilberg O, Lokke A. Screening tools for evaluation of depression in Chronic Obstructive Pulmonary Disease (COPD). A systematic review. Eur Clin Respir J. 2017;4(1):1332931. doi:10.1080/20018525.2017.1332931
29. Alqahtani JS, Oyelade T, Aldhahir AM, et al. Reduction in hospitalised COPD exacerbations during COVID-19: a systematic review and meta-analysis. PLoS One. 2021;16(8):e0255659. doi:10.1371/journal.pone.0255659
30. Berghaus TM, Karschnia P, Haberl S, Schwaiblmair M. Disproportionate decline in admissions for exacerbated COPD during the COVID-19 pandemic. Respir Med. 2022;191:106120. doi:10.1016/j.rmed.2020.106120
31. Rocha V, Jácome C, Martins V, Marques A. Are in Person and Telephone Interviews Equivalent Modes of Administrating the CAT, the FACIT-FS and the SGRQ in People With COPD? Original Research. Front Rehabilit Sci. 2021;2. doi:10.3389/fresc.2021.729190
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.