Back to Journals » Nature and Science of Sleep » Volume 14
The Concomitant Pattern of Association Between Subjective Global Sleep Quality and Daytime Dysfunction in Hypnotic-Treated Older Adults: The Yilan Study, Taiwan
Authors Lin CH, Hsu NW, Chen HC , Chou P
Received 8 December 2021
Accepted for publication 30 March 2022
Published 6 April 2022 Volume 2022:14 Pages 567—579
DOI https://doi.org/10.2147/NSS.S353141
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ahmed BaHammam
Chia-Heng Lin,1 Nai-Wei Hsu,2– 4 Hsi-Chung Chen,5 Pesus Chou6
1Department of General Psychiatry, Taoyuan Psychiatric Center, Ministry of Health and Welfare, Taoyuan, Taiwan; 2Division of Cardiology, Department of Internal Medicine & Community Medicine Center, National Yang Ming ChiaoTung University Hospital, Yilan, Taiwan; 3Faculty of Medicine, School of Medicine, National Yang Ming ChiaoTung University, Taipei, Taiwan; 4Public Health Bureau, Yilan, Taiwan; 5Department of Psychiatry & Center of Sleep Disorders, National Taiwan University Hospital, Taipei, Taiwan; 6Community Medicine Research Center & Institute of Public Health, National Yang Ming ChiaoTung University, Taipei, Taiwan
Correspondence: Hsi-Chung Chen, Department of Psychiatry and Center for Sleep Disorders, National Taiwan University Hospital, No. 7 Chung San South Road, Taipei, 10002, Taiwan, Tel +886 2-2312-3456 ext. 66787, Fax +886 2-2381-3208, Email [email protected]
Objective: The relationship between improvements in subjective sleep quality and restoration of daytime function remains unclear. This study aimed to examine the concomitant pattern between subjective sleep quality and daytime dysfunction in hypnotic-treated older adults.
Methods: This was a community-based, cross-sectional study. Participants comprised community-dwelling adults aged ≥ 65 years. Individual items from the Pittsburgh Sleep Quality Index (PSQI) were adopted to evaluate subjective global sleep quality and daytime dysfunction. Daytime dysfunction included composite scores of daytime dysfunction in the PSQI and its two sub-components: “staying awake” and “maintaining enthusiasm.” Based on hypnotic use and status in subjective sleep quality, participants were categorized into four groups: “healthy control,” “treated with good sleep quality (T+GSQ),” “treated with poor sleep quality (T+PSQ),” and “not treated with poor sleep quality (NT+PSQ)”. The associations between these four groups and daytime dysfunction were analyzed using logistic regression.
Results: In total, 2622 individuals participated in the study. After controlling for covariates, the T+PSQ group was more likely to have daytime dysfunction, including “composite daytime dysfunction” (OR: 6.41; 95% CI: 3.90– 10.55), “poor at staying awake” (OR: 3.04; 95% CI: 1.45– 6.37), and “poor at maintaining enthusiasm” (OR: 7.42; 95% CI: 4.33– 12.70) compared to the T+GSQ group. However, the healthy control group was less likely than the T+GSQ group to present with daytime dysfunction, including “composite daytime dysfunction” (OR: 0.43; 95% CI: 0.26– 0.72) and “poor at maintaining enthusiasm” (OR: 0.39; 95% CI: 0.22– 0.68).
Conclusion: Subjective sleep quality attributed to hypnotic use did not necessarily indicate restoration of daytime dysfunction.
Keywords: daytime dysfunction, sleep, public health, quality of care, drug related
Introduction
Maintenance of function is key to successful aging.1 Older adults with good levels of functioning exhibit preservation of cognitive function, expression skills, activities, self-care, lifestyles, and social participation.2 To improve the well-being of older adults, it is crucial to identify factors associated with functional impairments.3 In this regard, sleep quality is a key factor that influences daytime function.4 Older adults are vulnerable to experiencing poor sleep quality due to normal aging-related changes in sleep physiology and various mental and physical comorbidities.5 Insomnia is the most common primary sleep disorder that compromises sleep quality in older adults.6 Indeed, more than one-third of community-dwelling older adults experience chronic insomnia.7 Therefore, mitigating the adverse impact of insomnia to improve sleep quality in older adults is an imperative public health issue.
Ensuring adequate treatment for comorbidities and primary sleep disorders is fundamental in the management of insomnia. Ideally, non-pharmacological interventions are employed as the first-line choice over hypnotic use.8 However, owing to refractory comorbidities,9 low accessibility,10 and smaller effect sizes of non-pharmacological interventions in older populations,11 physicians may resort to administering hypnotics to older adults in real-world settings despite the numerous adverse effects of hypnosedatives.12
In previous studies, outcome measurements of hypnotic efficacy focused predominantly on changes in nighttime symptoms of insomnia4 rather than improvements in subjective sleep quality and next-day function.13 Thus, “improving sleep quality” and “restoring daytime function” have recently been suggested as two essential outcome indicators for optimal treatment of insomnia.14 Notably, improvements in nighttime insomnia symptoms after taking hypnotics may not necessarily correlate with improved subjective sleep quality in older adults.15 In hypnotic-treated older adults, improvements in nighttime symptoms may not necessarily mitigate daytime dysfunction, such as daytime sleepiness,16 or fall risks.17 Furthermore, although it is well established that worsening of sleep quality and declining daytime function occur concurrently,18–20 the associations between these measures under hypnotic treatment in older adults remains unclear. In nursing home residents, hypnotics use has been shown to explain the positive associations between poor sleep quality and impaired activity of daily living, social activity, and quality of interactions. Conversely, hypnotics use seemed mitigate the extent of association between poor sleep quality and poor mental health.21 Furthermore, as compared to hypnotics-naïve, community-dwelling older adults who had good sleep quality, those who used hypnotics but continued to have poor sleep quality had a higher fall risk. In contrast, the fall risks for older adults who took hypnotics and had good sleep quality was comparable with that of the counterpart group.17 Therefore, hypnotics use might influence the association patterns between sleep quality and various measurements of daytime functioning in older adults. In addition, the extent that hypnotics use influences the relationship between sleep quality and daytime function seems dependent on specific domains of daytime function.
Accordingly, elucidating the association pattern between sleep quality and specific daytime functions by considering the role of hypnotics is highly relevant in older populations. In terms of sleep quality, measuring objective sleep parameters has limited practicality from the perspective of community medicine. In contrast, paper-based instruments that inquire about sleep conditions are more feasible. To evaluate subjective sleep quality in community-dwelling older adults, answering a complete questionnaire, such as the Pittsburgh Sleep Quality Index (PSQI), remains a labor-intensive and time-consuming task. In this regard, a single global question is a more feasible alternative for measuring subjective global sleep quality. In parallel, “function” is defined as an individual’s ability to perform daily activities and typical daily tasks as per their social role.18 In the literature, studies on the impact of sleep disturbances or corresponding interventions have predominantly used objective indicators of daytime function, such as hand grip;19 walking speed;19 risks of fall;17 and instrumental activities of daily living.20 In contrast, there are few subjective studies of global functional status in the literature.
Thus, this study intended to examine the pattern of association between subjective sleep quality and daytime function in a large cohort of older community-dwelling adults. By using a global evaluation of subjective sleep quality and subjective daytime function, the study aims of this study were twofold. First, we examined the concomitant association between subjective sleep quality and daytime dysfunction in older adults treated with hypnotics. Second, we examined whether the daytime function in hypnotics-treated older adults was comparable to that of healthy individuals who did not use hypnotics. We hypothesized that improved subjective sleep quality and better daytime function would appear concomitantly among older adults under hypnotics treatment. Moreover, we also hypothesized that good subjective sleep quality under hypnotics treatment would indicate a full restoration of subjective daytime function when compared with healthy controls.
Participants and Methods
Participants
This cross-sectional study is part of the Yilan study series. The Yilan study is a community-based comprehensive geriatric assessment study conducted in Yilan City, Taiwan. The details of the study methods have been described elsewhere.22 As participants’ personal data were protected by the Personal Data Protection Act in Taiwan, no detailed household registration data could be acquired. Thus, participants who were 65 years or older and living in the community of Yilan City were randomly recruited by convenient sampling method. Face-to-face interviews were conducted by trained interviewers from August 2013 to November 2016. Older adults who were unable to complete the interview or anthropometric measurements for any reason were excluded from our analysis. Sociodemographic characteristics, information on comorbidities, and sleep-wake-related variables were collected. A final total of 2622 participants were enrolled in the study. All participants provided written informed consent, and the study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki. This study was approved by the Institutional Review Board of the National Yang-Ming University Hospital (IRB No. 2011A016) and National Taiwan University Hospital (IRB No. 201812192RINC). This study was conducted in accordance with the Helsinki Declaration of 1975, as revised in 2008.
Assessments of Daytime Dysfunction
Pittsburgh Sleep Quality Index items were used to evaluate daytime dysfunction.23 The reliability and validity of the Chinese version of the PSQI have been verified.24 The Cronbach’s alpha of the PSQI in this study was 0.74. Component 7, comprising daytime functional assessments in PSQI, were a composite score of the two individual constructs “staying awake” and “maintaining enthusiasm.” The question used to evaluate “staying awake” was “During the past month, how often have you had trouble staying awake while driving, eating meals, or engaging in social activity?” The scores assigned to the response “not during the past month,” “less than once a week,” “once or twice a week,” and “three or more times each week,” were 0, 1, 2, and 3, respectively. Participants who scored ≥ 1 were classified as “poor at staying awake”. The construct of “maintaining enthusiasm” was evaluated using the question “During the past month, how much of a problem has it been for you to keep up enough enthusiasm to get things done?” The scores assigned to the responses “no problem at all,” “only a very slight problem,” “somewhat of a problem,” and “a very big problem” were 0, 1, 2, and 3, respectively. Participants who scored ≥ 1 were defined as “poor at maintaining enthusiasm”. According to the scoring rule of the PSQI, raw scores of “staying awake” plus raw scores of “maintaining enthusiasm” yield the total scores that are subsequently rescaled to generate the final scores of Component 7. In this study, the raw total composite scores were used, and participants with a composite score ≥ 1 of the above two constructs were classified as “composite daytime dysfunction.” The associations of these three dimensions of daytime dysfunction with subjective sleep quality with and without hypnotic use were investigated.
Subgrouping Treatment Responses to Hypnotics
In this study, Component 6 of medication use in the PSQI (“During the past month, how often have you taken medication to help you sleep?”) was used to evaluate hypnotic use and to define the “treated” groups. In the Yilan study, data on the total days of hypnotic use in the preceding months were acquired. In the present study, “treated” was defined as the use of hypnotics three or more times per week. Hypnotic use more than 3 times a week implied that the participants had chronic and severe insomnia, which required stronger hypnotic treatments.25 Component 1 of the PSQI was used to evaluate global subjective sleep quality in participants, using the question “During the past month, how would you rate your sleep quality overall?” The possible responses to the question included “very good,” “fairly good,” “fairly bad,” and “very bad”. “Fairly bad” and “very bad” were defined as “poor sleep quality”; “very good” and “fairly good” were categorized as “good sleep quality”. Based on the above classifications, the participants were assigned into four mutually exclusive subgroups: (1) healthy controls: participants who did not use hypnotics and had good sleep quality, (2) treated with good sleep quality (T+GSQ): participants who used hypnotics and had good sleep quality, (3) treated with poor sleep quality (T+PSQ): participants who used hypnotics but still had poor sleep quality, and (4) not treated with poor sleep quality (NT+PSQ): participants who did not use hypnotics and had poor sleep quality. These four groups were examined, and their relationship with daytime dysfunction was compared.
Other Variables
In addition to sociodemographic data and lifestyle information, data on potential confounders were also collected. Physical diseases were recorded as present only if the participants reported both pertinent diagnoses and corresponding treatments,26 including diabetes, hypertension, cardiac disease, hyperlipidemia, stroke, and snoring. Any fall experience in the previous year was defined as the presence of a fall. Because older adults typically have various physical comorbidities, their symptoms may overlap with the somatic symptoms of depression and anxiety. Thus, the Hospital Anxiety and Depression Scale (HADS) was used to evaluate non-somatic symptoms of depression and anxiety to prevent overestimation.27 The use of HADS in older individuals has been validated,28 and the validity of the Chinese version of the HADS has been verified.29 Among older Chinese individuals, the cutoff of HADS for syndromal depression and anxiety are ≥ 6 and ≥ 3 points, respectively.29 In addition, the Epworth Sleepiness Scale (ESS) was used to measure daytime sleepiness with a cutoff defined as ≥ 11. The validity of the Chinese version of the ESS has been verified.30
Statistical Analysis
SPSS software (version 17.0; SPSS Inc., Chicago, IL, USA) was used for statistical analyses. The chi-square test was used for univariate analysis. A goodness-of-fit test was used to examine the sociodemographic differences between the participants and registered residents in Yilan City. Pearson correlation analyses were performed to examine the bivariate relationships among measurements of daytime dysfunction. Multiple logistic regressions were used to examine the independent associations between each subgroup of treatment response to hypnotics with daytime dysfunction. All covariates were included to specify the statistical model using the forced enter method. All reported p-values were two-tailed and considered significant if p < 0.05.
Results
Table 1 summarizes the sociodemographic data, lifestyle information, and clinical characteristics of the participants. A total of 2622 participants were enrolled in this study, 61.2% of whom were ≥ 75 years old and 59.0% were female. Of these participants, the prevalence of prominent symptoms of depression and anxiety was 9.5% and 34.4%, respectively. Compared with the registered household data in Yilan City in 2013,31 participants in this study were significantly older (χ2= 9.2, df = 1, p = 0.002) and less literate (χ2= 20.2, df = 1, p < 0.001) but had a similar sex distribution (χ2= 1.3, df = 1, p = 0.26).
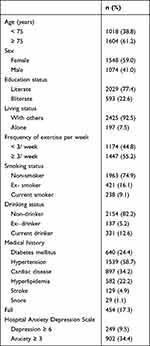 |
Table 1 Sociodemographic and Clinical Characteristics of Participants (n=2622) |
Table 2 presents the sleep-wake-related variables of the participants. Of participants, 11.8% had composite daytime dysfunction, 4.2% were poor at staying awake, and 10.2% had poor enthusiasm. To examine the mutual associations between three measurements of daytime dysfunction, correlation coefficients between the raw scores of the three measurements were examined. The correlation for the composite daytime dysfunction with “poor at staying awake” and “poor enthusiasm” were moderate (r=0.68, p<0.001) and high (r=0.89, p<0.001), respectively. The correlation between “poor at staying awake” and “poor enthusiasm” was low (r=0.33, p<0.001). Of these participants, 15.9%, 58.0%, 21.9%, and 4.2% self-reported having good, fairly good, fairly poor, and very poor of sleep quality, respectively. Thus, according to the cutoff used to determine sleep quality in this study, 26.1% of participants had poor subjective sleep quality. A total of 19.9% of older adults in this study received hypnotic treatments. The healthy control, T+GSQ, T+PSQ, and NT+PSQ subgroups comprised 63.0%, 10.9%, 9.0%, and 17.1% of the treatment response subgroups, respectively.
 |
Table 2 Sleep-Wake Related Variables of Participants (n=2622) |
Table 3 presents the univariate analysis of factors associated with various dimensions of daytime dysfunction. Participants with composite daytime dysfunction were more likely to be ≥ 75 years old (p = 0.01), female (p < 0.001), exercised less (p = 0.02), and non-current alcohol drinkers (p = 0.02). Older adults with hyperlipidemia (p = 0.001), cardiac disease, stroke, falling down, depression, anxiety, and daytime sleepiness were more likely to have composite daytime dysfunction (all p < 0.001). In terms of subgroups of treatment response to hypnotics, significantly different distributions were observed in the dimension of composite daytime dysfunction among subgroups (p < 0.001). Subgroups T+PSQ and NT+PSQ were prone to composite daytime dysfunction. For the other two independent constructs, with the exception of alcohol use, cardiac disease, and hyperlipidemia, factors associated with “poor at staying awake” were comparable to those for composite daytime dysfunction. The factors associated with “poor at maintaining enthusiasm” were identical to those associated with composite daytime dysfunction.
 |
Table 3 Univariate Analyses for Factors Associated with Daytime Dysfunction (n=2622) |
Table 4 summarizes the independent relationships between the treatment response groups and daytime dysfunction. In terms of composite daytime dysfunction, compared with the T+GSQ subgroup, healthy controls were less likely to have composite daytime dysfunction (OR: 0.43; 95% CI: 0.26–0.72). In contrast, T+PSQ (OR: 6.41; 95% CI: 3.90–10.55) and NT+PSQ (OR: 4.98; 95% CI: 3.11–7.99) subgroups were both more likely to have composite daytime dysfunction. With regard to the factor of “poor at staying awake”, compared with the T+GSQ subgroup, T+PSQ (OR: 3.04; 95% CI: 1.45–6.37) and NT+PSQ (OR: 2.60; 95% CI: 1.29–5.24) subgroups still had a higher likelihood of having daytime dysfunction. However, there was no significant difference between the healthy control and T+GSQ subgroups. The pattern of associations between the four subgroups and “poor at maintaining enthusiasm” was identical to that for composite daytime dysfunction. Healthy controls demonstrated lower risks (OR: 0.39; 95% CI: 0.22–0.68), while T+PSQ (OR: 7.42; 95% CI: 4.33–12.70) and NT+PSQ (OR: 5.75; 95% CI: 3.43–9.63) subgroups exhibited higher risks of poor enthusiasm. To examine the impact of hypnotic treatment intensity, sensitivity analyses were conducted using different frequencies of hypnotic use to define “treated.” The results demonstrated that regardless of exposure to hypnotics that was greater or less than three times per week, the pattern of associations between the four subgroups and various dimensions of daytime dysfunction remained unchanged (see Supplementary Table S1).
 |
Table 4 Logistic Regression Analyses for Factors Associated with Daytime Dysfunction (n=2622) |
Discussion
This study examined the relationships between subjective sleep quality and daytime dysfunction in a large cohort of hypnotic-treated older adults while controlling for potential confounders. Our findings partly support our hypotheses. In this study, the T+GSQ group did not exhibit comparable daytime function compared to healthy controls in the domains of composite daytime dysfunction and enthusiasm. In contrast, T+GSQ had better daytime function in all three dimensions compared with the T+PSQ and NT+PSQ subgroups. Our findings suggest that older adults who took hypnotics and reported good sleep quality also functioned better in the daytime. However, subjective sleep quality attributed to hypnotic use does not necessarily indicate full restoration of daytime function to the level of healthy individuals. To the best of our knowledge, this is the first study to explore the concomitant pattern between subjective sleep quality and daytime dysfunction in hypnotics-treated older adults.
Complex Associations Among Hypnotic Use, Sleep Quality, and Daytime Function
In this study, we observed that subjective sleep quality and daytime function were poor simultaneously, but they did not necessarily appear in an improved status concomitantly. The original components of the PSQI were used in this study to define “treated” (hypnotic use, Component 6), global subjective sleep quality (Component 1), and daytime dysfunction (Component 7). In the literature, factor analyses of PSQI in geriatric groups have consistently demonstrated that the PSQI is a multi-dimensional instrument.32 In these studies, global sleep quality and daytime dysfunction were consistently loaded to different factors. However, reports on the relationship of medication use with global sleep quality33 and daytime dysfunction34 have been inconsistent. Generally, our findings are in accordance with the findings of previous studies. Specifically, “medication use,” “subjective global sleep quality,” and “daytime dysfunction” may have concomitant or independent relationships with each other in older adults.
The Role of Hypnotic Use in the Concomitant Pattern Between Sleep Quality and Daytime Function
In the present study, regardless of hypnotic treatment, participants with poor sleep quality demonstrated pervasive concomitant daytime dysfunction compared to participants with good sleep quality after using hypnotics. The concomitant deterioration pattern between poor sleep quality and consequent daytime dysfunction is well-established in older adults who have not received treatment.35 In contrast, the association between poor subjective sleep quality and daytime dysfunction in individuals who have been treated with hypnotics may be bi-directional. In this regard, inadequate use of hypnotics can result in residual daytime dysfunction, but older adults may also be dissatisfied with their sleep quality due to the adverse effects of hypnotics that may perpetuate daytime dysfunction.
Inadequate treatments are underscored by quantitative and qualitative aspects. In terms of quantity, the T+PSQ and NT+PSQ subgroups exhibited daytime dysfunction. In the sensitivity analyses, different cutoffs for the frequency of hypnotic use did not alter the main findings. This suggests that daytime dysfunction in the T+PSQ subgroup is less likely due to quantitative inadequacy to treat sleep disturbances by hypnotics. In terms of quality, compared with the T+GSQ subgroup, the T+PSQ subgroup still demonstrated poor ability to stay awake after controlling for ESS-defined daytime sleepiness. This finding suggests that staying awake in older adults does not simply reflect the absence of residual sedative effects of hypnotics in the daytime. Further, the T+PSQ subgroup exhibited an independent association with poor enthusiasm after controlling for various confounders. These findings underscore the limitations of using hypnotics to treat insomnia. Indeed, most hypnosedatives do not sufficiently enhance slow-wave sleep, which is essential for gaining subjective restorative sleep.15 In addition, hypnotics have numerous adverse side effects, including daytime sleepiness, muscle relaxation, general weakness,15 poor coordination,15 and decline in cognitive function.36 These side effects may further compromise participation in social activities and interpersonal interactions among older adults.4,37 As a result, older adults who take hypnotics may attribute these side effects to non-restorative sleep and devaluate their sleep quality accordingly. We also observed that although with a non-significant difference, T+GSQ subgroup had poor function of staying awake when compared with healthy controls. Besides, T+GSQ did have inferior scores to those of healthy controls regarding to “maintaining enthusiasm”. This finding supports our argument regarding the qualitative inadequacy of hypnotic treatment on subjective sleep quality.
Definitions of Subjective Global Sleep Quality and Daytime Dysfunction
In this study, a single item in the PSQI was used to evaluate global sleep quality. The literature indicates that compared to objective sleep parameters, subjective sleep quality has a stronger relationship with non-sleep phenomena, such as subjective well-being, depression, and quality of life.38 Poor subjective sleep quality may originate from mood-modified perceptions of sleep, rather than genuine sleep quality.39 In this study, confounding effects of mood status were adjusted for, and poor global subjective sleep quality remained associated with daytime dysfunction. This suggests that a single question that evaluates global subjective sleep quality is a valid indicator of daytime dysfunction in older adults. Moreover, because subjective global functional status has been less studied, our use of Component 7 of the PSQI as the measurement of daytime function is highly novel and adds to the literature. The two constructs in Component 7 (“staying awake” and “maintaining enthusiasm”) used in this study are sensitive to aging23 and reflect the core entity underpinning daytime function in older populations, whose social roles change continuously with age. In addition, our correlation analyses also illustrated a low level of correlation between these two constructs (r=0.33), suggesting the need to examine their independent associations with sleep quality in hypnotic-treated older adults.
In this study, when compared with T+GSQ, healthy controls had significantly fewer problems in terms of “staying awake” in the univariate analysis, with this significance vanishing after controlling for covariates. There were two reasons that may underlie this finding. First, in this study, the prevalence of a problematic “staying awake” response (4.2%) was much lower than that of ESS ≥ 11 (11.2%). The ESS was designed to measure the sleep propensity under various soporific circumstances. The question inquiring about participants’ “staying awake“ in the PSQI evaluates problems with staying awaking while driving, eating, and engaging in social activities. According to the ESS, the somnificity for these circumstances in “staying awake“ is low. This suggests that “poor at staying awake” in this study is a more stringent form of daytime sleepiness than that of the ESS measures. As the ESS and “poor at staying awake” question share an underlying construct, specifying the ESS in the model may partially mitigate the difference between T+GSQ and healthy controls in terms of “staying awake”. Second, compared with the composite daytime dysfunction and “poor at maintaining enthusiasm” response, our study found fewer cases numbers of “poor at staying awake”. Therefore, inadequate statistical power may also contribute to the vanishing lower risk for problematic “staying awake” in healthy controls.
Clinical Implications
This study demonstrated that poor sleep quality was a key correlate of daytime dysfunction regardless of medication use. Further, daytime function may not necessarily return to premorbid levels after hypnotic treatment. Accordingly, when clinicians prescribe hypnotics for older adults, both subjective sleep quality and daytime function should be actively and simultaneously evaluated in order to ensure quality of care. Moreover, in both clinical settings and the community, individual questions of the PSQI may serve as valid and simple instruments to evaluate subjective global sleep quality and daytime function in older adults.
Limitations
This study has several limitations. First, this was a cross-sectional study, and causal inferences could not be drawn. Second, older adults in this study were community-dwelling and relatively healthier compared to institutionalized older adults. The sociodemographic characteristics of the study participants differed to those of the registered residents of Yilan City. In addition, Yilan City is an agricultural urban area that is distinct to metropolitan areas. This may limit the generalizability of our findings. Third, medications, decline in cognitive function, other primary sleep disorders, and chronic pain may have introduced confounding factors in this study. The side effects of various medications that result in daytime sleepiness and consequently affect daytime function may bias our findings. In the present study, various comorbidities were included in the statistical models to control for confounding effects. In order to prevent collinearity and confounding by indications, we believe that specifying morbidities alone at least partly controlled for the influence of their respective medications. However, we were still unable to ascertain other forms of confounders derived from medications, such as drug-drug or drug-condition interactions. In terms of cognitive function, although we did not formally evaluate cognitive function of participants, the cognitive demand required to complete the questionnaires of the Yilan study are indicative of the cognitive levels of participants. However, this study was still subject to information bias in cases in which the participants might have mild cognitive impairment. Primary sleep disorders were not formally diagnosed in this study, such as insomnia and sleep apnea. We used snoring and ESS as proxies to partially control for confounding factors that may be introduced by primary sleep disorders, such as disordered breathing in sleep.40 Fourth, subjective global evaluation of sleep quality and daytime function in this study limited the scope of the clinical implications of our findings. Finally, we did not collect information regarding other treatment modalities for insomnia, such as Chinese herbs, acupuncture, and cognitive behavioral therapy. The lack of information on these treatment modalities may have biased our findings.
Conclusions
In aging societies, maintaining function in older adults is essential. Given the close association between sleep quality and daytime function, improving sleep quality may be a key factor for improving function in older populations. In real-world settings, hypnotic use remains prevalent among older adults. Our findings illustrate that older adults who use hypnotics may continue to report poor sleep quality. In addition, hypnotic use may not sufficiently restore daytime function, which should be the main goal of such therapeutics. As multiple etiologies contribute to the unsatisfactory sleep quality and impaired daytime function in older adults, comprehensive assessment of patients’ somnological history is always necessary. Detailed evaluations of physical and mental comorbidities, the adequacy and adverse side effects of treatments, chronic distress conditions (such as pain and frailty), and psychosocial issues can help clinicians to formulate an optimal intervention to enhance both sleep quality and daytime function in older adults. In the future, follow-up studies and randomized controlled trials are warranted to further explore specific regimens that genuinely improve sleep quality and promote daytime function in older populations.
Abbreviations
CI, confidence interval; ESS, the epworth sleepiness scale; HADS, the hospital anxiety and depression scale; NT+PSQ, not treated with poor sleep quality; OR, odds ratios; PSQI, pittsburgh sleep quality index; T+GSQ, treated with good sleep quality; T+PSQ, treated with poor sleep quality.
Acknowledgments
The authors would also like to thank Yang-Ming Crusaders, Mr. Da-Wei Lin, Ms Yu-Hui Lin, Mr. Chia-Hsiang Lin, and Ms Tzu-Chun Lo for their help with data collection.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Ministry of Science and Technology, Taiwan [MOST-107-2314-B-010 −049] and partially supported by the Ministry of Science and Technology, Taiwan [MOST-107-2314-B-002 −219, MOST-108-2314-B-002 −110 -MY2, and MOST-110-2314-B-002 −096 -MY3].
Disclosure
The authors report no conflicts of interest for this work.
References
1. Rowe JW, Kahn RL. Successful aging. Gerontologist. 1997;37(0016–9013(Print)):433–440.
2. Bousquet J, Malva J, Nogues M, Mañas LR, Vellas B, Farrell J. Operational Definition of Active and Healthy Aging (AHA): the European Innovation Partnership (EIP) on AHA Reference Site Questionnaire: montpellier October 20- 21, 2014, Lisbon July 2, 2015. J Am Med Dir Assoc. 2015;16(12):1020–1026.
3. Delle Fave A, Bassi M, Boccaletti ES, Roncaglione C, Bernardelli G, Mari D. Promoting Well-Being in Old Age: the Psychological Benefits of Two Training Programs of Adapted Physical Activity. Front Psychol. 2018;28:
4. Kunz D. Rethinking the use of hypnotics for treatment of insomnia in the elderly. Expert Opin Pharmacother. 2021;22(8):953–957.
5. McHugh JE, Casey Am Fau Lawlor BA, Lawlor BA. Psychosocial correlates of aspects of sleep quality in community-dwelling Irish older adults. Aging Ment Health. 2011;15(6):749–755.
6. Chiou JH, Chen HC, Chen KH, Chou P. Correlates of self-report chronic insomnia disorders with 1-6 month and 6-month durations in home-dwelling urban older adults - the Shih-Pai Sleep Study in Taiwan: a cross-sectional community study. BMC Geriatr. 2016;16:119.
7. Wang P, Song L, Wang K, et al. Prevalence and associated factors of poor sleep quality among Chinese older adults living in a rural area: a population-based study. Aging Clin Exp Res. 2020;32(1):125–131.
8. Riemann D, Baglioni C, Bassetti C, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26(6):675–700.
9. Ustinov Y, Lichstein KL, Wal GSV, Taylor DJ, Riedel BW, Bush AJ. Association between report of insomnia and daytime functioning. Sleep Med. 2010;11(1):65–68.
10. Montgomery P, Dennis J. A systematic review of non-pharmacological therapies for sleep problems in later life. Sleep Med Rev. 2004;8(1):47–62.
11. Kwon CY, Lee B, Cheong MJ, et al. Non-pharmacological Treatment for Elderly Individuals With Insomnia: a Systematic Review and Network Meta-Analysis. Front Psychiatry. 2021;11:1544.
12. Rossman J. Cognitive-Behavioral Therapy for Insomnia: an Effective and Underutilized Treatment for Insomnia. Am J Lifestyle Med. 2019;13(6):544–547.
13. Buscemi N, Vandermeer B, Friesen C, et al. The efficacy and safety of drug treatments for chronic insomnia in adults: a meta-analysis of RCTs. J Gen Intern Med. 2007;22(9):1335–1350.
14. Edinger JD, Buysse DJ, Deriy L, et al. Quality measures for the care of patients with insomnia. J Clin Sleep Med. 2015;11(3):311–334.
15. Schroeck JL, Ford J, Conway EL, et al. Review of Safety and Efficacy of Sleep Medicines in Older Adults. Clin Ther. 2016;38(11):2340–2372.
16. Chen L, Bell JS, Visvanathan R, et al. The association between benzodiazepine use and sleep quality in residential aged care facilities: a cross-sectional study. BMC Geriatr. 2016;16:196.
17. Min Y, Kirkwood CK, Mays DP, Slattum PW. The Effect of Sleep Medication Use and Poor Sleep Quality on Risk of Falls in Community-Dwelling Older Adults in the US: a Prospective Cohort Study. Drugs Aging. 2016;33(2):151–158.
18. Košćec Bjelajac A, Holzinger B, Despot Lučanin J, Delale EA, Lučanin D. Sleep Quality and Daytime Functioning in Older European Adults. Eur Psychol. 2020;25(3):186–199.
19. Kim M, Yoshida H, Sasai H, Kojima N, Kim H. Association between objectively measured sleep quality and physical function among community-dwelling oldest old Japanese: a cross-sectional study. Geriatr Gerontol Int. 2015;15(8):1040–1048.
20. Zisberg A, Nurit GY, Shochat T. Contribution of routine to sleep quality in community elderly. Sleep. 2010;33(4):509–514.
21. Valenza MC. Nursing homes: impact of sleep disturbances on functionality. Arch Gerontol Geriatr. 2013;56:3.
22. Hsu NW, Tsao HM, Chen HC, Chou P. Anxiety and depression mediate the health-related quality of life differently in patients with cardiovascular disease and stroke-preliminary report of the Yilan study: a population-based community health survey. PLoS One. 2014;9(9):e107609.
23. Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213.
24. Tsai PS, Wang SY, Wang MY, et al. Psychometric evaluation of the Chinese version of the Pittsburgh Sleep Quality Index (CPSQI) in primary insomnia and control subjects. Qual Life Res. 2005;14(8):1943–1952.
25. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders.
26. Wu CS, Lai MS, Gau SS, Wang SC, Tsai HJ. Concordance between patient self-reports and claims data on clinical diagnoses, medication use, and health system utilization in Taiwan. PLoS One. 2014;9(12):e112257.
27. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370.
28. Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52(2):69–77.
29. Leung CM, Ho S, Kan CS, Hung CH, Chen CN. Evaluation of the Chinese version of the Hospital Anxiety and Depression Scale. A cross-cultural perspective. Int J Psychosom. 1993;40(1–4):29–34.
30. Chen NH, Johns MW, Li HY, et al. Validation of a Chinese version of the Epworth sleepiness scale. Qual Life Res. 2002;11(8):817–821.
31. Yilan City Household Registration Office. Household registration statistics data. Yilan City Household Registration Office, Yilan County; 2021. Available from: https://ilhhr.e-land.gov.tw/cp.aspx?n=2B3911AF933DF44D.
32. Mollayeva T, Thurairajah P, Burton K, Mollayeva S, Shapiro CM, Colantonio A. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: a systematic review and meta-analysis. Sleep Med Rev. 2016;25:52–73.
33. Cole JC, Motivala SJ, Buysse DJ, Oxman MN, Levin MJ, Irwin MR. Validation of a 3-factor scoring model for the Pittsburgh sleep quality index in older adults. Sleep. 2006;29(1):112–116.
34. Koh HW, Lim RB, Chia KS, Lim WY. The Pittsburgh Sleep Quality Index in a multi-ethnic Asian population contains a three-factor structure. Sleep Breathing. 2015;19(4):1147–1154.
35. Malinowska KB, Okura M, Ogita M, et al. Effect of self-reported quality of sleep on mobility in older adults. Geriatr Gerontol Int. 2016;16(2):266–271.
36. Bastien CH, LeBlanc M, Carrier J, Morin CM. Sleep EEG power spectra, insomnia, and chronic use of benzodiazepines. Sleep. 2003;26(3):313–317.
37. Roth T, Ancoli-Israel S. Daytime consequences and correlates of insomnia in the United States: results of the 1991 National Sleep Foundation Survey. II. Sleep. 1999;22(Suppl 2):S354–358.
38. Fabbri M, Beracci A, Martoni M, Meneo D, Tonetti L, Natale V. Measuring Subjective Sleep Quality: a Review. Int J Environ Res Public Health. 2021;18(3):35.
39. Krystal AD, Edinger JD. Measuring sleep quality. Sleep Med. 2008;9(Suppl 1):S10–S17.
40. Alakuijala A, Salmi T. Predicting Obstructive Sleep Apnea with Periodic Snoring Sound Recorded at Home. J Clin Sleep Med. 2016;12(7):953–958.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
