Back to Journals » Cancer Management and Research » Volume 13
The Combination of Neoadjuvant Therapy and Surgical Resection: A Safe and Effective Treatment for Rectal Gastrointestinal Stromal Tumors
Authors Liu Y, Chang W, Tang W, Wei Y , Liu T, Chen Y, Ji M, Liang F, Ren L, Xu J
Received 8 March 2021
Accepted for publication 7 May 2021
Published 14 June 2021 Volume 2021:13 Pages 4671—4678
DOI https://doi.org/10.2147/CMAR.S307426
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bilikere Dwarakanath
Yu Liu,1,2,* Wenju Chang,1– 3,* Wentao Tang,1– 3,* Ye Wei,1– 3 Tianyu Liu,1,2 Yijiao Chen,1,2 Meiling Ji,1– 3 Fei Liang,4 Li Ren,1– 3 Jianmin Xu1– 3
1Colorectal Cancer Center, Zhongshan Hospital, Fudan University, Shanghai, People’s Republic of China; 2Department of General Surgery, Zhongshan Hospital, Fudan University, Shanghai, People’s Republic of China; 3Shanghai Engineering Research Center of Colorectal Cancer Minimally Invasive, Shanghai, People’s Republic of China; 4Department of Biostatistics, Zhongshan Hospital, Fudan University, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jianmin Xu
Department of General Surgery, Zhongshan Hospital, Fudan University, Shanghai, People’s Republic of China
Tel +86 13501984869
Email [email protected]
Background: Rectal gastrointestinal stromal tumors (GISTs) are rare digestive tumors. The treatment methods of rectal GISTs are diverse, while no standardized treatment was recommended. The aim of this study was to report the treatment mode of rectal GISTs in our center.
Methods: Patients with pathologically confirmed rectal GISTs who received neoadjuvant therapy and surgical resection were retrospectively collected. Perioperative complications and long-term prognosis were evaluated.
Results: From January 2010 to December 2019, 36 patients were pathologically diagnosed with primary rectal GISTs. After excluding patients who were treated with surgery or imatinib alone, 21 patients received neoadjuvant therapy and surgery was enrolled. During neoadjuvant treatment, tumors shrank significantly (6.53cm to 4.68cm, p< 0.001) without toxicities over grade 2. The total postoperative complication rate was 42.9% (all grade). R0 resection was achieved in 76.2% patients. Transanal resection had advantages on anus preservation and postoperative recovery. No patients died during the follow-up period, 4 patients relapsed and the relapse-free survival was 81.0%.
Conclusion: The combination of neoadjuvant therapy and surgical resection was a safe and effective treatment for rectal gastrointestinal stromal tumors.
Keywords: rectal gastrointestinal stromal tumors, GISTs, neoadjuvant therapy, surgical resection
Introduction
Gastrointestinal stromal tumors (GISTs) occur anywhere in the digestive tract. Rectal GISTs are rare, and the proportion is less than 5% of all GISTs.1,2 Compared with GISTs occurring elsewhere, rectal GISTs are more malignant and associated with poor prognosis.3 Due to the low incidence, there were limited randomized trial and large cohorts providing evidence for the treatment of rectal GISTs. Current National Comprehensive Cancer Network (NCCN)4 and European Society of Medical Oncology (ESMO)5 guidelines recommended that all rectal GISTs should be treated with surgery, but no clear recommendations were made regarding the neoadjuvant treatment and surgical approach.
GISTs rarely have lymph node metastasis, thus lymph node dissection is usually unnecessary. Transanal resection which is minimally invasive and has less complications was reported to be feasible in treating rectal GISTs.6 As for cases with large lesions, low anterior resection (LAR) or abdomino-perineal resection (APR) is still required considering limited pelvic space.
Since imatinib was approved for the treatment of GISTs, it has been reported that neoadjuvant imatinib can downsize tumors, improve patient OS and RFS and increase anus preservation rate.7–9 Moreover, Neoadjuvant imatinib enables patients who cannot be resected locally or even cannot be resected to achieve R0 resection by local resection.10,11 However, few studies focused on the combination of neoadjuvant imatinib and surgical resection.
In the present study, we reported a 10-year single-center cohort that received neoadjuvant imatinib and surgical resection and evaluated the safety and efficacy of this treatment strategy. We present the following article in accordance with the STROBE reporting checklist.
Patients and Methods
We retrospectively collected 50 patients with pathologically confirmed colorectal GISTs from 2010 to 2019 at Zhongshan hospital. We excluded 14 patients for the following reasons: secondary GISTs from other sites (n=11), colon GISTs (n=3). The rest of patients with localized rectal GISTs were classified into three groups according to the treatment method: neoadjuvant imatinib and surgery (n=21); surgery alone (n=7), imatinib alone (n=8). The study was approved by the ethics committee of Fudan University Zhongshan Hospital and was performed according to Good Clinical Practice guidelines and the Declaration of Helsinki. Informed consent was signed by all patients before study commencement.
The diagnosis of patients was based on pathology review. Biopsy specimens were acquired before treatment and were evaluated by experienced gastrointestinal pathologists. Tumor size was measured by pre-treatment imaging examination. Risk classification was evaluated according to the modified National Institutes of Health (NIH) consensus criteria.12,13 Since both the mitotic counts based on preoperative biopsies and the mitotic counts after neoadjuvant therapy could not be used for risk stratification. Tumor size >5 cm was classified as high risk and tumor size ≤5 cm was reported as “unspecified”. Mutations in c-kit exons 9, 11, 13 and 17 as well as PDGFRα exons 12, 14 and 18 were detected by polymerase chain reaction (PCR) and sequencing methods.
The response rate was assessed according to the Response Evaluation Criteria In Solid Tumors (RECIST) Criteria. Postoperative complications were classified according to Clavien-Dindo classification. Toxicity associated with neoadjuvant therapy was assessed using the National Cancer Institute’s Common Terminology Criteria for Adverse Events version 3.0.
All patients were followed up either at our institution or in partnership with their referring institutions. Patients who underwent neoadjuvant therapy and surgical resection were recommended to receive adjuvant treatment for at least 3 years. Abdominal-pelvic computed tomography was carried out every three months in first 3 years, then every six months until five years and annually for an additional 5 years. The follow-up data were based on the outpatient record, telephone calls were made when patients have no updated outpatient follow-up records for more than 6 months. Relapse-free survival (RFS) and overall survival (OS) were calculated from the day of surgery.
Clinicopathological and treatment data, including age, sex, tumor characteristics, R0 resection rates, perioperative complications, recurrence and survival were collected and analyzed. Statistical analysis was performed using a chi-squared test or Fisher’s exact test as appropriate. Survival curves were estimated by Kaplan–Meier method and compared using the Log rank test. Commercial software (IBM SPSS Statistics 21; SPSS, Chicago, IL, US) was used for statistical analysis (P < 0.05 was considered statistically significant).
Results
Patients and Tumors Characteristics
During the ten-year period, 36 patients were diagnosed with primary rectal GISTs. (Figure 1) 21 of them underwent neoadjuvant imatinib and surgery; 8 of them underwent surgery alone, and the rest 8 patients underwent imatinib alone. Among 21 patients receiving neoadjuvant therapy, 15 (71.4%) underwent transanal resection. The baseline characteristics are shown in Table 1. Fifteen (71.4%) patients had tumors larger than 5cm and they were classified as high risk. c-kit mutation was detected in 80.0% patients, while no PDGFRA mutation was detected. Among 12 patients with c-kit mutation, most had exon 11 mutation (10/12). The rest 2 patients, 1 had exon 13 mutation, another had both exon 11 and 13 mutations. The incidence of risk factors, including tumor size and mitotic count, were not significantly different between patients who underwent transanal and non-transanal surgery.
 |
Table 1 Baseline Characteristics of Patients Undergoing Neoadjuvant Therapy |
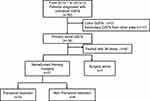 |
Figure 1 Diagram of the study. Abbreviations: GISTs, gastrointestinal stromal tumors; IM, imatinib. |
Neoadjuvant Therapy
Twenty-one patients underwent neoadjuvant therapy. The mean duration of neoadjuvant treatment was 10.6 months. According to RESIST Criteria, PR was observed in 13 patients (61.9%), SD in 7 patients (33.3%) and PD in 1 patient (4.8%). The overall response rate (ORR) was 61.9% and the disease control rate (DCR) was 95.2%. During neoadjuvant treatment, the mean tumor size was decreased from 6.53cm±2.45cm to 4.68cm±1.53cm (p<0.001). (Figure 2) Adverse events occurred to 4 patients (19.0%) during neoadjuvant therapy. 75% were grade I–II according to CTCAE 3.0. Eyelid edema was the most common adverse event. Table 2. One patient withdrew imatinib and switched to sunitinib due to allergy.
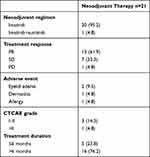 |
Table 2 Efficacy and Safety of Neoadjuvant Therapy |
 |
Figure 2 The maximum diameter of rectal GISTs before and after neoadjuvant therapy. Abbreviations: GISTs, gastrointestinal stromal tumors; IM, imatinib. |
Surgical Treatment
Fifteen patients underwent transanal resection. Among patients underwent non-transanal resection, 4 underwent low anterior resection and 2 underwent abdomino-perineal resection. Compared with non-transanal resection, transanal resection was associated with more anus preservation and less prevention stoma. Transanal resection achieved R0 resection in 66.7% patients. No tumor rupture occurred in any group. Seventeen of 21 patients (81.0%) had the mitotic counts ≤5/HPF and 16 of 21 patients (76.2%) patients had tumor viability ≤50%. Postoperative complications occurred to 8 patients (38.1%). Table 3. Complications more than Clavien-Dindo grade 3 were not observed. The fasting time (2.9d vs 4.7d), indwelling catheterization time (3.0d vs 7.8d) and hospital stay time (6.2d vs 9.3d) in the transanal resection group were shorter than that of non-transanal group. After surgical resection, 10 of 21 (47.6%) patients received the adjuvant treatment as recommended.
 |
Table 3 Surgical and Postoperative Outcomes |
Long-Term Outcomes
During the median follow-up of 37 months, 4 patients (19.0%) relapsed and no patient died. The RFS rate was 81%. (Figure 3) The characteristics of these 4 patients are shown in Table 4. Three patients had local recurrence and 1 patient had liver and abdomen metastasis. Among them, 1 patient did not receive adjuvant IM because of patient choice, 2 patients stopped IM therapy before relapsed and 1 patient persisted IM therapy and converted to sunitinib after relapsed. The median adjuvant therapy duration was 17 months and the median interval between drug withdrawal and recurrence was 21 months.
 |
Table 4 Clinical Characteristics of Patients Who Relapsed |
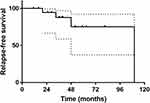 |
Figure 3 The recurrence free survival of all patients. |
Discussion
Neoadjuvant imatinib in rectal GISTs was firstly reported in 2005.14 However, due to the low incidence of rectal GISTs, the evidence of neoadjuvant therapy was derived from case reports15,16 and the subgroup analyses of cohort studies10,17,18 for this specific site. Few studies focused on the efficacy and safety of the combination of neoadjuvant therapy and surgical resection. Due to absence of high-level evidence, current National Comprehensive Cancer Network (NCCN)4 and European Society of Medical Oncology (ESMO)5 guidelines still not had clear recommendation on the indication of neoadjuvant imatinib for rectal GISTs. The current study reported the ten-year experience of the neoadjuvant therapy in our center and proved the efficacy and safety of the combination of neoadjuvant therapy and surgical resection.
The efficacy of imatinib treatment has been verified. The DCR in the current study was 95.2%, which was consistent with previous studies.17–19 Due to the heterogeneity of baseline characteristics, it has been reported that the response rate of imatinib treatment was from 60% to 100%.7,17,20–22 Neoadjuvant therapy enabled transanal resection in 8 patients who had tumors larger than 5cm and 2 patients who had tumors larger than 10cm. They might have undergone LAR or APR without neoadjuvant therapy. Seven (70%) of them were R0 resected by local resection. Besides, in 13 patients, the tumor was less than 5cm from the anal margin. They might have received LAR or even APR, but after neoadjuvant therapy, their tumors shrank and were resected transanally.
It has been reported that the most common adverse events of imatinib were fluid retention, nausea, fatigue, skin rash.23 Eyelid edema was the most common adverse event in our cohort. Allergy occurred in 1 patient and imatinib was then replaced by sunitinib. To our knowledge, this was the first study that reported the adverse events of neoadjuvant therapy in rectal GISTs. The results of this study proved that the neoadjuvant treatment of rectal GISTs was safe. The optimal duration of neoadjuvant therapy remains controversial.7,17 The treatment duration in our cohort was 10.6±7.8 months. The decision of surgical resection was made by our multidisciplinary team. In the experience of our center, the optimal response may require at least 6 months.
It has been reported that neoadjuvant therapy did not increase the complications of surgery in all-site GISTs.24 In rectal GISTs, the postoperative complications were 17.2%-33%.25,26 The complication rate (38.1%) of current study was higher than the previous studies. However, no complications more than Clavien-Dindo grade 3 was observed. This indicated that the combination of neoadjuvant therapy and resection was safe. The complication rate was not significantly different between transanal and non-transanal resection. However, consistent with previous study,6 transanal resection had advantages on anus preservation and postoperative recovery. In the current study, local excision of GISTs were all achieved by transanal approach and segmental resection were achieved transabdominally. As for operation methods, Lee et al reported the robotic transabdominal local excision27 and the combination of transabdominal resection and transanal excision in treating rectal GISTs has been reported more recently.28 These new surgical approaches might be able to reduce intra-operative injury (robotic resection) or resect large tumors that are difficult to be resected transanally (the combination of transabdominal and transanal resection). However, these studies had limited sample size or was even a case report. The efficacy and safety of transanal resection and these new approaches need to be compared in further study.
For long-term outcomes, previous studies that covered patients undergoing neoadjuvant therapy reported the RFS from 77.8% to 100.0%.17–19 Our cohort that had a long follow-up period of 37 months has 100.0% OS and 81.0% RFS. Yang et al6 reported that DFS and OS were not significantly different between the transanal and non-transanal group. But the neoadjuvant therapy and the risk grade were not balanced between these two groups in their research. The current studies had balanced baseline characteristics and got same results. Neoadjuvant therapy enabled transanal R0 resection for patients with large lesions. But it is worth noting that patients who had local recurrence were all in the transanal group. Whether transanal resection can achieve the same long-term outcomes as non-transanal resection needs to be confirmed by large sample studies. Recently, several studies that including all GISTs reported that R1 resection did not negatively influence prognosis when excluded tumor rupture.29–31 In the current study focused on rectal GISTs, we came to a similar result that the recurrence rates of R0 and R1 resection were not significantly different (18.8% vs 20.0%, p=1.000).
PDGFRA mutation was not observed in cases of the current study. It has been reported that the rate of PDGFRA mutation in rectal GISTs was from 0% to 9%,32–34 lower than that of gastric or intestinal GISTs (about 12%).35,36 PDGFRA mutation was considered to be one of the earliest events in GISTs. Whether the difference in PDGFRA mutation rate was due to different driven gene and signaling pathway in rectal GISTs or was simply an accidental phenomenon caused by the limited sample size need further studies.
This study has some limitations. First, this is a single center retrospective study. The results might be affected by selecting bias. A multi-center prospective study would be better to be conducted. Second, with the limitation of sample size, the statistic power was limited. It is necessary to validate the long-term outcomes with larger samples in the future.
In summary, neoadjuvant therapy was safe and could downsize the tumor. After neoadjuvant therapy, most tumors could be resected locally. The surgical resection after neoadjuvant therapy was safe and had a satisfactory rate of R0 resection. Compared to non-transanal resection, transanal resection had advantages on anus preservation and postoperative recovery. The combination of neoadjuvant therapy and surgical resection could achieve relatively good long-term outcomes.
Acknowledgements
Supported by The National Natural Science Foundation of China (81602035, 81472228); The Shanghai Municipal Health Commission: Shanghai Outstanding Youth Specialist Training Program (Q2017-059); Clinical Science and Technology Innovation Project of Shanghai (SHDC12016104); Shanghai Engineering Research Center of Colorectal Cancer Minimally Invasive (17DZ2252600); Youth Fund of Zhongshan Hospital (2019ZSQN28).
Disclosure
The authors report no conflicts of interest.
References
1. Tran T, Davila JA, El-Serag HB. The epidemiology of malignant gastrointestinal stromal tumors: an analysis of 1458 cases from 1992 to 2000. Am J Gastroenterol. 2005;100(1):162–168. doi:10.1111/j.1572-0241.2005.40709.x
2. Ulanja MB, Rishi M, Beutler BD, et al. Racial disparity in incidence and survival for Gastrointestinal Stromal Tumors (GISTs): an analysis of SEER database. J Racial Ethn Health Disparities. 2019;6(5):1035–1043. doi:10.1007/s40615-019-00605-9
3. Bischof DA, Kim Y, Dodson R, et al. Conditional disease-free survival after surgical resection of gastrointestinal stromal tumors: a multi-institutional analysis of 502 patients. JAMA Surg. 2015;150(4):299–306. doi:10.1001/jamasurg.2014.2881
4. von Mehren M, Randall RL, Benjamin RS, et al. Soft tissue sarcoma, version 2.2018, NCCN clinical practice guidelines in oncology. JNCCN. 2018;16(5):536–563. doi:10.6004/jnccn.2018.0025
5. Casali PG, Abecassis N, Aro HT, et al. Gastrointestinal stromal tumours: ESMO-EURACAN clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv267. doi:10.1093/annonc/mdy320
6. Yang Z, Guo W, Huang R, Hu M, Wang H, Wang H. Transanal versus nontransanal surgery for the treatment of primary rectal gastrointestinal stromal tumors: a 10-year experience in a high-volume center. Ann Transl Med. 2020;8(5):201. doi:10.21037/atm.2020.01.55
7. Wilkinson MJ, Fitzgerald JE, Strauss DC, et al. Surgical treatment of gastrointestinal stromal tumour of the rectum in the era of imatinib. Br J Surg. 2015;102(8):965–971. doi:10.1002/bjs.9818
8. Li YS, Li W, Zeng QS, Fu WH. Effect of the imatinib treatment regimen on the postoperative prognosis of patients with high-risk gastrointestinal stromal tumors. Onco Targets Ther. 2019;12:4713–4719. doi:10.2147/OTT.S198129
9. Tang S, Yin Y, Shen C, et al. Preoperative imatinib mesylate (IM) for huge gastrointestinal stromal tumors (GIST). World J Surg Oncol. 2017;15(1):79. doi:10.1186/s12957-017-1143-2
10. Cavnar MJ, Wang L, Balachandran VP, et al. Rectal Gastrointestinal Stromal Tumor (GIST) in the era of imatinib: organ preservation and improved oncologic outcome. Ann Surg Oncol. 2017;24(13):3972–3980. doi:10.1245/s10434-017-6087-9
11. Kramp KH, Omer MG, Schoffski P, d’Hoore A. Sphincter sparing resection of a large obstructive distal rectal gastrointestinal stromal tumour after neoadjuvant therapy with imatinib (Glivec). BMJ Case Rep. 2015;2015:bcr2014207775–bcr2014207775. doi:10.1136/bcr-2014-207775
12. Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol. 2002;33(5):459–465. doi:10.1053/hupa.2002.123545
13. Rutkowski P, Bylina E, Wozniak A, et al. Validation of the Joensuu risk criteria for primary resectable gastrointestinal stromal tumour - the impact of tumour rupture on patient outcomes. Eur J Surg Oncol. 2011;37(10):890–896. doi:10.1016/j.ejso.2011.06.005
14. Lo SS, Papachristou GI, Finkelstein SD, Conroy WP, Schraut WH, Ramanathan RK. Neoadjuvant imatinib in gastrointestinal stromal tumor of the rectum: report of a case. Dis Colon Rectum. 2005;48(6):1316–1319. doi:10.1007/s10350-004-0922-3
15. Kenno S, Inagaki M, Funakoshi T, et al. [A case of rectal Gastrointestinal Stromal Tumor(GIST)treated with imatinib mesylate neoadjuvant therapy to preserve the anus]. Gan to Kagaku Ryoho. 2018;45(6):981–984. Japanese.
16. Ebihara Y, Okushiba S, Kawarada Y, Kitashiro S, Katoh H, Kondo S. Neoadjuvant imatinib in a gastrointestinal stromal tumor of the rectum: report of a case. Surg Today. 2008;38(2):174–177. doi:10.1007/s00595-007-3585-6
17. Jakob J, Mussi C, Ronellenfitsch U, et al. Gastrointestinal stromal tumor of the rectum: results of surgical and multimodality therapy in the era of imatinib. Ann Surg Oncol. 2013;20(2):586–592. doi:10.1245/s10434-012-2647-1
18. Tielen R, Verhoef C, van Coevorden F, et al. Surgical management of rectal gastrointestinal stromal tumors. J Surg Oncol. 2013;107(4):320–323. doi:10.1002/jso.23223
19. Machlenkin S, Pinsk I, Tulchinsky H, et al. The effect of neoadjuvant Imatinib therapy on outcome and survival after rectal gastrointestinal stromal tumour. Colorect Dis. 2011;13(10):1110–1115. doi:10.1111/j.1463-1318.2010.02442.x
20. Fujimoto Y, Akiyoshi T, Konishi T, Nagayama S, Fukunaga Y, Ueno M. Laparoscopic sphincter-preserving surgery (intersphincteric resection) after neoadjuvant imatinib treatment for gastrointestinal stromal tumor (GIST) of the rectum. Int J Colorectal Dis. 2014;29(1):111–116. doi:10.1007/s00384-013-1769-7
21. Huynh TK, Meeus P, Cassier P, et al. Primary localized rectal/pararectal gastrointestinal stromal tumors: results of surgical and multimodal therapy from the French Sarcoma group. BMC Cancer. 2014;14:156. doi:10.1186/1471-2407-14-156
22. Liu H, Yan Z, Liao G, Yin H. Treatment strategy of rectal gastrointestinal stromal tumor (GIST). J Surg Oncol. 2014;109(7):708–713. doi:10.1002/jso.23562
23. Cohen MH, Farrell A, Justice R, Pazdur R. Approval summary: imatinib mesylate in the treatment of metastatic and/or unresectable malignant gastrointestinal stromal tumors. Oncologist. 2009;14(2):174–180. doi:10.1634/theoncologist.2008-0255
24. Eisenberg BL, Harris J, Blanke CD, et al. Phase II trial of neoadjuvant/adjuvant imatinib mesylate (IM) for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumor (GIST): early results of RTOG 0132/ACRIN 6665. J Surg Oncol. 2009;99(1):42–47. doi:10.1002/jso.21160
25. Pai VD, Demenezes JL, Patil PS, Saklani AP. Multimodality therapy of rectal gastrointestinal stromal tumors in the era of imatinib-an Indian series. J Gastrointest Oncol. 2016;7(2):262–268. doi:10.3978/j.issn.2078-6891.2015.088
26. Guo W, Yang Z, Wei Y, et al. Radical excision versus local resection for primary rectal gastrointestinal stromal tumors. Cohort Study Int J Surg. 2020;77:190–197. doi:10.1016/j.ijsu.2020.03.068
27. Lee H, Gachabayov M, Dong X, Bergamaschi R. Robotic transabdominal excision of pelvic gastrointestinal stromal tumour - a video vignette. Colorect Dis. 2019;21(9):1099. doi:10.1111/codi.14681
28. Emoto S, Akiyoshi T, Fukata K, Nagasaki T, Fukunaga Y. Laparoscopic transabdominal dissection and transection of the distal rectum followed by local excision through the anus for rectal gastrointestinal stromal tumor. Dis Colon Rectum. 2020;63(11):e542. doi:10.1097/DCR.0000000000001814
29. Pantuso G, Macaione I, Taverna A, et al. Surgical treatment of primary gastrointestinal stromal tumors (GISTs): management and prognostic role of R1 resections. Am J Surg. 2020;220(2):359–364. doi:10.1016/j.amjsurg.2019.12.006
30. Hølmebakk T, Bjerkehagen B, Hompland I, Stoldt S, Boye K. Relationship between R1 resection, tumour rupture and recurrence in resected gastrointestinal stromal tumour. Br J Surg. 2019;106(4):419–426. doi:10.1002/bjs.11027
31. Gronchi A, Bonvalot S, Poveda Velasco A, et al. Quality of surgery and outcome in localized gastrointestinal stromal tumors treated within an international intergroup randomized clinical trial of adjuvant imatinib. JAMA Surg. 2020;155(6):e200397. doi:10.1001/jamasurg.2020.0397
32. Ohshima K, Fujiya K, Nagashima T, et al. Driver gene alterations and activated signaling pathways toward malignant progression of gastrointestinal stromal tumors. Cancer Sci. 2019;110(12):3821–3833. doi:10.1111/cas.14202
33. Zhou Z, Chen Z, Chen M, Wang R, Yin Y, Yao Y. Clinicopathologic factors predicting outcomes in patients with gastrointestinal stromal tumors of the rectum and colon. Tumour Biol. 2014;35(5):4357–4362. doi:10.1007/s13277-013-1572-7
34. Jiaxin L, Peiyun Z, Zheng T, et al. Comparison of prognostic prediction models for rectal gastrointestinal stromal tumor. Aging (Albany NY). 2020;12(12):11416–11430. doi:10.18632/aging.103204
35. Nishida T, Blay JY, Hirota S, Kitagawa Y, Kang YK. The standard diagnosis, treatment, and follow-up of gastrointestinal stromal tumors based on guidelines. Gastric Cancer. 2016;19(1):3–14. doi:10.1007/s10120-015-0526-8
36. Joensuu H, Wardelmann E, Sihto H, et al. Effect of KIT and PDGFRA mutations on survival in patients with gastrointestinal stromal tumors treated with adjuvant imatinib: an exploratory analysis of a randomized clinical trial. JAMA Oncol. 2017;3(5):602–609. doi:10.1001/jamaoncol.2016.5751
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
