Back to Journals » International Journal of General Medicine » Volume 17
The Combination of circEPSTI1 and MIF Offers Diagnostic Value for Endometrial Cancer
Authors Cui Z , Zhou L, An X, Liu W, Li J, Zhang Y, Zhang W
Received 17 October 2023
Accepted for publication 4 March 2024
Published 8 April 2024 Volume 2024:17 Pages 1395—1403
DOI https://doi.org/10.2147/IJGM.S441861
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Zhili Cui,1 Liyuan Zhou,1 Xin An,2 Wenli Liu,1 Jingxia Li,1 Yueping Zhang,3 Wei Zhang4
1Department of Gynecology, Affiliated Hospital of Hebei University of Engineering, Handan, Hebei, 056002, People’s Republic of China; 2Department of Pathology, Handan First Hospital, Handan, Hebei, 056000, People’s Republic of China; 3Shexian Maternal and Child Health Hospital, Shexian, Hebei, 056004, People’s Republic of China; 4Department of Gynecology, Handan Traditional Chinese Medicine Hospital, Handan, Hebei, 056001, People’s Republic of China
Correspondence: Zhili Cui, Department of Gynecology, Affiliated Hospital of Hebei University of Engineering, No. 81 Congtai Road, Congtai District, Handan, Hebei, 056002, People’s Republic of China, Tel + 86-3103962266, Email [email protected]
Background: Circular RNAs (circRNAs) exhibit unique patterns of expression and high levels of stability in patient plasma samples such that they represent ideal non-invasive biomarkers that can be leveraged to detect a wide array of diseases including endometrial cancer (EC). This study was designed to identify circRNAs with potential diagnostic utility in serum samples from EC patients while also evaluating the utility of macrophage migration inhibitory factor (MIF) as a biomarker when screening for this form of cancer in the clinic.
Methods: Levels of circEPSTI1 and MIF were assessed in the plasma of EC patients and healthy subjects (n=186 each) through qPCR and ELISAs. The diagnostic utility of these biomarkers was assessed with receiver operating characteristic curve (ROC) analyses.
Results: Relative to healthy subjects, EC patient serum contained significantly elevated circEPSTI1 and MIF. An association was noted between circEPSTI1 expression in stages, histologic grade, and residual tumor. ROC curves confirmed that serum circEPSTI1 levels distinguished between controls and patients with EC with an Area of 0.835 and serum MIF levels distinguished between controls and patients with EC with an Area of 0.6646. When instead diagnosing patients based on the combination of MIF and circEPSTI1, the Area further rose to 0.8604.
Conclusion: Assessing the combination of circEPSTI1 and MIF may be a viable approach to reliably diagnosing EC.
Keywords: circEPSTI1, MIF, diagnostic biomarker, endometrial cancer
Introduction
Endometrial cancer (EC) is the third most prevalent malignancy among females worldwide,1 yet no reliable approaches to detecting EC in its early stage currently exist for women at average risk who are free of any symptoms.2 Under most established guidelines, initial detection strategies most commonly include endometrial biopsy or transvaginal ultrasonography, but both of these approaches are subject to limitations. Diagnostic curettage is also commonly employed when seeking to definitively identify this tumor type, but this procedure is inherently invasive and requires the scraping of tissue specimens without clear visibility such that missed diagnoses may occur, while also exposing patients to the potential for endometrial bleeding of varying severity levels. EC tumors can also invade the muscle layer, contributing to metastatic progression and adverse patient outcomes.3 This procedure is also highly specialized such that it can only be conducted by experienced gynecologists. As such, validated biomarkers that can more reliably aid in the diagnosis of EC are urgently needed in order to improve the prognosis of this form of cancer.
Circular RNAs (circRNAs) are a unique subset of transcripts that are extremely stable and resistant to degradation owing to their structural characteristics, defined by a closed covalent loop of RNA.4–6 Particular circRNAs have increasingly been shown to regulate a wide array of cancers and other diseases through their ability to modulate biological processes such as differentiation, glycolytic metabolism, survival, proliferation, and migration.7,8 These findings have spurred growing interest in the application of circRNAs as therapeutic targets or diagnostic biomarkers in a range of pathological contexts.9,10 Widespread circRNA dysregulation has recently been reported in EC, with certain circRNAs serving as important regulators of disease-related processes.11,12 Of note, circEPSTI1 can reportedly enhance certain oncogenic activities in cervical cancer,13 oral squamous cell carcinoma,14 ovarian cancer,15 non-small cell lung cancer,16 and breast cancer.17 How circEPSTI1 functions in EC, however, remains poorly understood. One recent study also determined that macrophage migration inhibitory factor (MIF) levels are closely associated with EC patient prognostic outcomes.18 While the bulk of prior MIF research has centered on inflammatory diseases,19 an increasing number of studies suggest that it may function as a key factor that links inflammation to oncogenesis owing to the importance of inflammatory activity and tissue remodeling in the context of cancer development.20
Here, circEPSTI1 and MIF levels were compared between samples of serum obtained from patients with EC and healthy subjects, while also assessing these expression levels across a range of tumor stages. To more fully assess the link between these biomarkers and the biology of EC, the associations between circEPSTI1 and MIF levels and the clinicopathological characteristics of EC patients were additionally examined. Receiver operating characteristic (ROC) curves were also constructed to test their diagnostic performance. Overall, these findings provided strong evidence for the importance of circEPSTI1 and MIF in the pathogenesis of EC, while also suggesting that they can be leveraged to help diagnose this form of cancer.
Materials and Methods
Sample Collection
This study received approval from the Ethics Committee of the Affiliated Hospital of Hebei University of Engineering, and was performed in accordance with the principles of the Helsinki Declaration. Serum samples were obtained from patients with EC and healthy individuals (n=186 each) between May 2021 and December 2022. The informed consent were gained from the patients and their families. Blood samples were collected in RNAse-free tubes for RNA isolation, which was performed with 30 minutes after collection. Patient clinicopathological and molecular characteristics were additionally recorded.
qPCR
RNA was isolated from 500 uL of serum per patient with an RNA Isolation Kit (Vazyme Biotech), after which cDNA was synthesized with a Prime Script RT reagent Kit (Takara). Then, qPCR reactions were conducted with SYBR Green (Takara) by 7500 Fast Real-Time PCR System, utilizing the GAPDH for the normalization of expression with the 2−ΔΔCt method. The primers used in this study were as follows: circEPSTI1, Forward (F), AAGCTGAAGAAGCTGAACTC, Reverse (R), CCGGTTTATATTTGGTGCTATC. GAPDH, F, GGAGCGAGATCCCTCCAAAAT, R, GGCTGTTGTCATACTTCTCATGG. The qPCR conditions used in this study were as follows: Initial 1 step at 95°C for 30 sec, followed by 40 cycles at 95°C for 5 sec and at 60°C for 34 sec.
ELISAs
MIF levels in appropriate samples were analyzed with an ELISA kit (R&D Systems) based on provided directions, with all samples being analyzed in triplicate.
Statistical Analysis
GraphPad Prism 8.0 (GraphPad Software) was used to analyze all data, which were compared with Student’s t-tests or one-way ANOVAs. Survival analyses were conducted as per the Kaplan–Meier method. P < 0.05 served as the cut-off when defining statistical significance.
Results
Participant Characteristics
For this study, plasma samples from 186 EC patients and an equal number of normal control individuals were analyzed. The characteristics of these patients are summarized in Table 1, and no differences in age were detected between these two groups of study subjects (P > 0.05).
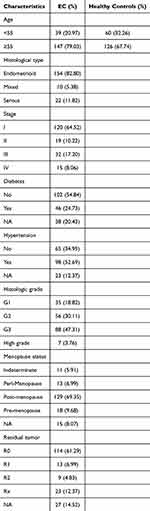 |
Table 1 Clinicopathological Characteristics of EC Patients and Controls |
CircEPSTI1 Levels in Serum Samples are Correlated with the Clinicopathological Characteristics of Patients with EC
To confirm the identity of circEPSTI1, the genomic loci of circEPSTI1 and the Sanger sequence of junction site of circEPSTI1 were detected by Sanger sequencing (Figure 1A). EC patient serum samples were found to exhibit higher levels of circEPSTI1 when analyzed via qPCR (Figure 1B). The EC patients were allocated to two groups according to the median circEPST11 level (n=93/group). Under these conditions, high circEPSTI1 expression in the serum was significantly related to the presence of stages (p=0.031), histologic grade (p=0.029), and residual tumor (p=0.022), as shown in Table 2. No significant relationships were seen with age (p=0.652), histological type (p=0.352), diabetes (p=0.186), hypertension (p=0.854), or menopause status (p=0.309), but patients expressing high circEPSTI1 did exhibit a reduction in both progression-free survival (PFS, Figure 2A) and overall survival (OS, Figure 2B) when compared to those patients exhibiting low expression levels. Consistently, univariate analyses detected a close relationship between the levels of this circRNA, stage, histologic grade and residual tumor, as shown in Tables 3 and 4. These associations remained significant when performing multivariate analyses.
 |
Table 2 Relationships Between circEPSTI1 Expression Levels and Patient Clinicopathological Characteristics |
 |
Table 3 Univariate and Multivariate Cox Analyses of PFS-Related Factors in EC Patients |
 |
Table 4 Univariate and Multivariate Cox Analyses of OS-Related Factors in EC Patients |
Serum MIF Levels Over Values for the Diagnosis of EC
There is prior evidence for an association between MIF concentrations and the prognosis of individuals with EC.18 Here, the mean respective concentrations of MIF in the serum of EC patients and healthy subjects were 4.825±1.05 ng/mL and 5.871±3.37 ng/mL (P < 0.0001) (Figure 3A). As revealed by constructed ROC curves, levels of serum MIF were capable of reliably distinguishing between patients with EC and healthy individuals, with an Area of 0.6646 (95% CI; 0.6086–0.7205) (Figure 3B).
EC Can Be Effectively Diagnosed Based on Serum circEPSTI1
To similarly assess the diagnostic performance of the serum levels of circEPSTI1, ROC curves were again constructed, effectively allowing for differentiation between samples from individuals with EC and healthy subjects. At the optimal cut-off of 3.9496, the Area for the ROC curve was 0.8351 (95% CI: 0.7941–0.8761) (Figure 4A). CircEPSTI1 may thus outperform MIF with respect to its sensitivity when leveraged as a biomarker to diagnose EC. These analyses were further expanded by examining the diagnostic performance of a combination of serum circEPSTI1 and MIF as a means of detecting EC. The combined AUC when using these two biomarkers was 0.8604 (95% CI: 0.8217–0.8991) (Figure 4B). Predictive probability levels above this cut-off were indicative of EC positivity.
Discussion
EC is among the most frequently diagnosed malignancies among women. While many patients are diagnosed while the disease is in its early stages, the overall survival of individuals with recurrent disease or who are only diagnosed when the disease is relatively advanced is substantially shorter. Analyses of biomarkers in the peripheral blood thus hold great promise as a noninvasive and cost-effective means of aiding in EC diagnosis.
Many studies have emphasized the value of circRNAs in the diagnosis of a variety of cancers as these molecules are both highly stable and abundant, in addition to playing important roles in key cancer-associated processes.21,22 The stability of circRNAs in patient serum and plasma remains consistent even with freezing and thawing or shifts in pH,23,24 making them optimal minimally invasive targets for clinical assessment. Serum Circ-FAF1/Circ-ELP3 offers great utility as a biomarker when applied in the context of diagnosing breast cancer,25 while hsa_circ_0000437 can promote gastric cancer pathogenesis and metastasis to the lymph nodes.26 Levels of hsa_circ_0000702 in patient serum have been proposed to be an effective gastric cancer-related biomarker.27 Although identified only recently, circEPSTI1 has been noted to support tumorigenesis and progression in cervical cancer,13 oral squamous cell carcinoma,14 ovarian cancer,15 non-small cell lung cancer,16 and breast cancer.17 The functions and diagnostic performance of circEPSTI1 in EC, however, have not been previously documented.
This study is the only one we are aware of examining the relevance of MIF in EC patients. MIF has also been tied to angiogenic activity and the induction of tumor progression and growth in various cancers, although not in EC. In patients with malignant ascites, for example, Hagemann et al noted strong MIF expression, while ovarian cancer cell-derived MIF was capable of stimulating cytokine, chemokine, and pro-angiogenic factor production, contributing to angiogenic activity and vascularization.28 Nishihira et al further highlighted a close association between MIF and both angiogenesis and tumor growth through studies in which they used antisense MIF to treat murine colon cancer cells.29 These results support the use of MIF as a biomarker for diagnosing EC.
Here, serum circEPSTI1 levels in EC patients were evaluated to gauge their diagnostic value. These analyses revealed that higher circulating serum levels of this circRNA were related to EC patient stages, histologic grade, and residual tumor. This suggests that circEPSTI1 may play a role in the development or malignant progression of this form of cancer. The OS and PFS of patients expressing higher circEPSTI1 levels were worse than those of patients in which this circRNA was not highly abundant, further underscoring its potential application in the context of patient management. The ROC curves showed that serum circEPSTI1 levels could reliably differentiate between patients with EC and healthy serum samples at high levels of sensitivity and specificity better than those for MIF alone. When combining serum circEPSTI1 and MIF analyses together, EC could be accurately diagnosed with even greater specificity and sensitivity, indicating that this combined biomarker panel may be a robust strategy for EC diagnosis in the clinic.
Conclusion
In conclusion, EC patients exhibit pronounced circEPSTI1 upregulation that may be closely tied to oncogenic activity such that efforts to measure circulating levels of this circRNA can aid in the more effective and timely diagnosis of this disease. The combination of circEPSTI1 with a range of other clinical biomarkers that are part of standard analyses may further enhance its diagnostic performance. While these results are subject to some limitations, they nonetheless underscore important opportunities for research focused on assessing the clinical utility of circEPSTI1 in combination with MIF when seeking to screen for EC.
Funding
This study is supported by Handan City Science and Technology Research and Development Plan Project (No. 19422083029ZC).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Neacsu A, Marcu ML, Stanica CD, et al. Clinical and morphological correlations in early diagnosis of endometrial cancer. Rom J Morphol Embryol. 2018;59(2):527–531.
2. Tarney CM, Wang G, Bateman NW, et al. Biomarker panel for early detection of endometrial cancer in the prostate, lung, colorectal, and ovarian cancer screening trial. Am J Obstet Gynecol. 2019;221(5):472 e471–472 e410. doi:10.1016/j.ajog.2019.06.005
3. Gottwald L, Kubiak R, Pasz-Walczak G, et al. The value of progesterone and estrogen receptors expression in tissue microarray method in prognosis of patients with endometrioid endometrial cancer. Ginekol Pol. 2013;84(2):95–101. doi:10.17772/gp/1602
4. Panda AC, Grammatikakis I, Munk R, Gorospe M, Abdelmohsen K. Emerging roles and context of circular RNAs. Wiley Interdiscip Rev RNA. 2017;8(2). doi:10.1002/wrna.1386
5. Muller S, Appel B. In vitro circularization of RNA. RNA Biol. 2017;14(8):1018–1027. doi:10.1080/15476286.2016.1239009
6. Ebbesen KK, Kjems J, Hansen TB. Circular RNAs: identification, biogenesis and function. Biochim Biophys Acta. 2016;1859(1):163–168. doi:10.1016/j.bbagrm.2015.07.007
7. Chen RX, Liu HL, Yang LL, et al. Circular RNA circRNA_0000285 promotes cervical cancer development by regulating FUS. Eur Rev Med Pharmacol Sci. 2019;23(20):8771–8778.
8. Chen Y, Wang J, Wang C, Liu M, Zou Q. Deep learning models for disease-associated circRNA prediction: a review. Brief Bioinform. 2022;23(6). doi:10.1093/bib/bbac364
9. Zhang HD, Jiang LH, Sun DW, Hou JC, Ji ZL. CircRNA: a novel type of biomarker for cancer. Breast Cancer. 2018;25(1):1–7. doi:10.1007/s12282-017-0793-9
10. Jiang B, Zhang J, Sun X, et al. Circulating exosomal hsa_circRNA_0039480 is highly expressed in gestational diabetes mellitus and may be served as a biomarker for early diagnosis of GDM. J Transl Med. 2022;20(1):5. doi:10.1186/s12967-021-03195-5
11. Liu Y, Chen S, Zong ZH, Guan X, Zhao Y. CircRNA WHSC1 targets the miR-646/NPM1 pathway to promote the development of endometrial cancer. J Cell Mol Med. 2020;24(12):6898–6907. doi:10.1111/jcmm.15346
12. Zhang J, Chen S, Wei S, et al. CircRAPGEF5 interacts with RBFOX2 to confer ferroptosis resistance by modulating alternative splicing of TFRC in endometrial cancer. Redox Biol. 2022;57:102493. doi:10.1016/j.redox.2022.102493
13. Wu P, Li C, Ye DM, et al. Circular RNA circEPSTI1 accelerates cervical cancer progression via miR-375/409-3P/515-5p-SLC7A11 axis. Aging. 2021;13(3):4663–4673. doi:10.18632/aging.202518
14. Wang J, Jiang C, Li N, et al. The circEPSTI1/mir-942-5p/LTBP2 axis regulates the progression of OSCC in the background of OSF via EMT and the PI3K/Akt/mTOR pathway. Cell Death Dis. 2020;11(8):682. doi:10.1038/s41419-020-02851-w
15. Xie J, Wang S, Li G, et al. circEPSTI1 regulates ovarian cancer progression via decoying miR-942. J Cell Mol Med. 2019;23(5):3597–3602. doi:10.1111/jcmm.14260
16. Xie Y, Wang L, Yang D. CircEPSTI1 promotes the progression of non-small cell lung cancer through miR-145/HMGB3 Axis. Cancer Manag Res. 2020;12:6827–6836. doi:10.2147/CMAR.S252893
17. Zhang Y, Tan D, Xie Y, et al. CircEPSTI1 Promotes the Proliferation of HER2-Positive Breast Cancer Cells via circEPSTI1/miR-145/ERBB3 Axis. J Oncol. 2022;2022:1028851. doi:10.1155/2022/1028851
18. Xiao W, Dong X, Zhao H, et al. Expression of MIF and c-erbB-2 in endometrial cancer. Mol Med Rep. 2016;13(5):3828–3834. doi:10.3892/mmr.2016.4992
19. Mizue Y, Ghani S, Leng L, et al. Role for macrophage migration inhibitory factor in asthma. Proc Natl Acad Sci U S A. 2005;102(40):14410–14415. doi:10.1073/pnas.0507189102
20. Bucala R, Donnelly SC. Macrophage migration inhibitory factor: a probable link between inflammation and cancer. Immunity. 2007;26(3):281–285. doi:10.1016/j.immuni.2007.03.005
21. Ma Y, Li Z, Ma D, Guo J, Sun W. Hsa_circ_0003195 as a biomarker for diagnosis and prognosis of gastric cancer. Int J Clin Oncol. 2022;27(2):354–361. doi:10.1007/s10147-021-02073-w
22. Lei B, Tian Z, Fan W, Ni B. Circular RNA: a novel biomarker and therapeutic target for human cancers. Int J Med Sci. 2019;16(2):292–301. doi:10.7150/ijms.28047
23. Vea A, Llorente-Cortes V, de Gonzalo-Calvo D. Circular RNAs in Blood. Adv Exp Med Biol. 2018;1087:119–130.
24. Guo S, Hu C, Zhai X, Sun D. Circular RNA 0006602 in plasma exosomes: a new potential diagnostic biomarker for hepatocellular carcinoma. Am J Transl Res. 2021;13(6):6001–6015.
25. Omid-Shafaat R, Moayeri H, Rahimi K, et al. Serum Circ-FAF1/Circ-ELP3: a novel potential biomarker for breast cancer diagnosis. J Clin Lab Anal. 2021;35(11):e24008. doi:10.1002/jcla.24008
26. Shen X, Kong S, Ma S, et al. Hsa_circ_0000437 promotes pathogenesis of gastric cancer and lymph node metastasis. Oncogene. 2022;41(42):4724–4735. doi:10.1038/s41388-022-02449-w
27. Yuan W, Fang R, Mao C, Chen H, Tai B, Cong H. Serum circular RNA hsa_circ_0000702 as a novel biomarker for diagnosis of gastric cancer. J Clin Lab Anal. 2023;37(3):e24842. doi:10.1002/jcla.24842
28. Hagemann T, Robinson SC, Thompson RG, Charles K, Kulbe H, Balkwill FR. Ovarian cancer cell-derived migration inhibitory factor enhances tumor growth, progression, and angiogenesis. Mol Cancer Ther. 2007;6(7):1993–2002. doi:10.1158/1535-7163.MCT-07-0118
29. Nishihira J, Ishibashi T, Fukushima T, Sun B, Sato Y, Todo S. Macrophage migration inhibitory factor (MIF): its potential role in tumor growth and tumor-associated angiogenesis. Ann N Y Acad Sci. 2003;995(1):171–182. doi:10.1111/j.1749-6632.2003.tb03220.x
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




