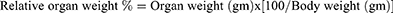Back to Journals » Drug Design, Development and Therapy » Volume 16
The Combination of Zinc and Melatonin Enhanced Neuroprotection and Attenuated Neuropathy in Oxaliplatin-Induced Neurotoxicity
Received 13 August 2022
Accepted for publication 22 September 2022
Published 4 October 2022 Volume 2022:16 Pages 3447—3463
DOI https://doi.org/10.2147/DDDT.S385914
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Tuo Deng
Mayyadah Ali,1 Tavga Aziz2
1Hiwa Cancer Hospital, Sulaimani, Kurdistan Region, Iraq; 2Department of Pharmacology and Toxicology, College of Pharmacy, University of Sulaimani, Sulaimani, Kurdistan Region, Iraq
Correspondence: Tavga Aziz, Tel +9647701523544, Email [email protected]
Objective: The present study was designed to investigate the possible synergistic effects of melatonin with zinc in the prevention and treatment of oxaliplatin-induced neurotoxicity in rats.
Methodology: Forty-eight male Wistar albino rats were used and randomly allocated into six groups: The negative control group, oxaliplatin group, zinc + oxaliplatin group, melatonin + oxaliplatin group, zinc + melatonin + oxaliplatin prevention-approach group, and zinc + melatonin + oxaliplatin treatment-approach group. The thermal nociceptive/hyperalgesia tests were performed. Brain tissue homogenate was used for measuring GFAP, NCAM, TNF α, MAPK 14, NF-kB, GPX, and SOD. Brain tissue was sent for histopathological and immunohistochemistry studies.
Results: The combination therapies showed improvement in the behavioral tests. A significant increase in GPX and SOD with a significant decrease in GFAP levels resulted in the prevention approach. TNF α decreased significantly in the treatment approach. No significant changes were seen in NCAM, NFkB, and MAPK-14. The histopathological findings support the biochemical results. Additionally, immunohistochemistry revealed a significant attenuation of p53 and a non-significant decrease in Bcl2 levels in the combination groups.
Conclusion: The combination of zinc with melatonin for the prevention approach was effective in attenuating neurotoxicity induced by oxaliplatin. The proposed mechanisms are boosting the antioxidant system and attenuating the expression of p53, GFAP, and TNF-α.
Keywords: melatonin, zinc, oxaliplatin, neurotoxicity, antioxidant activity, GFAP, p53
Introduction
Antineoplastic chemotherapy may produce various types of neurotoxicity ranging from dysfunction, and pain to peripheral neuropathy secondary to injury of sensory, motor, and/or autonomic nerves that directly lessen the quality of life of the patients.1,2 Oxaliplatin-induced peripheral neuropathy (OIPN) is an account for 90% of cancer patients.3 Hyperexcitation of sensory and motor nerves by the use of oxaliplatin produces neuromyotonic-like syndrome, which may disappear within a few days, this is considered an acute effect of the drug.4 Chronic use of oxaliplatin (OXL) may end up with sensory symmetrical symptoms, sensory ataxia, and falls.5,6 Peripheral neurotoxicity induced by OXL may continue even after finishing the treatment for more than 5 years in 25–30% of the patients including psychological problems and depression.7,8 The anticipated unwanted responses of OXA have been reported as the clinical use of OXA has grown. These OXA side effects may result in the termination of treatment plans, as well as a reduction in treatment compliance of the patients.9
Several mechanisms were proposed for the neurotoxic effects of OXL.10 OXL can induce neuropathy by damaging numerous cells in different ways. Damage in the nuclear DNA is one of the mechanisms of OIPN,11 where platinum compounds create DNA adducts that hinder DNA base excision repair and transcription finally leading to cell damage.12 Oxidative stress is the most prominent mechanism; in which oxaliplatin increases the generation of reactive oxygen species.13 Activation of transcription factor14 and change in gene expression that trigger neuroinflammation also contribute to OIPN.15 Mitochondrial dysfunction is another mechanism of OIPN and usually, and this is usually accompanied by the generation of reactive species that further aggravate the condition.16 Furthermore, disruption of ion channels like sodium, potassium, and calcium channels,17 especially, dysfunction of voltage-gated sodium channel18 along with neuroinflammation also attributed to OIPN.19
Given the severity of OIPN and the fact that it is irreversible in a substantial percentage of patients, efforts to treat this pharmacoresistant clinical illness have been ongoing for many years.20 Many drugs and nutraceuticals have been tried to control OIPN and most of these agents possess antioxidant and anti-inflammatory activities.21–23 The fact that most medications only address one feature of the disease may contribute to the inability to create treatments that effectively combat brain injury-induced neurodegeneration.24 Melatonin is tryptophan derivatives produced primarily by the pineal gland in mammals throughout the night.25 It has pleiotropic effects in the human body, which include regulation of important metabolic processes such as regulation of sleep–wake cycle, bone metabolism, fertility and reproduction, as well as protection against a variety of illnesses like preventive effects against oxidative stress, aberrant immunological activation and/or inflammation, in addition to, obesity, cardiovascular problems, cancer development, and neurodegeneration.26 Melatonin has been reported to have a prolonged and strong antinociceptive impact in neuropathic pains.27 There is a bidirectional relationship between melatonin and zinc. Zinc is shown to be involved in the production and action of melatonin and the later in turn increases the absorption of zinc.28,29 Co-supplementation of zinc and melatonin has been found to have many beneficial effect both in animal experiments and clinical study.30,31
Although various studies have indicated melatonin’s significance in the treatment of diabetic neuropathy,32,33 there is no investigations on its influence on neuropathy prevention and treatment in combination with zinc. Therefore, the present study was designed to investigate the possible synergistic effects of melatonin with zinc in both the prevention and treatment approaches against OXL-induced neurotoxicity in rats.
Materials and Methods
Experimental Animals
Forty-eight male Wistar albino rats weighing (150–200g) were obtained from the College of Medicine/Tikrit Medical University. The rats were housed in the College of Pharmacy/University of Sulaimani animal house in well-ventilated plastic cages under regular conditions; temperature 25 ± 2°C and humidity of 55 ± 5%, and 12 hours’ dark/light cycle. They were fed a conventional pellet diet and were given unlimited access to water. The animals were kept for one week before the experiment for acclimatization. The experimental protocols met the Guidelines for Animal Experimentation and approved by the Ethical Committee of the University of Sulaimani, College of Pharmacy (Certificate no. PH33-21 on 14th November 2021) following the institutional Animal Ethics Committee. The study was performed by the Canadian Council on Animal Care (CCAC) guidelines.
Study Design
Forty-eight rats used in the current study and kept in cages (8 cubic feet in volume); each cage contained two rats during the study, and they were allocated randomly to six groups each consisting of 8 rats as described below:
1. Negative control group: Received 0.5 mL glucose water (GW) intraperitoneally on the first, second, fifth, and sixth day of treatment course.
2. Positive control group: Received 4mg/kg OXL intraperitoneally after diluting it in a 5% dextrose solution on the first, second, fifth, and sixth days of therapy (total cumulative dose: 16 mg/kg).
3. Zinc + oxaliplatin group: Received 15 mg/kg zinc orally every day for two weeks before starting oxaliplatin induction, and then daily for 1 week along with OXL protocol.
4. Melatonin + oxaliplatin group: Received 10 mg/kg melatonin orally every day for two weeks before starting oxaliplatin induction, and then daily for 1 week along with OXL protocol.
5. Zinc + melatonin + oxaliplatin prevention-approach group: Received 15 mg/kg zinc with 10 mg/kg melatonin orally every day for two weeks before starting OXL induction, and then daily for 1 week along with OXL protocol.
6. Zinc + melatonin + oxaliplatin treatment-approach group: Received 15 mg/kg zinc with 10 mg/kg melatonin orally every day for 1 week along with OXL protocol.
The doses of oxaliplatin, zinc and melatonin and the treatment regimen used in the current study have been chosen depending on the previous studies.21,34,35 The rats were euthanized 24 hours after the last dose of OXL administration using diethyl ether as anesthetic agent.
Measurement of the Weight and Relative Organ Weight of the Rats
The rats utilized in the experiment were weighed before starting the treatment and on scarification day, using a weight measurement scale. The brain was removed carefully, cleaned of all other tissue and weighed. Relative organ weight was measured using the following equation:
Tests of Thermal Nociceptive/Hyperalgesia
Test of Tail Immersion in Hot Water
This experiment was based on a modification of Adeyemi et al protocol in 2011.36 The tail-immersion in hot water test was used to examine thermal sensitivity and measure pain sensitivity in response to a heat stimulus. The middle tails of the rats were immersed in a hot water beaker (55◦C ± 0.5◦C) until tail withdrawal (flicking reaction) or evidence of a struggle was seen in this experiment (cut-off time 30 sec). The reaction time of the animals to the heat stimuli was measured in seconds by tail flick delay. Sensitivity/hyperalgesia was suggested by a shorter tail pullout time, which was linked to a central mechanism.37 Using a stopwatch, the time interval between the commencement of tail heating and the withdrawal response was manually measured in seconds. To avoid tissue damage, tail heating was halted after 30 seconds (cut off time) in the absence of a reaction. The average of the three consecutive tail-flick latency measurements was used to compute tail-flick latency for each rat.
Test of Tail Immersion in Cold Water
The cold-water tail flick test was developed using a modified version of Pizziketti et al's original method (1985). Cold stimulation, tail-immersion in cold water test was used to examine thermal sensitivity and measure pain sensitivity. The lower half of the tails were immersed in a beaker of cold water (4°C± 0.5°C) until tail withdrawal (flicking reaction) or evidence of a struggle was noticed in this test (cut-off time 150 sec). The reaction time of the animals to the cold stimuli was measured in seconds by the tail flick delay. Allodynia is indicated by a decrease in the tail withdrawal time.35 Using a stopwatch, the time interval between the commencement of tail heating and the withdrawal response was manually recorded in seconds.
Brain Tissue Preparation
After the rats were euthanized, the brain was externalized and carefully cleansed with ice-cold phosphate buffer saline, before the whole brain was weighed and divided into two pieces. One piece was used for histopathological study, while the other was weighed, minced, and mixed with ice-cold phosphate saline (PBS) at 4°C at a concentration of 1:9 wt/v (1 g of tissue/9 mL of PBS) and with protease inhibitors (PI) at a concentration of 1:100 v/v (1 mL of PI for 100mL of PBS and brain tissue mixture) for biochemical testing. The tissue homogenizer was then used to homogenize the solution until it was clear. The tissue homogenate solution was then centrifuged for 5 minutes at 8620 g in a cold centrifuge (at 4°C), and the resulted supernatant was separated and frozen at (– 65°C) until used.
Biochemical Tests
Tissue homogenate was used using ELISA kits (Bioassay Technology Laboratory, Shanghai, China) for measuring glial fibrillary acidic protein (GFAP), neural cell adhesion molecule (NCAM), tumor necrosis factor α (TNF α), mitogen activated protein kinase 14 (MAPK 14), nuclear factor-kappa B (NF-kB), superoxide dismutase (SOD) and glutathione peroxidase (GPX).
Histotechnique Protocol
Next to the termination point of each group, animals were fast for at least 12 hours then euthanized humanely with anesthetic agent in a specialized scarification chamber. Following necropsy, brain samples were taken for histological preparation. Briefly, brain tissues were cut and aligned into tissue cassettes and fixed in 10% buffered formalin for 72 hours. Then after, tissue sections were dehydrated from the undue water using a series of ascending concentrations of ethanol (50%, 60%, 70%, 90%, and 100%), followed by couple steps of xylene cleaning. After that, the brain sections were embedded in melted paraffin at (60 −70°C) using an automated wax embedder. Embedded tissue blocks were sectioned to 6 µm using a semi-automated rotary microtome. Following that, the tissue sections were placed in a warm water bath for few minutes and then hunted and mounted on glass slides using a hot plate slide holder. Later, the glass slide-mounted tissue sections were deparaffinized and cleaned up with 2 steps of xylene, 15 minutes each and then oven dried at 50°C for 10 minutes. Finally, the tissue sections were stained with alcoholic solutions of Harris’s hematoxylin and eosin, and then tissue slides were cleaned as a final step in xylene and cover slipped with glassy cover slides using DPX. At the end of this procedure, brain tissues were viewed and examined under bright field light microscope.
Semi Quantitative Histological Assessment
As a morphometric measure, the lesion scoring for brain tissues was estimated semi quantitatively and calculated in µm then statistically evaluated as mean percentage for different morphometric values. Briefly, area of perivascular edema together with inflammatory exudates, as well as the area of vascular hemorrhage and capillary engorgement with microthrombi were measured in µm, and semi quantitatively evaluated in mean percentage. On the other hand, pyknotic cells in the cerebrum and cerebellum were counted in a randomly chosen ten fields tissue sections under high power magnification (100X), then the mean average was calculated statistically in percentage. Finally, all morphometric values were analyzed under the light microscope (NOVEL XSZ-N107, China) via image analyzer software (AmScope Ver. 3.7) using a microscope digital camera (MU300, 2019). The mean percentage of the estimated values were expressed as the following lesion scores (score 0–10% as no lesions; score 10–50% as mild to moderate lesions; score 50–70% as moderate lesions; score 70–85% as moderate to severe lesions; score 85–100% as severe to critical lesions).
Immunohistochemistry (IHC) Screening
The immunohistochemistry (IHC) analysis was performed using a Dako REALTM EnvisionTM Detection System-HRP, Peroxidase/(DAB+), Rabbit/Mouse kit (Dako K5007, Denmark) following the manufacturer’s instruction. Paraffin-embedded brain tissues were cross-sectioned into 4 µm-thick pieces at 5-mm intervals, heated in oven 70°C overnight, deparaffinized at room temperature, and rehydrated for two times, once with xylene for five minutes and once with ethanol in a graduation series (absolute ethanol for five minutes, 90% ethanol for two minutes, 70% ethanol for two minutes). Then, the slides were washed with wash buffer (PBS manufactured by Bio SB, USA) in slide jars and circled around each tissue after being boiled by (TpLink) at 100c for 45 minutes and cooled to room temperature. For the detection of p53 and Bcl2 proteins, a monoclonal mouse p53 antibody (DO-7, code M 7001, DAKO, Denmark) and monoclonal rabbit Bcl2 antibody (EP36, code CA 93117, Bio SB, USA) were applied. Nonspecific antibody binding was blocked by incubation of the tissue section for 10 minutes with (H2O2) in humidity chamber at room temperature. Endogenous peroxidase activity in the sections was extinguished by incubation of the sections in 3% hydrogen peroxide for 5 minutes. Primary antibodies, Mouse-monoclonal anti-human p53 antibody and rabbit-monoclonal anti-human vascular Bcl2 antibody were applied to the sections at a concentration of 1:50 in accordance with the produced protocol and incubated in a humidity chamber at room temperature, after being rinsed twice with wash buffer for three minutes each. Following the same washing process, sections were incubated for 30 minutes in room temperature with Dextran backbone peroxidase-conjugated coupled with goat secondary antibody against rabbit and mouse immunoglobulins (Dako REAL, EnVision/HRP, Rabbit/Mouse). The reaction was then observed by staining the sections with 50 microchromogen brown color for 5 minutes; after adding linked for 15 minutes, waiting for conjugation to occur for 15 minutes, and washing with wash buffer each time. After dehydrating in graded ethanol (alcohol %70 for 2 minutes, alcohol %90 for 2 minutes, and alcohol absolute (%100) for 3 minutes) and xylene (for 5 minutes), respectively, sections were counterstained with hematoxylin. Utilizing DPX, specimens were mounted and coverslips applied.
Immuno-Semi Quantitative Evaluation of p53 and Bcl2
Evaluation of P53 and Bcl2 immunohistochemical staining was done based on the Allred combinative semi-quantitative scoring system,38 in which the percentage of positive cells and the strength of the reaction product are combined. The final score has eight possible values because the two scores are combined. Scores between 0 and 2 are regarded as negative. Scores between 3 and 8 are regarded as positive. In our study, value between 3 and 4, 5 and 6, 7 and 8 regarded as (+, ++, +++), respectively. The immuno-quantitative assessment of P53 and Bcl2 was measured by analyzing the average percentage of immune-positive cells together with the intensity of staining in the following manner: immune-positive population scored as 0, <1% of cells population scored as 1, 1–10% as 2, 11–33% as 3, 34–66% as 4, and >67% as 5. The staining intensity scored as 0, 1–33% of cells stained scored as 1, 34–66% as 2, >67% as 3. Representative images were read by a histopathologist who was unaware of the treatment groups using light microscope (OLYMPUS/U-TV0.5XC-3, JAPAN) with the aid of Image J program.
Statistical Analysis
The statistical analysis was performed using GraphPad Prism 8. The values of the measured parameters were expressed as mean ± standard deviation (S.D.). For the comparisons between different groups, one-way analysis of variance (ANOVA). Unpaired t-tests were used to compare each group with the positive control group. The results were considered statistically significant when the p-value was less than 0.05.
Results
Effect of Zinc and Melatonin Alone or in Combination on Total Weight and Relative Weight of the Rats
In the present study, a significant reduction was seen in the positive control group in comparison with the negative control group (p-value = 0.0002). Meanwhile, zinc and melatonin each alone and in combination in the prevention group and the treatment group significantly increased the weight of the rats when compared to the positive control group (p-value = 0.0001) (p-value = 0.0014), (p-value = 0.029) and (p-value = 0.045), respectively (Figure 1A). Regarding the relative weight, no significant changes have been detected in all the treatment groups (Figure 1B).
Effect of Zinc and Melatonin Alone or in Combination on the Behavioral Tests
OXL resulted in a significant decrease in the reaction time to the heat stimuli in the positive control group when compared to the negative control group (p-value = 0.0069). Zinc and melatonin each alone and in combination in the prevention group significantly increased the reaction time to heat stimuli when compared to the positive control group (p-value = 0.0014), (p-value = 0.0055) and (p-value = 0.036), respectively (Figure 2A). The combination of the treatment approach also significantly increased the reaction time (p-value = 0.0023).
Regarding the response to cold stimuli; oxaliplatin decreased the reaction time in the positive control group compared to the negative control group (p-value = 0.026). The combination of zinc with melatonin for the prevention and treatment approach was able to increase the reaction time significantly when compared to the negative control (p-value = 0.014), and (p-value = 0.025). Each zinc and melatonin alone and the prevention-combination group also increased the reaction time but it did not reach a significant level (p-value = 0.21) and (p-value = 0.82), respectively (Figure 2B).
Effect of Zinc and Melatonin Alone or in Combination on GFAP and NCAM Levels in Brain Tissue
The use of oxaliplatin produced in a significant increase in the level of GFAP in comparison with the negative control group (p-value = 0.0001). Zinc and melatonin each alone and in combination in the prevention group significantly decreased the level of GFAP when compared to the positive control group (p-value = 0.0026), (p-value = 0.0001) and (p-value = 0.01), respectively. The combination for the treatment approach also decreased the level of GFAB, but it was statistically not significant (p-value = 0.07) (Figure 3A). Concerning NCAM level; oxaliplatin produced non-significant decrease when compared with the negative control group (p-value = 0.5). Meanwhile, no significant change has been observed with zinc (p-value = 0.44), melatonin (p-value = 0.21), combination-prevention (p-value = 0.52) and combination-treatment group (p-value = 0.62) (Figure 3B).
Effect of Zinc and Melatonin Alone or in Combination on Inflammatory Markers in Brain Tissue
The study revealed that the level of NF-kB increased significantly after the treatment with OXL in comparison with the negative control group (p-value = 0.008). No significant change has been observed with zinc (p-value = 0.07), melatonin (p-value = 0.18), combination-prevention (p-value = 0.39) and combination-treatment group (p-value = 0.16) (Figure 4A).
Furthermore, TNF-α also increased significantly by the administration of OXL (p-value = 0.001) when compared with the negative control group. However, none of the treatment groups showed any significant change: zinc (p-value = 0.74), melatonin (p-value = 0.9), and combination-prevention group (p-value = 0.72), except for the combination-treatment group; which was able to decrease the level of TNF-α when compared to the positive control group (p-value = 0.023) (Figure 4B). Additionally, MAPK-14 level increased by the use of OXL; however, the change was statistically not significant (p-value = 0.48), and all the treatment groups did not demonstrate any significant change when compared with the positive control group: zinc (p-value = 0.5), melatonin (p-value = 0.8), combination-prevention (p-value = 0.72) and combination-treatment group (p-value = 0.74) (Figure 4C).
Effect of Zinc and Melatonin Alone or in Combination on Oxidative Stress Parameters in Brain Tissue
The level of GPX and SOD has decreased significantly in the positive control group when compared to the negative control group (p-value = 0.013) and (p-value = 0.011), respectively. Each of zinc and the treatment combination groups increased the level of GPX but it did not reach a significant level (p-value = 0.99) and (p-value = 0.15), respectively. Melatonin and the prevention combination groups were able to increase GPX level significantly (p-value = 0.032), and (p-value = 0.046), respectively (Figure 5A). Regarding the level of SOD, a non-significant increase was observed by the zinc and the treatment combination (p-value = 0.37) and (p-value = 0.2), respectively. Meanwhile, melatonin and the prevention combination groups significantly increased the level of SOD when compared to the positive control group (p-value = 0.028), and (p-value = 0.011), respectively (Figure 5B).
 |
Figure 5 Effect of zinc and melatonin alone or in combination on (A) GPX, (B) SOD; *(p < 0.05), significantly different compared to the positive control group using one-way ANOVA and unpaired t-test. |
Histopathology
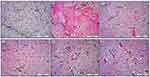
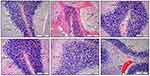
Initially, Table 1 establishes the morphometric semiquantitative assessment of brain tissue, with different histological lesions, and shows a significant P < 0.05 reduction in the percentage of the area of edema together with inflammatory exudates in G5 animals (Zinc and Melatonin prevention group) in comparison with other treatment groups particularly with G2 OXL group, evident by the tremendous decrease in the mean percentage of hemorrhagic area in addition to the reduction in the amount of microthrombi together with the numbers of pyknotic neuroglial cells which has been shown clearly in Figure 6. Moreover, Figure 6 reveals a significant reduction in most morphological lesions in the treatment groups in comparison to the control positive group G2; however, it is moderately much significant in G3 and G4 (zinc and melatonin groups) in comparison to the treatment-approach group (G6). On the other hand, Figure 7 demonstrates the same morphological improvements in the cerebellum sections as in Figure 6. Particularly, animals treated with zinc and melatonin as a preventive measure (G5), show a significant reduction in nearly most of the inflammatory biostatistical markers in comparison with the G2 group. Overall, the semi-quantitative morphometric calculation and lesion scoring in all treatment groups has been clearly changed and significantly reduced in comparison with the oxaliplatin group (G2), thus it is much more significant in prevention-approach trial G5 in both cerebrum and cerebellum, followed by Zinc and Melatonin groups G3 and G4. However, lesion scoring is minimized as well in treatment-approach group G6 in all brain tissues as shown in Table 1, yet it is not significant in comparison to the other treatment groups, especially G5. Therefore, zinc and melatonin supplementation as a prevention measure in calculated doses show eminent amelioration in the morphometric values (Table 1) and histopathological lesions (Figures 6 and 7).
 |
Table 1 Histological Quantitative Evaluation of Brain |
Immunohistochemistry
In general, the combinative semi-quantitative assay of immuno-positive reactions with the P53 antibodies is illustrated in (Table 2), which shows lower percentage of P53 cell expression and stain intensity in zinc plus melatonin prevention and treatment groups (+ for both groups). Furthermore, moderate cell expression and stain intensity of P53 antibody in zinc, melatonin, and negative control groups G1 (++ in all groups). In terms of statistical analysis for P53 biomarker, zinc plus melatonin prevention and treatment groups showed significant (P < 0.01) decrease in the expression of immune-positive cells and in stain intensity in comparison with oxaliplatin group as shown in (Table 2). Although zinc alone and melatonin alone groups showed non-significant (p > 0.05) decrease in immune-positive cell expression and stain intensity, but they revealed lower value when compared to positive control group (Figure 8). On the other hand, Table 3 semi-quantitatively demonstrates the effect of different treatment platforms on expression of Bcl2 protein. Generally speaking, zinc alone and zinc plus melatonin prevention approach group produced comparable effect and nearly the same percentage of Bcl2 expression and stain intensity in comparison to the positive control group (+++ in all groups); in contrast, melatonin alone group and zinc plus melatonin in the treatment approach groups show lower immune positive cell expression and weak, moderate stain intensity when compared with positive control group (++ in both groups) (Figure 9).
 |
Table 2 Immunohistochemical Quantitative Assay of P53 |
 |
Table 3 Immunohistochemical Quantitative Assay of Bcl2 |
Discussion
Many studies showed the effectiveness of combining melatonin with zinc in boosting the immune system,31,39 increasing body energy and enhancing good quality of life,40 as well as improving antioxidant capacity.30 To the best of our knowledge, this is the first study on the use of this combination in neurotoxicity induced by oxaliplatin for the possible synergistic effect in both prevention and treatment approaches. Melatonin has been known as a powerful biological antioxidant in neutralizing free radicals,41 in addition to physiologic tasks such as governing day-night cycle, inducing sleep, seasonal regulation of reproduction, and enhancing immune system activity.39 Because of these properties, melatonin is a prospective therapy for neurodegenerative disease prevention and treatment. In the present study, both melatonin and zinc alone or in combination protected the tested animals from the deleterious effects of oxaliplatin that appeared as the normal growth of the rats. These impacts may be contributed to the modification of the circadian cycle, expanding sleep length and resulting in more growth hormone release.42,43 Additionally, zinc supplementation has been shown in many studies to boost growth hormone secretion in school-aged children.44,45 Response to pain sensitivity also improved by using hot-plate and cold allodynia test, and the combination groups demonstrated a better effect. Thermal hyperalgesia has long been recognized as an important indicator of peripheral neuropathy.46 Exaggerated hyperalgesic behavior is thought to occur in animals in reaction to noxious stimuli, and this behavior may mimic characteristics of painful neuropathy.47 Furthermore, the analgesic effect of melatonin could be attributed to the interference with the action of glutamate, gamma-aminobutyric acid, and, in particular, opioid neurotransmission.48 Melatonin also showed to exhibit a neuroprotective effects when used in animal diabetic model of neuropathy35 and attenuated the neuropathic pain induced by paclitaxel.49 Additionally, melatonin was found to enhance autophagy in oxaliplatin-induced neuropathy.50 Furthermore, degeneration and cognitive decline issues, such as increased neuronal death and reduced learning and memory, are caused by zinc deficiency. Zinc, as a biological component, has a physiological effect in the central nervous system as well as a pathological impact in neurological diseases.51 GFAP has been proven to play a role in CNS damage repair. More specifically, it is known for its function in the creation of glial scars in a variety of CNS sites, including the brain.52 The intermediate filament protein GFAP is found in glial cells such as astrocytes and ependymal cells. Overexpression of GFAP has been linked to astrocyte homeostasis disruption in neuropathological situations and in response to neurotoxins in this environment.53,54 In the current study, melatonin and zinc alone and in combination for the prevention approach were able to decrease the level of GFAP with no significant effect on NCAM. NCAMs are membrane glycoproteins that are involved in the growth of axons and synapses, as well as the activation of signal pathways, and the adhesion and migration of nervous system cells.55 This study confirmed that NCAM levels were reduced due to OXL-induced damage in the brain. Although, the reduction was not significant; all the treatment groups increased its level when compared to the OXL group. This effect could be attributed to the neuroprotective effect of each of zinc56 and melatonin.57
Oxaliplatin increased the levels of the inflammatory markers and the treatment combination of zinc and melatonin demonstrated a better effect in this regard. The NF-κB cascade underlies the pathophysiology of various inflammatory conditions and is aberrantly activated by oxidative stress, cytokines, and MAPK14.58 Furthermore, MAPK14, is considered as a central organizer of the inflammatory reaction in numerous cells.59 TNF-α is another inflammatory cytokine, which plays a pivotal role in the inflammatory response,60 and it is released when NFk-B is activated, which starts the inflammatory response in the body.61 Several studies proved the role of melatonin in reducing both chronic and acute inflammations62 through regulating proinflammatory and anti-inflammatory cytokines,63 and recently melatonin has been shown to attenuate neuroinflammation associated with parkinsonism.64 On the other hand, zinc also showed a beneficial effect in reducing the inflammatory reactions via decreasing NF-κB, TNF-α and IL-1β activation.65 Moreover, the antioxidant effect of melatonin alone and in the combination for the prevention approach was clearly demonstrated through elevating the levels of both GPX and SOD. Various studies proved the role of melatonin in attenuating oxidative stress,24,66 and demonstrated its role in the treatment of neurodegenerative illnesses because of its multiple roles in scavenging free radicals, regulating oxidant and prooxidant enzymes, and inhibiting the production of mitochondrial radicals.41
The histopathological finding revealed a protective effect in all the treatment groups with maximum protection produced by the combination group for the protective approach. This effect was clearly demonstrated through attenuating the mean percentage of hemorrhagic and edematous area along with the decline of the inflammatory and lesion scoring. These findings are in tune with other studies that proved the anti-inflammatory activities of each of zinc and melatonin alone67,68 and in combinations.69
Regarding the findings of the immunohistochemistry, OXL-treated animals resulted in higher levels of p53 proteins than control animals. Meanwhile, zinc plus melatonin in both the prevention and treatment groups attenuated the level of p53 protein when compared to OXL-treated animals. Moreover, Bcl2 level was significantly lower in OXL-treated group in comparison with control animals and this was comparable with the prevention-combination group. While both melatonin alone and the treatment-combination resulted in lower level of Bcl2 when compared to OXL-treated group. Apoptosis is a type of controlled cell death that is involved in many biological processes, including the regulation of cellular homeostasis.70 In tune with the present study, previous findings demonstrated that neuropathic pain could be mediated by increased apoptotic alterations in dorsal root ganglion neurons as a result of overexpression of the p53 gene and subsequent increases in caspase-3 expression.71,72 Furthermore, a new study on melatonin proved the antiapoptotic effect via p53 pathway,73 and melatonin was also shown to suppress neuronal apoptosis ameliorating neuronal damage.74 On the other hand, excess Zn has been linked with structure modification of the p53 protein inhibiting its ability to bind to DNA.75 Bcl2 value till the date is a matter of debate, and studies revealed the modulating role of melatonin in regulating autophagy and apoptosis through different mechanisms like downregulation of Bcl2 expression.76 On the other hand, zinc is involved in proliferation and apoptosis and consistent with the finding of the current work, zinc alone increased the expression of Bcl2.77 However, the combination groups lowered the expression of Bcl2. This modulatory effect could be the reason behind decreasing the neuropathic pain and may explain the neuroprotection achieved by this combination.
Conclusion
The combination of zinc with melatonin for both approaches was effective in improving the behavioral tests, and the prevention approach revealed a maximum effect in the histopathological findings than the use of each alone or the treatment approach. The proposed mechanism of decreasing neurotoxicity and neuropathic pain is via attenuating GFAP, TNF-α and boosting the antioxidant system. Additionally, our finding revealed a novel mechanism of protection against neuropathic pain provided by the aforementioned combination through ameliorating the expression of p53.
Acknowledgments
The authors appreciate the College of Pharmacy, University of Sulaimani for its support and for providing facilities to this project.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Aziz MT, Good BL, Lowe DK. Serotonin-norepinephrine reuptake inhibitors for the management of chemotherapy-induced peripheral neuropathy. Ann Pharmacother. 2014;48(5):626–632. doi:10.1177/1060028014525033
2. Piccolo J, Kolesar JM. Prevention and treatment of chemotherapy-induced peripheral neuropathy. Am J Health Syst Pharm. 2014;71(1):19–25. doi:10.2146/AJHP130126
3. Brown TJ, Sedhom R, Gupta A. Chemotherapy-Induced Peripheral Neuropathy. JAMA Oncol. 2019;5(5):750. doi:10.1001/JAMAONCOL.2018.6771
4. Velasco R, Bruna J. Oxaliplatin Neurotoxicity. Curr Colorectal Cancer Rep. 2014;10(3):303–312. doi:10.1007/S11888-014-0230-9
5. Yamaguchi K, Kusaba H, Makiyama A, et al. The risk factors for oxaliplatin-induced peripheral sensory neuropathy and thrombocytopenia in advanced gastric cancer. Cancer Chemother Pharmacol. 2018;82(4):625–633. doi:10.1007/S00280-018-3652-2
6. Beijers AJM, Mols F, Tjan-Heijnen VCG, Faber CG, Van De Poll-Franse LV, Vreugdenhil G. Peripheral neuropathy in colorectal cancer survivors: the influence of oxaliplatin administration. Results from the population-based PROFILES registry. Acta Oncol. 2015;54(4):463–469. doi:10.3109/0284186X.2014.980912/SUPPL_FILE/IONC_A_980912_SM2268.PDF
7. Argyriou AA, Kalofonou F, Litsardopoulos P, Anastopoulou GG, Kalofonos HP. Oxaliplatin rechallenge in metastatic colorectal cancer patients with clinically significant oxaliplatin-induced peripheral neurotoxicity. J Peripher Nerv Syst. 2021;26(1):43–48. doi:10.1111/JNS.12426
8. Selvy M, Pereira B, Kerckhove N, et al. Long-term prevalence of sensory chemotherapy-induced peripheral neuropathy for 5 years after adjuvant FOLFOX chemotherapy to treat colorectal cancer: a multicenter cross-sectional study. J Clin Med. 2020;9(8):2400. doi:10.3390/JCM9082400
9. Han CH, Kilfoyle DH, Hill AG, Jameson MB, McKeage MJ. Preventing oxaliplatin-induced neurotoxicity: rationale and design of phase Ib randomized, double-blind, placebo-controlled, cross-over trials for early clinical evaluation of investigational therapeutics. Expert Opin Drug Metab Toxicol. 2016;12(12):1479–1490. doi:10.1080/17425255.2016.1223625
10. Ali MM, Aziz TA. Toxic effect of platinum compounds: molecular mechanisms of toxicity. Al-Rafidain J Med Sci. 2021;1:81–88. doi:10.54133/AJMS.V1I.32
11. Yan F, Liu JJ, Ip V, Jamieson SMF, McKeage MJ. Role of platinum DNA damage-induced transcriptional inhibition in chemotherapy-induced neuronal atrophy and peripheral neurotoxicity. J Neurochem. 2015;135(6):1099–1112. doi:10.1111/JNC.13355
12. Kelley MR, Jiang Y, Guo C, Reed A, Meng H, Vasko MR. Role of the DNA base excision repair protein, APE1 in cisplatin, oxaliplatin, or carboplatin induced sensory neuropathy. PLoS One. 2014;9(9):e106485. doi:10.1371/JOURNAL.PONE.0106485
13. McQuade RM, Carbone SE, Stojanovska V, et al. Role of oxidative stress in oxaliplatin-induced enteric neuropathy and colonic dysmotility in mice. Br J Pharmacol. 2016;173(24):3502–3521. doi:10.1111/BPH.13646
14. Morucci G, Branca JJV, Gulisano M, et al. Gc-protein-derived macrophage activating factor counteracts the neuronal damage induced by oxaliplatin. Anticancer Drugs. 2015;26(2):197–209. doi:10.1097/CAD.0000000000000177
15. Ji RR, Chamessian A, Zhang YQ. Pain regulation by non-neuronal cells and inflammation. Science. 2016;354(6312):572. doi:10.1126/SCIENCE.AAF8924
16. Di Cesare Mannelli L, Zanardelli M, Landini I, et al. Effect of the SOD mimetic MnL4 on in vitro and in vivo oxaliplatin toxicity: possible aid in chemotherapy induced neuropathy. Free Radic Biol Med. 2016;93:67–76. doi:10.1016/J.FREERADBIOMED.2016.01.023
17. Egashira N, Hirakawa S, Kawashiri T, Yano T, Ikesue H, Oishi R. Mexiletine reverses oxaliplatin-induced neuropathic pain in rats. J Pharmacol Sci. 2010;112(4):473–476. doi:10.1254/JPHS.10012SC
18. Sittl R, Carr RW, Fleckenstein J, Grafe P. Enhancement of axonal potassium conductance reduces nerve hyperexcitability in an in vitro model of oxaliplatin-induced acute neuropathy. Neurotoxicology. 2010;31(6):694–700. doi:10.1016/J.NEURO.2010.07.006
19. Di Cesare Mannelli L, Pacini A, Micheli L, Tani A, Zanardelli M, Ghelardini C. Glial role in oxaliplatin-induced neuropathic pain. Exp Neurol. 2014;261:22–33. doi:10.1016/J.EXPNEUROL.2014.06.016
20. Hu S, Huang KM, Adams EJ, Loprinzi CL, Lustberg MB. Recent developments of novel pharmacologic therapeutics for prevention of chemotherapy-induced peripheral neuropathy. Clin Cancer Res. 2019;25(21):6295–6301. doi:10.1158/1078-0432.CCR-18-2152
21. Celik H, Kucukler S, Ozdemir S, et al. Lycopene protects against central and peripheral neuropathy by inhibiting oxaliplatin-induced ATF-6 pathway, apoptosis, inflammation and oxidative stress in brains and sciatic tissues of rats. Neurotoxicology. 2020;80:29–40. doi:10.1016/J.NEURO.2020.06.005
22. Kawashiri T, Kobayashi D, Egashira N, Tsuchiya T, Shimazoe T. Oral administration of Cystine and Theanine ameliorates oxaliplatin-induced chronic peripheral neuropathy in rodents. Sci Rep. 2020;10(1):1–8. doi:10.1038/s41598-020-69674-9
23. Schwingel TE, Klein CP, Nicoletti NF, et al. Effects of the compounds resveratrol, rutin, quercetin, and quercetin nanoemulsion on oxaliplatin-induced hepatotoxicity and neurotoxicity in mice. Naunyn Schmiedebergs Arch Pharmacol. 2014;387(9):837–848. doi:10.1007/S00210-014-0994-0
24. Ikram M, Park HY, Ali T, Kim MO. Melatonin as a potential regulator of oxidative stress, and neuroinflammation: mechanisms and implications for the management of brain injury-induced neurodegeneration. J Inf Res. 2021;Volume 14:6251–6264. doi:10.2147/JIR.S334423
25. Acuña-Castroviejo D, Escames G, Venegas C, et al. Extrapineal melatonin: sources, regulation, and potential functions. Cell Mol Life Sci. 2014;71(16):2997–3025. doi:10.1007/S00018-014-1579-2
26. Ferlazzo N, Andolina G, Cannata A, et al. Is melatonin the cornucopia of the 21st century? Antioxidants. 2020;9(11):1–29. doi:10.3390/ANTIOX9111088
27. Kumar A, Singh A. Possible involvement of GABAergic mechanism in protective effect of melatonin against sleep deprivation-induced behaviour modification and oxidative damage in mice. Fundam Clin Pharmacol. 2009;23(4):439–448. doi:10.1111/J.1472-8206.2009.00737.X
28. Bediz CS, Baltaci AK, Mogulkoc R. Both zinc deficiency and supplementation affect plasma melatonin levels in rats. Acta Physiol Hung. 2003;90(4):335–339. doi:10.1556/APHYSIOL.90.2003.4.7
29. Belviranli M, Baltaci AK. The relation between reduced serum melatonin levels and zinc in rats with induced hypothyroidism. Cell Biochem Funct. 2008;26(1):19–23. doi:10.1002/CBF.1384
30. Semercioz A, Baltaci AK, Mogulkoc R, Avunduk MC. Effect of zinc and melatonin on oxidative stress and serum inhibin-B levels in a rat testicular torsion-detorsion model. Biochem Genet. 2017;55(5–6):395–409. doi:10.1007/S10528-017-9826-5
31. Baltaci AK, Bediz CS, Mogulkoc R, Kurtoglu E, Pekel A. Effect of zinc and melatonin supplementation on cellular immunity in rats with toxoplasmosis. Biol Trace Elem Res. 2003;96(1–3):237–245. doi:10.1385/BTER:96:1-3:237
32. Mok JX, Ooi JH, Ng KY, Koh RY, Chye SM. A new prospective on the role of melatonin in diabetes and its complications. Horm Mol Biol Clin Investig. 2019;40(1). doi:10.1515/HMBCI-2019-0036
33. Pourhanifeh MH, Hosseinzadeh A, Dehdashtian E, Hemati K, Mehrzadi S. Melatonin: new insights on its therapeutic properties in diabetic complications. Diabetol Metab Syndr. 2020;12:30. doi:10.1186/S13098-020-00537-Z
34. Maryoosh TM, Al-Shawi NN, Salih ES. Effects of two different doses of zinc sulfate on serum troponin I 3 enzyme level and cardiac malondialdehyde contents in mitoxantrone-induced cardiotoxicity in rats. Iraqi J Pharm Sci. 2020;29(1):115–122. doi:10.31351/VOL29ISS1PP115-122
35. Zangiabadi N. Effects of melatonin in prevention of neuropathy in STZ-induced diabetic rats. Am J Pharmacol Toxicol. 2011;6(2):59–67. doi:10.3844/ajptsp.2011.59.67
36. Olaide Adeyemi O, Adewale Adeneye A, Elizabeth Alabi T. Analgesic activity of the aqueous seed extract of Hunteria umbellata (K. Schum.) Hallier f. in rodents. Indian J Exp Biol. 2011;49:698–703.
37. Kanaan SA, Saadé NE, Haddad JJ, et al. Endotoxin-induced local inflammation and hyperalgesia in rats and mice: a new model for inflammatory pain. Pain. 1996;66(2–3):373–379. doi:10.1016/0304-3959(96)03068-0
38. Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17(5):1474–1481. doi:10.1200/JCO.1999.17.5.1474
39. Baltaci SB, Mogulkoc R, Baltaci AK, Emsen A, Artac H. The effect of zinc and melatonin supplementation on immunity parameters in breast cancer induced by DMBA in rats. Arch Physiol Biochem. 2018;124(3):247–252. doi:10.1080/13813455.2017.1392580
40. Castro-Marrero J, Zaragozá MC, López-Vílchez I, et al. Effect of melatonin plus zinc supplementation on fatigue perception in myalgic encephalomyelitis/chronic fatigue syndrome: a randomized, double-blind, placebo-controlled trial. Antioxidants. 2021;10(7):1010. doi:10.3390/ANTIOX10071010
41. Hacışevki A, Baba B. An overview of melatonin as an antioxidant molecule: a biochemical approach. Melatonin. 2018. doi:10.5772/INTECHOPEN.79421
42. Mostafavi SA, Mohammadi MR, Hosseinzadeh P, et al. Dietary intake, growth and development of children with ADHD in a randomized clinical trial of Ritalin and Melatonin co-administration: through circadian cycle modification or appetite enhancement? Iran J Psychiatry. 2012;7(3):114.
43. Chen YH, Xu DX, Wang JP, et al. Melatonin protects against lipopolysaccharide-induced intra-uterine fetal death and growth retardation in mice. J Pineal Res. 2006;40(1):40–47. doi:10.1111/J.1600-079X.2005.00274.X
44. Park SG, Choi HN, Yang HR, Yim JE. Effects of zinc supplementation on catch-up growth in children with failure to thrive. Nutr Res Pract. 2017;11(6):487. doi:10.4162/NRP.2017.11.6.487
45. Rerksuppaphol S, Rerksuppaphol L. Zinc supplementation enhances linear growth in school-aged children: a randomized controlled trial. Pediatr Rep. 2017;9(4):7294. doi:10.4081/PR.2017.7294
46. Obrosova IG. Diabetic painful and insensate neuropathy: pathogenesis and potential treatments. Neurotherapeutics. 2009;6(4):638–647. doi:10.1016/J.NURT.2009.07.004
47. Thiagarajan VRK, Shanmugam P, Krishnan UM, Muthuraman A. Ameliorative effect of Vernonia cinerea in vincristine-induced painful neuropathy in rats. Toxicol Ind Health. 2014;30(9):794–805. doi:10.1177/0748233712463779
48. Srinivasan V, Pandi-Perumal SR, Spence DW, et al. Potential use of melatonergic drugs in analgesia: mechanisms of action. Brain Res Bull. 2010;81(4–5):362–371. doi:10.1016/J.BRAINRESBULL.2009.12.001
49. Galley HF, McCormick B, Wilson KL, Lowes DA, Colvin L, Torsney C. Melatonin limits paclitaxel-induced mitochondrial dysfunction in vitro and protects against paclitaxel-induced neuropathic pain in the rat. J Pineal Res. 2017;63(4):e12444. doi:10.1111/JPI.12444
50. Areti A, Komirishetty P, Akuthota M, Malik RA, Kumar A. Melatonin prevents mitochondrial dysfunction and promotes neuroprotection by inducing autophagy during oxaliplatin-evoked peripheral neuropathy. J Pineal Res. 2017;62(3):e12393. doi:10.1111/JPI.12393
51. Choi S, Hong DK, Choi BY, Suh SW. Zinc in the brain: friend or foe? Int J Mol Sci. 2020;21(23):1–24. doi:10.3390/ijms21238941
52. Xu S, Sleat DE, Jadot M, Lobel P. Glial fibrillary acidic protein is elevated in the lysosomal storage disease classical late-infantile neuronal ceroid lipofuscinosis, but is not a component of the storage material. Biochem J. 2010;428(3):355–362. doi:10.1042/BJ20100128
53. Kuzu M, Kandemir FM, Yildirim S, Kucukler S, Caglayan C, Turk E. Morin attenuates doxorubicin-induced heart and brain damage by reducing oxidative stress, inflammation and apoptosis. Biomed Pharmacother. 2018;106:443–453. doi:10.1016/J.BIOPHA.2018.06.161
54. Çelik H, Kandemir FM, Caglayan C, et al. Neuroprotective effect of rutin against colistin-induced oxidative stress, inflammation and apoptosis in rat brain associated with the CREB/BDNF expressions. Mol Biol Rep. 2020;47(3):2023–2034. doi:10.1007/S11033-020-05302-Z
55. Dec K, Łukomska A, Maciejewska D, et al. The influence of fluorine on the disturbances of homeostasis in the central nervous system. Biol Trace Elem Res. 2017;177(2):224–234. doi:10.1007/S12011-016-0871-4
56. Šulinskiene J, Bernotiene R, Baranauskiene D, et al. Effect of zinc on the oxidative stress biomarkers in the brain of nickel-treated mice. Oxid Med Cell Longev. 2019;2019:8549727. doi:10.1155/2019/8549727
57. Lee JG, Woo YS, Park SW, Seog DH, Seo MK, Bahk WM. The neuroprotective effects of melatonin: possible role in the pathophysiology of neuropsychiatric disease. Brain Sci. 2019;9(10):285. doi:10.3390/BRAINSCI9100285
58. Kandemir FM, Caglayan C, Aksu EH, et al. Protective effect of rutin on mercuric chloride-induced reproductive damage in male rats. Andrologia. 2020;52(3):e13524. doi:10.1111/AND.13524
59. Lo U, Selvaraj V, Plane JM, Chechneva OV, Otsu K, Deng W. p38α (MAPK14) critically regulates the immunological response and the production of specific cytokines and chemokines in astrocytes. Sci Rep. 2014;4:7405. doi:10.1038/SREP07405
60. Kandemir FM, Yildirim S, Caglayan C, Kucukler S, Eser G. Protective effects of zingerone on cisplatin-induced nephrotoxicity in female rats. Environ Sci Pollut Res. 2019;26(22):22562–22574. doi:10.1007/S11356-019-05505-3
61. Kandemir FM, Kucukler S, Caglayan C, Gur C, Batil AA, Gülçin İ. Therapeutic effects of silymarin and naringin on methotrexate-induced nephrotoxicity in rats: biochemical evaluation of anti-inflammatory, antiapoptotic, and antiautophagic properties. J Food Biochem. 2017;41(5):e12398. doi:10.1111/JFBC.12398
62. Tarocco A, Caroccia N, Morciano G, et al. Melatonin as a master regulator of cell death and inflammation: molecular mechanisms and clinical implications for newborn care. Cell Death Dis. 2019;10(4):1–12. doi:10.1038/s41419-019-1556-7
63. Yu GM, Kubota H, Okita M, Maeda T, Scavone C. The anti-inflammatory and antioxidant effects of melatonin on LPS-stimulated bovine mammary epithelial cells. PLoS One. 2017;12(5):e0178525. doi:10.1371/JOURNAL.PONE.0178525
64. Zheng R, Ruan Y, Yan Y, et al. Melatonin attenuates neuroinflammation by down-regulating NLRP3 inflammasome via a SIRT1-dependent pathway in MPTP-induced models of parkinson’s disease. J Inf Res. 2021;Volume 14:3063–3075. doi:10.2147/JIR.S317672
65. Jarosz M, Olbert M, Wyszogrodzka G, Młyniec K, Librowski T. Antioxidant and anti-inflammatory effects of zinc. Zinc-dependent NF-κB signaling. Inflammopharmacology. 2017;25(1):11–24. doi:10.1007/S10787-017-0309-4
66. Winiarska K, Fraczyk T, Malinska D, Drozak J, Bryla J. Melatonin attenuates diabetes-induced oxidative stress in rabbits. J Pineal Res. 2006;40(2):168–176. doi:10.1111/J.1600-079X.2005.00295.X
67. Hadj Abdallah N, Baulies A, Bouhlel A, et al. The effect of zinc acexamate on oxidative stress, inflammation and mitochondria induced apoptosis in rat model of renal warm ischemia. Biomed Pharmacother. 2018;105:573–581. doi:10.1016/J.BIOPHA.2018.06.017
68. Esposito E, Cuzzocrea S. Antiinflammatory activity of melatonin in central nervous system. Curr Neuropharmacol. 2010;8(3):228. doi:10.2174/157015910792246155
69. Torabi F, Shafaroudi MM, Rezaei N. Combined protective effect of zinc oxide nanoparticles and melatonin on cyclophosphamide-induced toxicity in testicular histology and sperm parameters in adult Wistar rats. Int J Reprod Biomed. 2017;15(7):403–412. doi:10.29252/IJRM.15.7.403
70. Turk E, Kandemir FM, Yildirim S, Caglayan C, Kucukler S, Kuzu M. Protective effect of hesperidin on sodium arsenite-induced nephrotoxicity and hepatotoxicity in rats. Biol Trace Elem Res. 2018;189(1):95–108. doi:10.1007/S12011-018-1443-6
71. Gao Y, Sun N, Wang L, et al. Bioinformatics analysis identifies p53 as a candidate prognostic biomarker for neuropathic pain. Front Genet. 2018;9. doi:10.3389/FGENE.2018.00320
72. Leng YF, Gao XM, Wang SX, Xing YH. Effects of tetramethylpyrazine on neuronal apoptosis in the superficial dorsal horn in a rat model of neuropathic pain. Am J Chin Med. 2012;40(6):1229–1239. doi:10.1142/S0192415X12500917
73. Li Z, Zhao J, Liu H, Wang J, Lu W. Melatonin inhibits apoptosis in mouse Leydig cells via the retinoic acid-related orphan nuclear receptor α/p53 pathway. Life Sci. 2020;246:117431. doi:10.1016/J.LFS.2020.117431
74. Wu H, Shao A, Zhao M, et al. Melatonin attenuates neuronal apoptosis through up-regulation of K+–Cl− cotransporter KCC2 expression following traumatic brain injury in rats. J Pineal Res. 2016;61(2):241–250. doi:10.1111/JPI.12344
75. Formigari A, Gregianin E, Irato P. The effect of zinc and the role of p53 in copper-induced cellular stress responses. J Appl Toxicol. 2013;33(7):527–536. doi:10.1002/JAT.2854
76. Mehrzadi S, Hossein Pourhanifeh M, Mirzaei A, Moradian F, Hosseinzadeh A. An updated review of mechanistic potentials of melatonin against cancer: pivotal roles in angiogenesis, apoptosis, autophagy, endoplasmic reticulum stress and oxidative stress. Cancer Cell Int. 2021;21:188. doi:10.1186/s12935-021-01892-1
77. Nesari F, Gholami M, Rezaian J, et al. Effects of zinc on expression of apoptosis-related genes in freezing thawing damage of adipose tissue derived mesenchymal stromal cells. Prep Biochem Biotechnol. 2022;52(6):640–647. doi:10.1080/10826068.2021.1983830
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.