Back to Journals » Journal of Inflammation Research » Volume 16
The Combined Effect of Systemic Immune-Inflammation Index and Type 2 Diabetes Mellitus on the Prognosis of Patients Undergoing Percutaneous Coronary Intervention: A Large-Scale Cohort Study
Authors Bian X, He J, Zhang R, Yuan S, Dou K
Received 19 October 2023
Accepted for publication 12 December 2023
Published 27 December 2023 Volume 2023:16 Pages 6415—6429
DOI https://doi.org/10.2147/JIR.S445479
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Ning Quan
Xiaohui Bian,1– 3,* Jining He,1– 3,* Rui Zhang,1– 3 Sheng Yuan,1– 3 Kefei Dou1– 4
1Cardiometabolic Medicine Center, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, People’s Republic of China; 2Department of Cardiology, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, People’s Republic of China; 3State Key Laboratory of Cardiovascular Disease, Beijing, People’s Republic of China; 4National Clinical Research Center for Cardiovascular Diseases, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Kefei Dou, Cardiometabolic Medicine Center, National Clinical Research Center for Cardiovascular Diseases, State Key Laboratory of Cardiovascular Disease, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, A 167 Beilishi Road, Xicheng District, Beijing, 100037, People’s Republic of China, Tel +86-13801032912, Fax +86-10-6831-3012, Email [email protected]
Background: Chronic low-grade inflammation is the common mechanism of both atherosclerosis and type 2 diabetes mellitus (T2DM), and systemic immune-inflammation index (SII) has been emerged as a novel and simple inflammatory biomarker. However, the association between SII and glycemic metabolism and their synergetic effect on the prognosis of coronary artery disease (CAD) patients remains unclear.
Methods: A total of 8602 patients hospitalized for percutaneous coronary intervention (PCI) were included. The primary endpoint was major adverse cardiovascular events (MACE), including all-cause death, myocardial infarction (MI), and target vessel revascularization. According to the optimal cut-off value of SII for MACEs, patients were grouped into higher levels of SII (SII-H) and lower levels of SII (SII-L) and further divided by the concomitance of T2DM into four groups: SII-H/T2DM, SII-H/Non-T2DM, SII-L/T2DM, SII-L/Non-T2DM.
Results: During a median 2.4-year follow-up, 522 MACEs occurred. The optimal cut-off value of SII for MACEs was 502.5. A 1-unit increase of SII (transformed by natural logarithm) was associated with a 29% increase of MACE risks in the T2DM cohort [adjusted hazard ratio (HR): 1.29, 95% confidence interval (CI): 1.03 to 1.61, P = 0.024], while had no effect in the non-T2DM cohort (HR: 1.03, 95% CI: 0.80 to 1.34, P = 0.800). Compared to those in SII-H/T2DM group, patients in SII-H/Non-T2DM, SII-L/T2DM, SII-L/Non-T2DM had significantly decreased risk of MACEs [adjusted HR: 0.77, 95% CI: 0.61 to 0.98, P = 0.036; adjusted HR: 0.66, 95% CI: 0.50 to 0.87, P = 0.003; adjusted HR: 0.58, 95% CI: 0.45 to 0.74, P < 0.001; respectively]. Multivariable Cox regression analysis also indicated the highest risk in T2DM patients with higher levels of SII than others (P for trend < 0.001).
Conclusion: In this large-scale real-world study, diabetic patients with elevated SII levels were associated with worse clinical outcomes after PCI.
Keywords: systemic immune-inflammation index, type 2 diabetes mellitus, coronary artery disease, percutaneous coronary intervention
Introduction
Atherosclerosis is identified as a chronic, low-grade inflammatory condition accompanied by autoimmune responses involving both the innate and adaptive immune systems.1,2 Numerous observational studies have probed the prognostic value of inflammatory biomarkers in patients with coronary artery disease (CAD),3,4 and several anti-inflammatory clinical trials have further confirmed the causal relationship between inflammation and CAD.2 The growth of atherosclerotic lesion is characterized by an imbalance between inflammation and its resolving, with various types of blood cells playing distinct roles in this intricate pathological process.5 A previous study has indicated that elevated neutrophil-to-lymphocyte ratio (NLR) was associated with adverse prognosis for CAD patients, especially in those with type 2 diabetes mellitus (T2DM).6 Recent research has also highlighted the involvement of platelets in the recruitment and activation of leukocytes and mediate leukocyte-endothelium adhesion, which play a crucial role in the progression of atherosclerosis.7,8 The uncontrolled and excessive activation of leukocytes and platelets generates a vicious circle, resulting in plaque instability and vessel occlusion.9 Therefore, both leukocytes and platelets significantly contribute to the initiation and progression of CAD.
Systemic immune-inflammation index (SII), a novel index calculated as (platelet*neutrophil)/lymphocyte, integrates the prognostic information of leukocytes and platelets into a single index, providing insights into the sophisticated interaction among host immunity, inflammation, and thrombosis. Previous studies have shown that SII was associated with the severity of coronary lesions.10,11 Furthermore, SII has been identified as an independent predictor for poor clinical outcomes in patients with either stable CAD or acute coronary syndrome (ACS).12,13
Patients with T2DM tended to have a severer plaque burden and increased cardiovascular risks.14 Sterile chronic inflammation, a well-recognized feature that is common to both diabetes and atherosclerosis, is considered as a possible link between T2DM and CAD. However, there is currently limited evidence regarding the prognostic value of SII in CAD patients with impaired glycemic metabolism status. Whether SII and T2DM had a synergetic effect on the prognosis remains unknown. Therefore, the present study aimed to investigate the relationship between SII and glycemic metabolism status and to further evaluate their synergetic effect on the prognosis of CAD patients undergoing percutaneous coronary intervention (PCI).
Methods
Study Design and Follow-Up
The design of the present study was a prospective, observational, single-center cohort study. From January 2013 to December 2013, a total of 10,724 consecutive CAD patients undergoing PCI was enrolled at Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences (Beijing, China). The exclusion criteria included: Patients with (1) missing crucial baseline data; (2) loss of follow-up; (3) no drug-eluting stent (DES) implantation; (4) unsuccessful PCI; (5) severe renal dysfunction [estimated glomerular filtration rate (eGFR) < 15 mL/(min*1.73m2)];15 (6) hematological disorders (eg, leukemia, aplastic anemia); (7) acute infection; (8) active tumor; (9) immuno-suppressant prescriptions (Supplementary Figure S1). Finally, a total of 8,602 patients were included in the present study and divided into the SII-H/T2DM (N = 1,440), SII-L/T2DM (N = 2,516), SII-H/Non-T2DM (N = 1,687) and SII-L/Non-T2DM (N = 2,959) groups, according to the optimal cut-off value of SII and whether they had T2DM or not.
The clinical status of enrolled patients was routinely followed up through telephone interviews or outpatient visits at 1, 6, and 12 months, and then annually for up to 3 years. The primary endpoint was major adverse cardiovascular events (MACEs), defined as a composite of all-cause death, myocardial infarction (MI), and target vessel revascularization (TVR). Secondary endpoints included a composite of all-cause death, MI, and individual components of MACEs. All-cause death was defined as death from any cause, whether cardiac or non-cardiac. MI was defined by clinical symptoms, electrocardiogram, and laboratory parameters based on the third universal definition of MI. TVR was defined as any repeat percutaneous intervention or surgical bypass of any segment of the target vessel. All events were adjudicated by 2 blinded independent specialists, and any disagreement was resolved by consensus or a third experienced cardiologist.
The study protocol underwent review and approval by the Ethics Committee of Fuwai Hospital (2016–847). The study adhered to the ethical standards outlined for medical research involving human subjects, as specified in the 1964 Declaration of Helsinki and its subsequent amendments. Relevant information, including the study’s purpose, procedures, potential risks and benefits, confidentiality measures, and the rights of the participants, was provided to all participants before including them in the study. All participants in this study provided written informed consent before intervention.
Treatment and Procedure
PCI was performed by experienced interventional cardiologists who were unaware of the study protocol, in line with recent practical guidelines. The details of equipment choice and intraoperative strategy were at the discretion of individual physicians. Before the selective PCI, all patients received aspirin (300 mg) and a loading-dose P2Y12 inhibitor (ticagrelor 180 mg or clopidogrel 300mg), unless they already received dual antiplatelet therapy; for patients presenting with acute coronary syndrome (ACS), the same dose of aspirin and ticagrelor (180 mg) or clopidogrel (300–600 mg) was given orally as soon as possible. To achieve procedural anticoagulation, unfractionated heparin (100 U/kg) was administered to all patients before PCI. After the procedure, aspirin (100 mg daily) and clopidogrel (75 mg daily) were prescribed for at least 12 months.
Definitions
SII mentioned in the present study was calculated by the following formula: 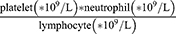 . T2DM was defined by fasting blood glucose (FBG) ≥7.0 mmol/L (126 mg/dL), or hemoglobin A1c (HbA1c) levels ≥6.5%, or 2-h blood glucose of oral glucose tolerance test (OGTT) ≥11.1 mmol/L (200 mg/dL), or known T2DM with current use of hypoglycemic drugs.16 Hypertension was determined as systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg or previous definite diagnosis of hypertension with antihypertensive medications.17 Dyslipidemia was diagnosed as fasting total cholesterol (TC) ≥5.2 mmol/L, low-density lipoprotein cholesterol (LDL-C) ≥3.4 mmol/L, high-density lipoprotein cholesterol (HDL-C) <1.0 mmol/L, triglyceride (TG) ≥1.7 mmol/L, and/or long-term use of lipid-lowering drugs.18 Renal dysfunction was defined as eGFR <90 mL/(min*1.73m2). Body mass index (BMI) was calculated as
. T2DM was defined by fasting blood glucose (FBG) ≥7.0 mmol/L (126 mg/dL), or hemoglobin A1c (HbA1c) levels ≥6.5%, or 2-h blood glucose of oral glucose tolerance test (OGTT) ≥11.1 mmol/L (200 mg/dL), or known T2DM with current use of hypoglycemic drugs.16 Hypertension was determined as systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg or previous definite diagnosis of hypertension with antihypertensive medications.17 Dyslipidemia was diagnosed as fasting total cholesterol (TC) ≥5.2 mmol/L, low-density lipoprotein cholesterol (LDL-C) ≥3.4 mmol/L, high-density lipoprotein cholesterol (HDL-C) <1.0 mmol/L, triglyceride (TG) ≥1.7 mmol/L, and/or long-term use of lipid-lowering drugs.18 Renal dysfunction was defined as eGFR <90 mL/(min*1.73m2). Body mass index (BMI) was calculated as 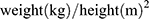 .
.
Coronary angiography data was interpreted and recorded by 2 independent cardiologists according to a clear and predefined criterion. Any identifying information or treatment assignment was concealed before being presented to the judges. If the 2 cardiologists disagreed, a third experienced physician was involved to reevaluate the case, and a consensus was reached to resolve the conflict. According to the coronary angiography, left main (LM) disease was defined as ≥50% stenosis of the LM coronary artery, and three-vessel disease was defined as ≥50% stenosis of all 3 main coronary arteries (ie, right coronary artery, left anterior descending artery and left circumflex artery). Chronic total occlusion (CTO) lesion was determined as thrombolysis in myocardial infarction (TIMI) flow grade of 0 in a native coronary artery for more than 3 months.19 The complexity of coronary lesions was evaluated by Synergy between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery (SYNTAX) score using an online calculator (http://www.syntaxscore.com/). Successful revascularization was determined as a residual stenosis <30% with TIMI flow grade 3 measured by visual estimation of post-PCI angiography.19
Laboratory tests
All the laboratory tests mentioned in this study were performed preoperatively on the day of admission. Fasting blood samples were collected from all patients within 24 h after admission and were stored at –80 °C until the test. An automatic blood cell analyzer (XT-1800i; Sysmex Corporation) was used to measure complete blood count including platelet, neutrophil, lymphocyte, and other parameters. Fasting blood glucose (FBG) was measured by the enzymatic hexokinase method. HbA1c was measured by Tosoh Automated Glycohemoglobin Analyzer (HLC-723G8, Tokyo, Japan). Other laboratory test indicators, including lipid profiles (TG, TC, LDL-C, and HDL-C), creatinine, and high-sensitivity C-reactive protein (hsCRP) were measured by standard operating procedures at the core laboratory in Fuwai Hospital. Chinese-modified Modification of Diet in Renal Disease (MDRD) equation was used to calculate eGFR.20
Statistical analysis
Continuous variables were presented as mean ± standard deviation or median (interquartile range [IQR]) and were compared by two-tailed unpaired Student’s t-test or Mann–Whitney U-test as appropriate. Categorical variables were presented as frequency with percentage and were compared by Pearson χ2 test or Fisher exact test as appropriate. Pearson correlation analysis was performed to evaluate the correlation between SII and glycemic (FBG and HbA1c) or inflammatory (hsCRP) parameters. The cumulative incidence of MACEs was depicted by Kaplan–Meier curves, and the risks of various groups were compared by Log rank test. The joint effect of T2DM and SII was analyzed by univariate and multivariate Cox proportional hazards models. Hazard ratios (HR) and 95% confidence interval (CI) were described. Univariate Cox regression analysis was conducted to identify variables with significant associations with the outcomes (P < 0.1, Supplementary Table S1), while other variables were selected according to previous studies.6,21 The included variates in multivariate Cox analysis were age, male, hypertension, dyslipidemia, prior MI, prior PCI/CABG, hsCRP, creatinine, LM/three-vessel disease, CTO, number of stents, SYNTAX score, total length of stents and average diameter of stents. An exploratory subgroup analysis was conducted to test the risk of MACEs among six different subgroups and the results were depicted by forest plot, while the choice of the subgroups was based on clinical significance and previous studies.22–24 Additionally, the linear correlation between SII and the risk of MACE was examined by restricted cubic splines. A two-tailed P value <0.05 was considered as statistical significance. All statistical analyses were performed using R (version 4.2.1), RStudio software (version 2022.07.1; http://www.rstudio.org/) and GraphPad Prism software (version 8.0.1; GraphPad Software, Inc., La Jolla, CA, United States). The mainly used R packages and their versions were “ggplot2” (3.3.6), “ezcox” (1.0.2), ‘rms’ (6.6–0), “survival” (3.3–1), and “survminer” (0.4.9).
Results
In general, 8,602 patients who met the inclusion and exclusion criteria were ultimately included in this study. The mean age of the total population was 58.41 ± 10.19 years, and 76.9% were male. During a median follow-up of 2.4 years (interquartile range: 2.2–2.6 years), 102 (1.2%) all-cause death, 90 (1.0%) MI, 393 (4.6%) TVR and 522 (6.1%) MACEs were recorded.
Baseline Characteristics
The baseline demographic and clinical characteristics of the entire study population as well as according to the occurrence of MACE are detailed in Table 1. Patients with any component of MACE tended to be older and had a higher proportion of T2DM and MI history. Besides, they also had higher levels of SII, FBG, HbA1c, and hsCRP, but lower LVEF in admission. As for angiography data, patients with MACE seemed to have more complicated lesions, which were more likely to present with LM/three-vessel disease or CTO lesions, and had higher SYNTAX scores. In addition, they tended to have more and longer stents implanted.
 |
Table 1 Baseline Characteristics for Patients Stratified by Primary Events |
Comparison of Baseline Characteristics Among Four Groups
According to the surv_cutpoint function of the R package survminer in the R programming language, the optimal cut-off point of SII for the risk of MACE was 502.5. Thus, patients were divided into four groups based on the level of SII and the presence of T2DM, and a comparison of baseline characteristic among the four groups are listed in Table 2. Compared with patients in the SII-H/T2DM subset, those in the other three groups tended to be younger and had a lower prevalence of comorbidities, such as hypertension, stroke history, PAD history, and clinical presentation as ACS. In the case of laboratory test results in admission, TG, TC, LDL-C, hsCRP, and creatinine were significantly higher in the SII-H/T2DM group, while HDL-C, eGFR, and LVEF were significantly lower. Patients with higher levels of SII combined with T2DM were more likely to have LM/three-vessel disease and CTO and present with higher SYNTAX scores.
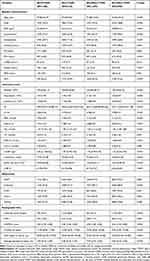 |
Table 2 Baseline Characteristics for Patients Stratified by the Level of SII and Different Glycemic Metabolism Status |
Predictive Value of SII on MACE
In the whole cohort, the incidence of MACE in the SII-L and SII-H groups were 5.5% (301/5475) and 7.1% (221/3127), respectively. The Kaplan–Meier survival curve indicated that patients with T2DM or higher levels of SII had significantly increased risk for MACE compared to the other groups (log-rank P < 0.001 and P = 0.0029) (Figures 1). The multivariate Cox analysis revealed a significant association between higher risks of MACE and elevated SII (adjusted HR: 1.21, 95% CI: 1.01–1.46, P = 0.037) (Table 3).
 |
Table 3 Relation of the SII Level and Primary Endpoint in Patients with Different Glycemic Metabolism Status |
 |
Figure 1 Kaplan–Meier curves for the cumulative incidence of MACE according to different SII levels (A), glycemic metabolism status (B). |
In the T2DM cohort, higher levels of SII were associated with increased risk of MACE, both as a continuous variable (HR: 1.36, 95% CI: 1.11–1.66 per 1-unit increase in SII, P = 0.003) and a categorical variable (HR: 1.39, 95% CI: 1.10–1.74, P = 0.005) in univariate analysis. This association remained statistically significant after adjustment for potential confounders in multivariate analysis (adjusted HR: 1.29, 95% CI: 1.03–1.61 per 1-unit increase in SII, P = 0.024; adjusted HR: 1.31, 95% CI: 1.03–1.67, P = 0.028). However, in the non-T2DM cohort, the risk of MACE did not differ significantly between groups with different levels of SII (adjusted HR: 1.11, 95% CI: 0.84–1.46, P = 0.476) (Table 3).
Clinical Outcomes of Different Groups Grouped by SII and Glycemic Metabolism Status
The incidence of MACE in SII-H subgroup with or without T2DM and SII-L subgroup with or without T2DM was 9.0% (129/1440), 5.5% (92/1687), 6.6% (166/2516) and 4.6% (166/2516), respectively (Figures 2a). The Kaplan–Meier survival curve indicated the highest risk of MACE in SII-H/T2DM group compared with other groups (log-rank P < 0.001) (Figures 2b). Furthermore, clinical outcomes of the four groups were compared by univariate and multivariate Cox regression analysis (SII-H/T2DM group as reference) (Table 4). Patients in SII-L/T2DM, SII-H/Non-T2DM, and SII-L/Non-T2DM groups had significantly decreased risk of MACE (HR: 0.72, 95% CI: 0.57–0.91, P = 0.005; HR: 0.60, 95% CI: 0.46–0.78, P < 0.001; HR: 0.50, 95% CI: 0.39–0.63, P < 0.001). The results remained consistent across multivariate Cox analysis (adjusted HR: 0.77, 95% CI: 0.61–0.98, P = 0.036; adjusted HR: 0.66, 95% CI: 0.50–0.87, P = 0.003; adjusted HR: 0.58, 95% CI: 0.45–0.74, P < 0.001). As for secondary endpoints, it is noteworthy that patients in the SII-L/Non-T2DM group had a 39% lower risk of MI and all-cause death compared with those in the SII-H/T2DM group (adjusted HR: 0.61, 95% CI: 0.38–0.98, P = 0.042). Moreover, multivariate Cox analysis indicated the highest risk of MACE in the SII-H/T2DM group (P for trend < 0.001) (Figure 3). Restricted spline curve analysis revealed that there was a monotonic increase in the risk of MACE with increasing SII levels in either univariable or multivariable model (P for non-linearity > 0.05 for both) (Supplementary Figure S2).
 |
Table 4 Predictive Value of the SII Level and Different Glycemic Metabolism Status for Primary and Secondary Endpoints |
 |
Figure 2 Incidence rate for MACE (A) and Kaplan–Meier curves for the cumulative incidence of MACE (B) according to four groups. |
Relationship between SII and HbA1c/FBG/hsCRP
Linear correlation analysis was conducted to assess the correlation between SII and glycemic metabolism status (Supplementary Table S2). SII was positively correlated with admission FBG in the whole cohort (R = 0.095, P < 0.001), and the results were consistent between the T2DM cohort and non-T2DM cohort (R = 0.127, P < 0.001; R = 0.109, P < 0.001). However, no significant correlation was observed between SII and HbA1c (R=−0.009, P = 0.416). Furthermore, we evaluated the correlation between SII and hsCRP, which is considered a well-established inflammatory biomarker. Results showed that there was a linear positive correlation between SII and hsCRP (R = 0.246, P < 0.001).
Subgroup Analysis
Subgroup analysis indicated a consistent tendency between four groups and risks of MACE across different subgroups (age, sex, BMI, hypertension, renal dysfunction, clinical presentation), with no significant interaction effect observed (all P for interaction > 0.05) (Figure 4; Supplementary Tables S3 and S4).
Discussion
In this large-scale, real-world, prospective observational study, we evaluated the association between SII and adverse outcomes in CAD patients with different glycemic metabolism state. The main findings of the present study are described below: (1) SII was an independent predictor for MACE in CAD patients undergoing completely coronary revascularization; (2) The prognostic value of SII could be modified by glycemic metabolism state; (3) Compared to patients in other three groups, T2DM patients with elevated SII had significantly higher risks for MACE. For the first time, we demonstrated the association between SII and T2DM in CAD patients undergoing PCI and revealed a synergetic prognostic effect of SII and T2DM on adverse cardiovascular outcomes.
In this study, SII was positively correlated to hsCRP, which is a well-established inflammatory biomarker. Compared to hsCRP, SII is derived from the examination of peripheral blood cells and is a cheaper and easily obtained biomarker. SII was first proposed by Hu et al25 and used as a well-performed prognostic factor in patients with different types of carcinomas.26–28 In recent years, numerous studies have been performed to evaluate the prognostic value of SII in the field of cardiology. SII was reported to be associated with the severity of lesions measured by SYNTAX scores in both ACS and stable CAD patients.10,11 A study from Turkey indicated that higher levels of SII were significantly related to poor coronary collateral circulation in patients with CTO.29 In a study including 3,561 three-vessel disease patients, SII was a useful predictive tool for worse clinical outcomes after adjusting for potential confounders.13 As for patients with ST-segment elevation myocardial infarction, several studies suggested that SII could serve as a predictor for in-hospital malignant ventricular arrhythmias, no-reflow phenomenon, and short-term mortality after primary PCI.12,30 A recent prospective study proved that SII was closely associated with all-cause death and cardiovascular death in a general population with no history of cardiovascular diseases through a 20-year follow-up.31 Further, both national cross-sectional and regional cohort studies showed that SII was an independent predictor for MACE in CAD patients,32–34 which was consistent with our findings.
Interestingly, we found that SII was an independent predictor in the T2DM cohort instead of the non-T2DM cohort, suggesting that the inflammatory status of the diabetic population should be paid more attention. As an integrated index derived from peripheral platelets, neutrophils, and lymphocytes, the synergetic effect of SII and T2DM on adverse cardiovascular outcomes may be attributed to the function of the above-mentioned different types of cells and their complicated interaction.
First, neutrophil extracellular traps (NETs) and the concomitant programmed cell death of neutrophils, termed NETosis, are direct evidence of links between atherosclerotic and diabetic processes through inflammatory pathways.35,36 Stimulated by pathogens or sterile inflammatory stimulators (eg, cholesterol crystals, activated platelets), neutrophils release enzymes and chromatin into the extracellular space to form a net-like structure during NETosis.37 T2DM patients were found to have increased levels of circulating biomarkers of NETosis.38,39 The absence of NETosis in atherosclerotic apoE−/− mice significantly reduced plaque size and pro-inflammatory cytokine levels.40 Moreover, the inhibition of NETosis in diabetic mice promoted plaque resolution after lipid lowering.41
Second, T2DM, characterized by hyperglycemia and insulin resistance, is a pro-thrombotic state with platelet dysfunction.42 Hyperglycemia may induce the overexpression of P-selection and glycating platelet surface proteins and activation of PKC, promoting platelet adhesion to impaired endothelium.43,44 Abnormally increased platelet aggregation in T2DM patients may be due to insulin resistance, in which the anti-aggregation effect of insulin is impaired.45
Mounting studies have pointed out that SII may be a useful diagnostic biomarker for detecting micro- or macrovascular complications in patients with T2DM, including diabetic macular edema, diabetic kidney disease, diabetic depression, and peripheral arterial disease.46–49 However, to date, the investigation of the prognostic value of SII for cardiovascular outcomes in T2DM patients has been scant. For the first time, we illustrated a modified effect of T2DM on the prognostic value of SII in CAD patients undergoing PCI, which testified to the complicated interaction among inflammation, T2DM, and CAD from another perspective.
In the present study, we found that SII was positively correlated to FBG in both T2DM and normoglycemic patients, while had no relationship with HbA1c. However, previous studies have reported that hsCRP was positively correlated to FBG and HbA1c in patients with or without T2DM.50,51 This may be since hsCRP and SII mediate the progression of diabetes through different inflammatory pathways, and we also proved that SII was a prognostic predictor for MACE independent of hsCRP. Notably, the correlation between SII and FBG should be interpreted cautiously on account of the relatively weak strength of correlation.
Our study has several limitations that need to be illustrated. First, SII and blood glucose levels were obtained only for one time before PCI, thus we cannot determine whether the dynamic changes will have an impact on the prognosis. In addition, the effect of antiplatelet drugs on SII cannot be evaluated. Second, although Fuwai hospital is a national center recruiting patients from all over the country, potential selection bias still exists on account of the single-center design. Third, due to the natural defect of observational study, there may exist unmeasured confounders leading to potential confounding bias to a certain extent, and no causal association could be established. Finally, despite a relatively large-scale sample size, this study only included the Chinese population in a single center, thus the generalization of our study results needs to be further verified.
Conclusions
In this large-scale real-world study, the combination of elevated SII and T2DM predicted worse clinical outcomes in CAD patients undergoing PCI. Test of SII may contribute to more precise risk stratification in this specific population.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics Approval and Informed Consent
The study process was in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of Fuwai Hospital. All subjects provided informed written consent for long-term follow-up before intervention.
Consent for Publication
The manuscript was approved by all authors for publication.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
CAMS Innovation Fund for Medical Sciences (CIFMS) (2021-I2M-1-008).
Disclosure
The authors declare that they have no competing interests.
References
1. Kong P, Cui ZY, Huang XF, Zhang DD, Guo RJ, Han M. Inflammation and atherosclerosis: signaling pathways and therapeutic intervention. Signal Transduct Target Ther. 2022;7(1):131. doi:10.1038/s41392-022-00955-7
2. Soehnlein O, Libby P. Targeting inflammation in atherosclerosis - from experimental insights to the clinic. Nat Rev Drug Discov. 2021;20(8):589–610. doi:10.1038/s41573-021-00198-1
3. Kazemian S, Ahmadi R, Rafiei A, et al. The serum levels of il-36 in patients with coronary artery disease and their correlation with the serum levels of IL-32, IL-6, TNF-α, and oxidative stress. Int Arch Allergy Immunol. 2022;183(10):1137–1145. doi:10.1159/000525845
4. Guedeney P, Claessen BE, Kalkman DN, et al. Residual inflammatory risk in patients with low LDL cholesterol levels undergoing percutaneous coronary intervention. J Am Coll Cardiol. 2019;73(19):2401–2409. doi:10.1016/j.jacc.2019.01.077
5. Bäck M, Yurdagul A, Tabas I, Öörni K, Kovanen PT. Inflammation and its resolution in atherosclerosis: mediators and therapeutic opportunities. Nat Rev Cardiol. 2019;16(7):389–406. doi:10.1038/s41569-019-0169-2
6. He J, Bian X, Song C, et al. High neutrophil to lymphocyte ratio with type 2 diabetes mellitus predicts poor prognosis in patients undergoing percutaneous coronary intervention: a large-scale cohort study. Cardiovasc Diabetol. 2022;21(1):156. doi:10.1186/s12933-022-01583-9
7. Petzold T, Zhang Z, Ballesteros I, et al. Neutrophil “plucking” on megakaryocytes drives platelet production and boosts cardiovascular disease. Immunity. 2022;55(12):2285–2299.e7. doi:10.1016/j.immuni.2022.10.001
8. Ed Rainger G, Chimen M, Harrison MJ, et al. The role of platelets in the recruitment of leukocytes during vascular disease. Platelets. 2015;26(6):507–520. doi:10.3109/09537104.2015.1064881
9. Stark K, Massberg S. Interplay between inflammation and thrombosis in cardiovascular pathology. Nat Rev Cardiol. 2021;18(9):666–682. doi:10.1038/s41569-021-00552-1
10. Akboga MK, Inanc IH, Sabanoglu C, et al. Systemic immune-inflammation index and c-reactive protein/albumin ratio could predict acute stent thrombosis and high syntax score in acute coronary syndrome. Angiology. 2023;74(7):693–701. doi:10.1177/00033197221125779
11. Candemir M, Kiziltunç E, Nurkoç S, Şahinarslan A. Relationship between systemic immune-inflammation index (sii) and the severity of stable coronary artery disease. Angiology. 2021;72(6):575–581. doi:10.1177/0003319720987743
12. Vatan MB, Çakmak AC, Ağaç S, Eynel E, Erkan H. The systemic immune-inflammation index predicts impaired myocardial perfusion and short-term mortality in st-segment elevation myocardial infarction patients. Angiology. 2023;74(4):365–373. doi:10.1177/00033197221106886
13. Zhao J, Lv H, Yin D, et al. Systemic immune-inflammation index predicts long-term outcomes in patients with three-vessel coronary disease after revascularization: results from a large cohort of 3561 patients. J Inflamm Res. 2022;15:5283–5292. doi:10.2147/JIR.S385990
14. Mosenzon O, Alguwaihes A, Leon JLA, et al. CAPTURE: a multinational, cross-sectional study of cardiovascular disease prevalence in adults with type 2 diabetes across 13 countries. Cardiovasc Diabetol. 2021;20(1):154. doi:10.1186/s12933-021-01344-0
15. Inker LA, Astor BC, Fox CH, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis. 2014;63(5):713–735. doi:10.1053/j.ajkd.2014.01.416
16. American Diabetes Association Professional Practice Committee. Classification and diagnosis of diabetes: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S17–S38. doi:10.2337/dc22-S002
17. Williams B, Mancia G, Spiering W, et al. ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021–3104. doi:10.1093/eurheartj/ehy339
18. Mach F, Baigent C, Catapano AL, et al. ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk: the task force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur Heart J. 2020;41(1):111–188. doi:10.1093/eurheartj/ehz455
19. Neumann FJ, Sousa-Uva M, Ahlsson A, et al. ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40(2):87–165. doi:10.1093/eurheartj/ehy394
20. Y-c M, Zuo L, Chen J-H, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. 2006;17(10):2937–2944. doi:10.1681/ASN.2006040368
21. He J, Song C, Zhang R, Yuan S, Li J, Dou K. Discordance between neutrophil to lymphocyte ratio and high sensitivity c-reactive protein to predict clinical events in patients with stable coronary artery disease: a large-scale cohort study. J Inflamm Res. 2023;16:5439–5450. doi:10.2147/JIR.S428734
22. He J, Yang M, Song C, et al. Lipoprotein(a) is associated with recurrent cardiovascular events in patients with coronary artery disease and prediabetes or diabetes. J Endocrinol Invest. 2023. doi:10.1007/s40618-023-02203-3
23. He J, Song C, Wang H, Zhang R, Yuan S, Dou K. Diabetes mellitus with mild or moderate kidney dysfunction is associated with poor prognosis in patients with coronary artery disease: a large-scale cohort study. Diabet Res Clin Pract. 2023;200:110693. doi:10.1016/j.diabres.2023.110693
24. He J, Yuan S, Song C, et al. High triglyceride-glucose index predicts cardiovascular events in patients with coronary bifurcation lesions: a large-scale cohort study. Cardiovasc Diabetol. 2023;22(1):289. doi:10.1186/s12933-023-02016-x
25. Hu B, Yang XR, Xu Y, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20(23):6212–6222. doi:10.1158/1078-0432.CCR-14-0442
26. Nøst TH, Alcala K, Urbarova I, et al. Systemic inflammation markers and cancer incidence in the UK biobank. Eur J Epidemiol. 2021;36(8):841–848. doi:10.1007/s10654-021-00752-6
27. Liu J, Li S, Zhang S, et al. Systemic immune-inflammation index, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio can predict clinical outcomes in patients with metastatic non-small-cell lung cancer treated with nivolumab. J Clin Lab Anal. 2019;33(8):e22964. doi:10.1002/jcla.22964
28. Jomrich G, Paireder M, Kristo I, et al. High systemic immune-inflammation index is an adverse prognostic factor for patients with gastroesophageal adenocarcinoma. Ann Surg. 2021;273(3):532–541. doi:10.1097/SLA.0000000000003370
29. Adali MK, Buber I, Sen G, Yilmaz S. Relationship between systemic immune-inflammation index and coronary collateral circulation in patients with chronic total Occlusion. Arq Bras Cardiol. 2022;119(1):69–75. doi:10.36660/abc.20210414
30. Aksakal E, Aksu U, Birdal O, et al. Role of systemic immune-inflammatory index in predicting the development of in-hospital malignant ventricular arrhythmia in patients with st-elevated myocardial Infarction. Angiology. 2023;74(9):881–888. doi:10.1177/00033197221121435
31. Xia Y, Xia C, Wu L, Li Z, Li H, Zhang J. Systemic immune inflammation index (sii), system inflammation response index (siri) and risk of all-cause mortality and cardiovascular mortality: a 20-year follow-up cohort study of 42,875 us adults. J Clin Med. 2023;12(3):1128. doi:10.3390/jcm12031128
32. Xiao S, Wang Z, Zuo R, et al. Association of systemic immune inflammation index with all-cause, cardiovascular disease, and cancer-related mortality in patients with cardiovascular disease: a cross-sectional study. J Inflamm Res. 2023;16:941–961. doi:10.2147/JIR.S402227
33. He L, Xie X, Xue J, Xie H, Zhang Y. Association of the systemic immune-inflammation index with all-cause mortality in patients with arteriosclerotic cardiovascular disease. Front Cardiovasc Med. 2022;9:952953. doi:10.3389/fcvm.2022.952953
34. Yang YL, Wu CH, Hsu PF, et al. Systemic immune-inflammation index (SII) predicted clinical outcome in patients with coronary artery disease. Eur J Clin Invest. 2020;50(5):e13230. doi:10.1111/eci.13230
35. Poznyak A, Grechko AV, Poggio P, Myasoedova VA, Alfieri V, Orekhov AN. The diabetes mellitus-atherosclerosis connection: the role of lipid and glucose metabolism and chronic inflammation. Int J Mol Sci. 2020;21(5):1835. doi:10.3390/ijms21051835
36. Fadini GP, Menegazzo L, Scattolini V, Gintoli M, Albiero M, Avogaro A. A perspective on NETosis in diabetes and cardiometabolic disorders. Nutr, Metab Cardiovasc Dis. 2016;26(1):1–8. doi:10.1016/j.numecd.2015.11.008
37. Döring Y, Soehnlein O, Weber C. Neutrophil extracellular traps in atherosclerosis and atherothrombosis. Circ Res. 2017;120(4):736–743. doi:10.1161/CIRCRESAHA.116.309692
38. Menegazzo L, Ciciliot S, Poncina N, et al. NETosis is induced by high glucose and associated with type 2 diabetes. Acta Diabetol. 2015;52(3):497–503. doi:10.1007/s00592-014-0676-x
39. Joshi MB, Ahamed R, Hegde M, Nair AS, Ramachandra L, Satyamoorthy K. Glucose induces metabolic reprogramming in neutrophils during type 2 diabetes to form constitutive extracellular traps and decreased responsiveness to lipopolysaccharides. Biochim Biophys Acta Mol Basis Dis. 2020;1866(12):165940. doi:10.1016/j.bbadis.2020.165940
40. Warnatsch A, Ioannou M, Wang Q, Papayannopoulos V. Inflammation. neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science. 2015;349(6245):316–320. doi:10.1126/science.aaa8064
41. Josefs T, Barrett TJ, Brown EJ, et al. Neutrophil extracellular traps promote macrophage inflammation and impair atherosclerosis resolution in diabetic mice. JCI Insight. 2020;5(7):e134796. doi:10.1172/jci.insight.134796
42. Neergaard-Petersen S, Hvas AM, Kristensen SD, Grove EL. Platelets and antiplatelet therapy in patients with coronary artery disease and diabetes. Semin Thromb Hemost. 2016;42(3):234–241. doi:10.1055/s-0036-1571308
43. Ferreiro JL, Angiolillo DJ. Diabetes and antiplatelet therapy in acute coronary syndrome. Circulation. 2011;123(7):798–813. doi:10.1161/CIRCULATIONAHA.109.913376
44. Keating FK, Sobel BE, Schneider DJ. Effects of increased concentrations of glucose on platelet reactivity in healthy subjects and in patients with and without diabetes mellitus. Am J Cardiol. 2003;92(11):1362–1365. doi:10.1016/j.amjcard.2003.08.033
45. Trovati M, Anfossi G. Influence of insulin and of insulin resistance on platelet and vascular smooth muscle cell function. J Diabetes Complications. 2002;16(1):35–40. doi:10.1016/s1056-8727(01)00196-9
46. Wang J, Zhou D, Dai Z, Li X. Association between systemic immune-inflammation index and diabetic depression. Clin Interv Aging. 2021;16:97–105. doi:10.2147/CIA.S285000
47. Guo W, Song Y, Sun Y, et al. Systemic immune-inflammation index is associated with diabetic kidney disease in type 2 diabetes mellitus patients: evidence from NHANES 2011–2018. Front Endocrinol. 2022;13:1071465. doi:10.3389/fendo.2022.1071465
48. Song Y, Zhao Y, Shu Y, et al. Combination model of neutrophil to high-density lipoprotein ratio and system inflammation response index is more valuable for predicting peripheral arterial disease in type 2 diabetic patients: a cross-sectional study. Front Endocrinol. 2023;14:1100453. doi:10.3389/fendo.2023.1100453
49. Elbeyli A, Kurtul BE, Ozcan SC, Ozarslan Ozcan D. The diagnostic value of systemic immune-inflammation index in diabetic macular oedema. Clin Exp Optom. 2022;105(8):831–835. doi:10.1080/08164622.2021.1994337
50. Seo JW, Park SB. The association of hemoglobin a1c and fasting glucose levels with hs-crp in adults not diagnosed with diabetes from the KNHANES, 2017. J Diabetes Res. 2021;2021:5585938. doi:10.1155/2021/558593851
51. Elimam H, Abdulla AM, Taha IM. Inflammatory markers and control of type 2 diabetes mellitus. Diabetes Metab Syndr. 2019;13(1):800–804. doi:10.1016/j.dsx.2018.11.061
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


