Back to Journals » International Journal of General Medicine » Volume 15
The Clinical Value of Systemic Inflammatory Response Index and Inflammatory Prognosis Index in Predicting 3-Month Outcome in Acute Ischemic Stroke Patients with Intravenous Thrombolysis
Authors Ma X, Yang J, Wang X, Wang X, Chai S
Received 2 August 2022
Accepted for publication 13 October 2022
Published 22 October 2022 Volume 2022:15 Pages 7907—7918
DOI https://doi.org/10.2147/IJGM.S384706
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Xin Ma *, Jie Yang *, Xiaolu Wang *, Xiang Wang *, Shuhong Chai
Department of Clinical Laboratory, Urumqi Friendship Hospital, Urumqi, Xinjiang Uygur Autonomous Region, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Shuhong Chai, Department of Clinical Laboratory, Urumqi Friendship Hospital, No. 558 Shengli Road, Tianshan District, Urumqi, Xinjiang Uygur Autonomous Region, 830049, People’s Republic of China, Tel +86-18997994493, Email [email protected]
Purpose: Systemic inflammatory response index (SIRI) was an independent predictor of the prognosis of many diseases. Inflammatory prognostic index (IPI) was a new inflammatory prognostic marker with certain clinical significance. We aimed to explore the association between SIRI, IPI and early stage severity of stroke as well as 3-month outcome of AIS patients.
Patients and Methods: A total of 63 AIS patients who treated with alteplase were selected. The patients were divided into mild group and moderate to severe group according to the National Institutes of Health Stroke Scale (NIHSS) scores. According to the modified Rankin scale (mRS) score, patients were divided into the good prognosis group and the poor prognosis group. Spearman correlation statistically analyzed the correlation between SIRI, IPI and NIHSS score. Univariate and multivariate logistic regression analyzed the risk factors of 3-month prognosis. ROC curve was adopted to predict the effect of SIRI and IPI levels on poor prognosis in AIS patients.
Results: Spearman analysis showed that there was positively correlated with NIHSS score and IPI in mild AIS group (r=0.541, P< 0.05). Compared with the mild group, SIRI and IPI in the moderate to severe group was significantly higher (P< 0.05). The SIRI and IPI in the poor prognosis group were significantly higher than those in the good prognosis group (P< 0.05). Univariate and multivariate logistic regression analysis showed that SIRI and IPI were independent prognostic factors for the 3-month prognosis of AIS patients (P< 0.05). The ROC curve showed that the areas under the 3-month prognosis curve predicted by SIRI and IPI were 0.685, 0.774 respectively.
Conclusion: IPI is correlated with stroke severity at admission. SIRI and IPI are independent predictors of short-term prognosis in AIS patients. SIRI and IPI can be a novel the good short-term prognostic biomarker for AIS patients treated with intravenous thrombolysis.
Keywords: acute ischemic stroke, systemic inflammatory response index, inflammatory prognosis index, short term prognosis
Introduction
Acute ischemic stroke (AIS) is mainly caused by cerebral ischemia and hypoxia caused by cerebral vascular thrombosis, resulting in the serious destruction of neurons, astrocytes and oligomutant glia. AIS has the characteristics of acute onset, rapid symptom progression and poor prognosis. It is one of the main causes of disability and death in middle-aged and elderly people.1 The 2018 edition of the guidelines for the diagnosis and treatment of acute ischemic stroke in China2 recommends that the AIS patients within 4.5 hours of onset should be treated with intravenous thrombolysis with alteplase (rt-PA). rt-PA is a thrombolytic drug, which can directly activate plasminogen and play a role in thrombolysis and restoring the blood flow supply of occluded vessels.3,4 Powers WJ had found that nearly half of AIS patients tend to have poor function outcomes after the therapy of intravenous thrombolysis.5 It was still of vital importance to establish accurate prognostic models of the functional outcome in patients after ischemic stroke.6
Inflammation has been increasingly recognized as a key factor in the pathophysiology of acute ischemic stroke.7 The components of immune system are closely related to the occurrence of ischemic brain injury. Immunosuppression secondary to cerebral ischemia may promote concurrent infection. Systemic inflammatory response index (SIRI), a new inflammatory marker based on the counts of neutrophils, monocytes and lymphocytes in peripheral blood, is an independent predictor of the prognosis of many diseases. Furthermore, SIRI has been widely considered in disease diagnosis and prognosis evaluation in recent years.8–11 Topkan et al12 have found that SIRI could be a novel, sound, and independent predictor of survival outcomes in patients with newly diagnosed glioblastoma multiforme who underwent postoperative Stupp protocol. The inflammatory prognostic index (IPI) establishes a new inflammatory prognostic model, which is an independent risk factor for the survival and prognosis of patients with non-small cell lung cancer (NSCLC).13
Now, we need through a better laboratory examination to quickly identify the type of AIS disease, evaluate the hypercoagulable state, and give precise treatment with early personalized intervention. So we aimed to systematically explore the relationship between SIRI, IPI and the severity of ischemic stroke and their prognostic value at 3-month follow-up in AIS patients treated with rt-PA.
Patients and Methods
Patients Selection
This prospective study consecutively selected 84 AIS patients who received intravenous thrombolysis with alteplase (rt-PA) in the stroke center (Department of Neurology) of Urumqi Friendship Hospital from May 30, 2020 to August 30, 2021. This study was reviewed and approved by the ethics committee of Urumqi friendship hospital.
Inclusion and exclusion criteria for AIS patients were as follows: (1) Inclusion criteria: ① age > 18 years old; ② Meet the diagnostic criteria of ischemic stroke in Chinese AIS diagnosis and treatment guidelines; ③ The onset-to-treatment (OTT) of the included patients received rt-PA intravenous thrombolysis within 4.5 hours; ④ The patients are with first-ever ischemic stroke event; ⑤ The patients are with first-ever ischemic stroke event. (2) Exclusion criteria: ①Previous intracranial hemorrhage, including suspected subarachnoid hemorrhage; Have a history of head trauma in the past 3 months; Gastroinr urinary bleeding in recent 3 weeks; Excessive surgical operations in the last 2 weeks; In retestinal recent 1 week Puncture the artery at the site where hemostasis is not easy to be pressed. ② There was a history of cerebral infarction or myocardial infarction in the past 3 months, but old small lacunar infarction was not included and no neurological signs were left. ③ Patients with severe heart, liver and kidney dysfunction or severe diabetes. ④ Physical examination found active evidence of blood or trauma (such as fracture). ⑤ Oral anticoagulants have been taken and INR > 15; Received heparin treatment within 48h (APTT exceeded Normal range). ⑥ Platelet count is lower than 100×109/L and blood glucose is lower than 27mmol/L. ⑦ Blood pressure: systolic blood pressure >180mmhg, or diastolic blood pressure >100mmhg. ⑧ Pregnancy. ⑨ I do not cooperate. ⑩ Those who died during follow-up. Overall,63 patients were included in our study. Figure 1 presents the selection of patients in a flow chart.
 |
Figure 1 Flow chart for patients’ selection. |
Data Collection
Outcome Measures
(1) before thrombolysis, 2 mL of venous blood was collected with EDTA-K2 anticoagulant vacuum tube. Within 30 minutes after reversing evenly, it was detected with automatic Mindray cal 8000 blood cell analyzer. (2) Before thrombolysis, collect 3~5 mL of venous blood with a coagulation promoting tube, centrifuge at 3500 rpm for 10 min, use Roche Cobas 8000 automatic biochemical analyzer to detect albumin, creatinine, urea nitrogen, blood glucose and other indicators, and use Roche diagnostic reagents to complete the detection within 4 hours. (3) Before thrombolysis, use sodium citrate anticoagulant vacuum tube to collect 3 mL of venous blood, centrifuge at 3500 rpm for 15 min, separate plasma, use STAGO compact Max Automatic Coagulation Analyzer to detect coagulation function indexes, and use STAGO matching reagent to complete the detection within 2 h.
Computing Method
SIRI (109/L) = Neutrophil (109/L) × Monocyte (109/L) / lymphocyte (109/L)
IPI = C-reactive protein (g/L) × Neutrophil (109/L) / lymphocyte (109/L) / albumin (g/L).
Diagnostic Criteria
(1) The severity of ischemic stroke was assessed by the National Institutes of Health Stroke Scale (NIHSS) scores on admission and stroke subtypes were evaluated by experienced clinicians. NIHSS > 5 was considered as moderate to severe stroke, and 0 < NIHSS ≤ 5 was considered as mild stroke.14 (2) The short-term prognosis was evaluated by modified Rankin Scale (mRS). mRS scores range from 0 to 6, mRS score of 0~2 was regarded as good prognosis, and the score of 3~6 was regarded as poor prognosis based on the 90-d mRS score at outpatient or telephone follow-up.15
Statistical Analysis
Kolmogorov–Smirnov test was used to check the distribution normality. Normally distributed continuous variables are summarized as mean ± SD, and nonnormally distributed continuous variables are expressed as median and interquartile range (IQR). Nominal variables were compared using Spearman’s chi-squared test, and comparisons of continuous variables were made using the Mann–Whitney U-test based on the data distribution. The count data were expressed by the number of cases or rate and analyzed by χ2 test. Logistic regression analysis was used to determine poor outcome at 3-months. Variables with P<0.05 in the univariate analysis were included in the multivariate logistic regression model.
Receiver operating characteristic (ROC) curves were used to evaluate the ability of the SIRI and IPI index to predict the clinical treatment outcome of different AIS patients. The optimal test cut-off point was established by calculating Youden’s index.
Statistical significance was set at P<0.05. Statistical analysis was performed using SPSS 22.0.
Results
Baseline Clinical Characteristics of AIS Subjects in the Two Groups
The prospective study had enrolled 63 AIS patients. The baseline characteristics are displayed in Table 1. Statistical analysis showed that there were significant differences in age, ADL score, swallowing function score, DNT, uric acid, triglyceride, glucose, platelet, INR, fibrinogen, D2 polymer, red blood cell, C-reactive protein (CRP), length of hospital stay, SIRI and IPI indexes between mild AIS group and moderate to severe AIS group. The average level of age, swallowing function score, platelet, INR, fibrinogen, D2 polymer, CRP, length of hospital stay, SIRI, IPI in moderate to severe AIS patients were significantly higher (P< 0.05) than mild AIS patients’.
 |
Table 1 Comparison of Baseline Clinical Indexes Between Mild AIS Group and Moderate to Severe AIS Group |
Correlation Between Admission NIHSS Score and SIRI Level in Two Groups of AIS Patients
Spearman correlation analysis showed that SIRI was not correlated with NIHSS score (rs = 0.226, P> 0.05) (Figure 2A). Mann–Whitney U-test (Wilcoxon rank sum test) showed that SIRI level in moderate to severe AIS patients (NIHSS score > 5) remained higher than mild AIS patients’ (0< NIHSSscore≤ 5) (median:1.77 vs 0.94, P< 0.05) (Figure 2B).
Correlation Between Admission NIHSS Score and IPI Level in Tow Groups of AIS Patients
Spearman correlation analysis showed that IPI was positively correlated with NIHSS score (rs = 0447, P< 0.05) (Figure 3A). Mann–Whitney U-test (Wilcoxon rank sum test) showed that IPI level in moderate to severe AIS patients (NIHSS score > 5) remained higher than mild AIS patients’ (0 < NIHSSscore ≤ 5) (median:0.31 vs 0.06, P< 0.05) (Figure 3B).
Baseline Clinical Characteristics of AIS Subjects in Good Prognosis Group and Poor Prognosis Group
Among the 63 AIS patients in this study, 45 patients had a good prognosis while 18 patients had a poor prognosis. The baseline characteristics are displayed in Table 2. There were significant differences in swallowing function score, uric acid, triglyceride, PT, INR, fibrinogen, D2 polymer, lymphocyte, red blood cell, CRP, SIRI and IPI between the poor prognosis group and the good one. The average level of swallowing function score, triglyceride, PT, INR, fibrinogen, D2 polymer, CRP, SIRI and IPI in poor prognosis group were significantly higher (P<0.05) than good prognosis group’s.
 |
Table 2 Baseline Clinical Characteristics of AIS Subjects in Good Prognosis Group and Poor Prognosis Group |
Univariate and Multivariate Logistic Analysis of SIRI, IPI and Poor 3-Month Outcome in AIS Patients
As is shown in the univariate regression analyses, swallowing function score, PT, fibrinogen, D2 polymer, CRP, GPS, SIRI and IPI were significantly associated with the poor outcome at three months. To figure out whether high SIRI and IPI were an independent prognostic indicator for poor outcome in three months. Variables with P<0.05 in the univariate analysis were included in the multivariate logistic regression model. After adjusting to potential confounders mentioned above, multivariate analysis showed that high SIRI remained independently associated with poor 3-month function outcome (OR=1.407, 95% CI=0.833~2.377, P<0.05) and high IPI also remained independently associated with poor 3-month function outcome (OR=1.306, 95% CI = 0.887~1.953, P< 0.05) (Table 3).
 |
Table 3 Univariate and Multivariate Logistic Analysis of SIRI, IPI and Poor 3-Month Outcome in AIS Patients |
The ROC Curve Analysis of SIRI and IPI in the Diagnosis of Short-Term Poor Outcome in AIS Patients
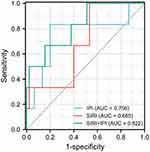
According to ROC analysis, the SIRI index had low accuracy. The SIRI cut-off value that distinguished 3-month poor outcome was 1.010×109 /L with a sensitivity of 1.000 and a specificity of 0.467. The area under curve (AUC) was 0.685 (95% CI=0.546~0.826, P<0.05). The IPI cut-off value that best-distinguished 3-month poor outcome was 0.343 with a sensitivity of 0.667 and a specificity of 0.867. The AUC was 0.756 (95% CI=0.604~0.826, P<0.05) (Figure 4).
Discussion
Acute ischemic stroke (AIS) has a high incidence rate, disability rate and mortality, and currently ranks first among the fatal diseases in China.16 Although antithrombotic and reperfusion therapy have improved the prognosis, there are still some AIS patients with poor prognosis.17 In view of the heavy burden of adverse prognostic outcomes of stroke, more attention is focused on the identification of new prognostic markers, so that personalized treatment schemes could be formulated to maximize the quality of life of patients. Studies have shown that inflammatory response played an important role in the progression of AIS patients. Animal experiments and clinical studies have confirmed that focal ischemia and hypoxia in the acute stage of stroke induces a cascade inflammatory reaction characterized by peripheral immune cell recruitment and cytokine secretion. Inflammatory factors accumulate in the focus and surrounding tissues for several weeks, leading to secondary brain injury of stroke, aggravation of patients’ condition and occurrence of adverse prognostic events.18,19 Previous studies on AIS have identified that some inflammatory factors such as the ratio of neutrophils to lymphocytes,20 C-reactive protein,21 interleukin 622 and other inflammatory factors were related to the development of AIS disease. Systemic inflammatory response index (SIRI) and inflammatory prognostic index (IPI) are new inflammatory markers, which are closely related to tumors and cardiovascular diseases.23 According to the relevant literature, there are few studies on the relationship between SIRI, IPI and the condition evaluation of AIS patients and the short-term prognosis after rt-PA intravenous thrombolysis. Based on the clinical data and related experimental indexes (SIRI, IPI, etc.) of AIS patients in the stroke center of Urumqi Friendship Hospital, the condition evaluation of AIS patients and the short-term prognosis of rt-PA intravenous thrombolysis. The results showed that IPI index could effectively and objectively evaluate the severity of AIS patients. Univariate and multivariate Logistic analysis that SIRI and IPI were independent risk factors for short-term prognosis of AIS patients. Results of ROC analysis demonstrated that SIRI and IPI indexes had certain accuracy in predicting the 3-month prognosis of AIS patients. SIRI and IPI were comprehensive in predicting the short-term prognosis of AIS patients, and might be used as a new index to assist in evaluating the poor short-term prognosis of AIS patients. Next, we will discuss the effects of SIRI and IPI on immune function in AIS patients and their interaction mechanism.
SIRI index is obtained according to the counts of neutrophils, monocytes and lymphocytes in peripheral blood, which is an independent prognostic factor for a variety of malignant tumors.24,25 SIRI represents innate immune system and adaptive immune system, and is easy to obtain, non-invasive and cheap marker. It can be routinely used to evaluate the inflammatory state of the body system in practical clinical work. When atherosclerosis occurs, neutrophils, as the first responding cells, quickly gather in the ischemic focus, destroy the endothelial cell membrane and basement membrane by releasing proinflammatory factors, such as inducible nitric oxide synthase, elastase, cathepsin and matrix metalloproteinase, and promote the destruction of blood-brain barrier and the occurrence of brain edema.26 Studies have shown that the increasing number of neutrophils were associated with stroke severity, infarct size and worse prognosis.27–29 In ischemic stroke, monocytes migrated and infiltrated in the early stage of tissue injury and reached a peak within 7 days after injury.30 Stimulated by oxidized LDL, monocytes differentiated into macrophages. Macrophages not only phagocytized low-density lipoprotein into foam cells, but also secreted interleukin-1, tumor necrosis factor and other inflammatory factors.31,32 Activated monocytes could secrete vascular endothelial growth factor, increase vascular permeability and aggravate the damage of blood–brain barrier.33 The number of monocytes might be a biomarker of clinical prognosis and could be used as an early indicator of disease severity in AIS patients.34 Lymphocytes have been shown to play an important role in curing inflammation.35 Experiments have shown that T lymphocytes and B lymphocytes mainly had regulatory function, reduced the volume of ischemic tissue in ischemic stroke and improved the function after neurological deficit.36,37 Clinical data confirmed that lower lymphocyte counts in AIS patients were associated with long-term poor prognosis.29 SIRI index is a composite marker of neutrophils, monocytes and lymphocytes, which reflects the ability of human immune surveillance to a certain extent and it is relatively stable, taking into account their respective cell functions. SIRI is a parameter in the combination of different inflammatory reactions, which can provide more information on the formation of immune activity in the immune process and the pathogenesis of ischemic stroke. Urbanowicz et al38 has been recently studied that Systemic Inflammatory Response Index (SIRI) Predict Mortality after Off-Pump Coronary Artery Bypass Surgery. A recent study39 has shown that SIRI was significantly higher in patients with coronavirus disease 2019 (COVID-19) than those in the control group, whereas its diagnostic value in COVID-19 was moderate. In this study, Mann–Whitney U-test was used to figure out the phenomenon that the SIRI index in moderate to severe group was higher than in mild group’s. The SIRI index in poor short-term prognosis group was significantly higher than good prognosis group’s. Univariate and multivariate binary logistic regression analysis showed that high-level SIRI index was an independent prognostic factor for the 3-month prognosis of AIS patients. According to ROC analysis, the SIRI index had low accuracy. SIRI played an important role in the development of acute ischemic stroke from the occurrence, development and recovery of stroke. According to this study, individualized treatment can be carried out for patients with SIRI > 1.010×109/L, so as to improve the prognosis, but I believe that still all AIS patients independent from the SIRI level should be treated early. Therefore, the clinical practice can dynamically observe the SIRI index of AIS patients, to accurately treat and improve the short-term prognosis of AIS patients.
Inflammatory prognostic index (IPI) is a new inflammatory prognostic marker with certain clinical significance based on C-reactive protein (CRP), neutrophils to lymphocytes ratio (NLR) and serum albumin (ALB). Dirican et al concluded that IPI was a low-cost, easy to obtain and reproducible index and an independent prognostic index for patients with non-small cell lung cancer.40 CRP was a sensitive indicator of the body’s inflammatory response in the early stage of infection. Studies have shown that the level of CRP was related to the severity of the disease, the size of infarct area and poor prognosis in AIS patients.41 In the future, it may be possible to control the level of inflammatory factor CRP as a new idea to reduce the risk of recurrent stroke in patients with cerebral infarction.42 NLR was a value obtained by comparing neutrophils with lymphocytes. Compared with single neutrophils or lymphocytes, NLR might be a more accurate indicator for predicting atherosclerosis related diseases.43 Several studies have confirmed that NLR was associated with short-term and long-term mortality in acute coronary syndrome.44–46 Meanwhile, Beomseok et al showed that the increase of NLR was an independent risk factor for ischemic stroke.47 Several articles reported that NLR was related to the severity of acute cerebral infarction, mortality, recurrence, hemorrhagic transformation, post-stroke infection, etc.48–51 ALB was the main protein in human blood, which could maintain body nutrition and balance colloidal osmotic pressure. ALB had many physiological functions, such as anti-oxidation, anti-inflammatory, inhibiting platelet aggregation and thrombosis.52 Animal model studies have shown that ALB has neuroprotective effect on ischemic stroke, and clinical studies have also confirmed that lower levels of ALB could increase the adverse prognosis of stroke.52–54 IPI, as a new type of inflammatory prognosis index, has the advantages of high sensitivity, specificity and more comprehensive. This study found that IPI was positively correlated with NIHSS score. Mann–Whitney U-test showed that IPI level in moderate to severe AIS patients had a significant higher than mild AIS patients’. The admission IPI index of AIS patients can effectively evaluate the severity of the patient’s condition, especially the patients with low NIHSS score, so as to take treatment in time, shorten the time from arrival to thrombolysis, and improve the prognosis of patients. The IPI index of the poor short-term prognosis group was significantly higher than that of the good prognosis group. Univariate and multivariate binary logistic regression analysis showed that IPI index was an independent prognostic factor for the 3-month prognosis of AIS patients. According to ROC analysis, the IPI index had a certain predictive value for the short-term good prognosis of patients with AIS undergoing rt-PA intravenous thrombolysis. IPI can be used as a new inflammatory marker for the short-term prognosis of AIS patients, and it also reveals that inflammation may provide accurate treatment for AIS treatment.
In conclusion, the joint examination of SIRI and IPI can effectively evaluate the body inflammation and carry out immune regulation. The implementation of personalized measures will help to improve the prognosis of AIS patients and contribute to the health management of stroke. SIRI and IPI are calculated by using objective laboratory test data, with good stability and strong comprehensiveness, which makes SIRI and IPI easy to be transformed into daily monitoring indicators. SIRI and IPI are more valuable in predicting the short-term prognosis of AIS patients, which provides a new idea for the treatment of AIS patients with rt-PA.
The limitations of present study are listed as follows: First, our study is a single-center study with 84 patients a relatively small sample size, which may cause selection bias and inaccuracy to. Secondly, there is no information about the vascular risk profile of the patients as it may interfere drastically with the final outcomes. Thirdly, I have not mRS at the baseline, so I cannot compare the same scale results. When I compare NIHSS and mRS there is always a level of uncertainty. Finally, further study is needed to combine multi regional stroke centers with large sample research.
Conclusion
Our study had figured out that IPI index of AIS patients could effectively evaluate the severity of patients’ condition. High levels of SIRI and IPI were independent risk factors for the short-term prognosis of AIS patients. SIRI and IPI were relatively more comprehensive inflammatory index, could be a short-term potential prognostic indicator for AIS patients undergoing rt-PA.
Data Sharing Statement
The study was approved by the Ethics Committee of Urumqi Friendship Hospital. All procedures performed in the study involving human participants were in accordance with the Ethics Committee of Urumqi Friendship Hospital, and patient consent to review their medical records was not required. This is because this was a prospective and non-interventional study, which collects experimental data in patients’ electronic medical records. This study is not greater than the minimum risk; Exemption from informed consent will not adversely affect the rights or welfare of subjects; The researcher’s use of the subject’s information will never have an adverse impact on it and should keep the patient’s information confidential. Therefore, the Ethics Committee of Urumqi Friendship Hospital approved the exemption of informed consent of patients in this study. The data were anonymized or maintained with confidentiality. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. All authors contributed equally to this manuscript.
Funding
This work was supported by the Special scientific research project for Young medical science and technology talents of Xinjiang Uygur Autonomous Region, China for financing this valuation (No. WJWY-202112).
Disclosure
The authors do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
References
1. Liu Y, Guo F, Wang J, et al. Correlation between comorbidity and functional recovery after stroke in middle-aged and elderly patients with acute ischemic stroke. Chin Gen Med. 2020;4:453–458.
2. Bin P, Bo W. Chinese guidelines for diagnosis and treatment of acute ischemic stroke 2018. Chin J Urol. 2018;51(09):666–682.
3. Mei D. Nursing discussion on acute ischemic stroke treated with alteplase thrombolysis. Everyone Healthy. 2020;2:135–136.
4. Benhan L, Yufu Z, Zhenyan W. Effect and safety analysis of intravenous thrombolytic therapy with alteplase in patients with acute ischemic stroke. Contemp Med Treatises. 2020;2:131–132.
5. Powers WJ, Solomon CG. Acute ischemic stroke. N Engl J Med. 2020;383(3):252–260. doi:10.1056/NEJMcp1917030
6. Jampathong N, Laopaiboon M, Rattanakanokchai S, et al. Prognostic models for complete recovery in ischemic stroke: a systematic review and meta-analysis. BMC Neurol. 2018;18(1):26. doi:10.1186/s12883-018-1032-5
7. Kim JY, Park J, Chang JY, Kim SH, Lee JE. Inflammation after ischemic stroke: the role of leukocytes and glial cells. Exp Neurobiol. 2016;25(5):241–251. doi:10.5607/en.2016.25.5.241
8. Ni J, Wang K, Zhang H, et al. Prognostic value of the systemic inflammatory response index in patients undergoing radical cystectomy for bladder cancer: a population-based study. Front Oncol. 2021;11:722151. PMID: 34485155; PMCID: PMC8416169. doi:10.3389/fonc.2021.722151
9. Wu J, Guo N, Zhang X, et al. HEV-LFS: a novel scoring model for patients with hepatitis E virus-related liver failure. J Viral Hepat. 2019;26(11):1334–1343. PMID: 31294523. doi: 10.1111/jvh.13174
10. Yun S, Yi HJ, Lee DH, et al. Systemic inflammation response index and systemic immune-inflammation index for predicting the prognosis of patients with aneurysmal subarachnoid hemorrhage. J Stroke Cerebrovasc Dis. 2021;30(8):105861. PMID: 34034125. doi: 10.1016/j.jstrokecerebrovasdis.2021.105861
11. Zhai GH, Zhang W, Xiang Z, et al. Diagnostic value of sIL-2R, TNF-α and PCT for sepsis infection in patients with closed abdominal injury complicated with severe multiple abdominal injuries. Front Immunol. 2021;12:741268. PMID: 34745113; PMCID: PMC8569904. doi:10.3389/fimmu.2021.741268
12. Topkan E, Kucuk A, Ozdemir Y, et al. Systemic inflammation response index predicts survival outcomes in glioblastoma multiforme patients treated with standard stupp protocol. J Immunol Res. 2020;2020:8628540. PMID: 33274245; PMCID: PMC7683150. doi:10.1155/2020/8628540
13. Zhishang W. Effects of C-reactive protein, neutrophil lymphocyte ratio and serum albumin based inflammatory prognosis index on the prognosis of non-small cell lung cancer. J Clin Lung. 2018;23(01):160–164.
14. Sulter G, Steen C, De KJ. Use of the Barthel index and modified Rankin scale in acute stroke trials. Stroke. 1999;30(8):1538–1541. doi:10.1161/01.STR.30.8.1538
15. Shi K, Tian DC, Li ZG, et al. Global brain inflammation in stroke. Lancet Neurol. 2019;18(11):1058–1066. doi:10.1016/S1474-4422(19)30078-X
16. Zerna C, Thomalla G, Campbell BCV, et al. Current practice and future directions in the diagnosis and acute treatment of ischaemic stroke. Lancet. 2018;392(10154):1247–1256. PMID: 30319112. doi:10.1016/S0140-6736(18)31874-9
17. Wang W, Jiang B, Sun H, et al. Prevalence, incidence, and mortality of stroke in China: results from a nationwide population-based survey of 480687 adults. Circulation. 2017;135(8):759–771. PMID: 28052979. doi: 10.1161/CIRCULATIONAHA.116.025250
18. Becker KJ. Inflammation and the silent sequelae of stroke. Neurotherapeutics. 2016;13(4):801–810. PMID: 27324389; PMCID: PMC5081115. doi:10.1007/s13311-016-0451-5
19. Anrather J, Iadecola C. Inflammation and stroke: an overview. Neurotherapeutics. 2016;13(4):661–670. PMID: 27730544; PMCID: PMC5081118. doi:10.1007/s13311-016-0483-x
20. Kotfis K, Bott-Olejnik M, Szylińska A, et al. Could Neutrophil-to-Lymphocyte Ratio (NLR) serve as a potential marker for delirium prediction in patients with acute ischemic stroke? A prospective observational study. J Clin Med. 2019;8(7):1075. PMID: 31336587; PMCID: PMC6679160. doi:10.3390/jcm8071075
21. Guo J, Su W, Fang J, et al. Elevated CRP at admission predicts post-stroke cognitive impairment in Han Chinese patients with intracranial arterial stenosis. Neurol Res. 2018;40(4):292–296. PMID: 29451096. doi: 10.1080/01616412.2018.1438224
22. Kwan J, Horsfield G, Bryant T, et al. IL-6 is a predictive biomarker for stroke associated infection and future mortality in the elderly after an ischemic stroke. Exp Gerontol. 2013;48(9):960–965. PMID: 23872300. doi: 10.1016/j.exger.2013.07.003
23. Xueyang H, Zhan G, Yong L. Effects of preoperative systemic inflammatory response index and fibrinogen on tumor prognosis after nephrectomy. Clin Urol. 2021;36(07):567–572. doi:10.13201/j.issn.1001-1420.2021.07.012
24. Wei L, Xie H, Yan P. Prognostic value of the systemic inflammation response index in human malignancy: a meta-analysis. Medicine. 2020;99(50):e23486. PMID: 33327280; PMCID: PMC7738007. doi:10.1097/MD.0000000000023486
25. Zhang Y, Liu F, Wang Y. Evidence of the prognostic value of pretreatment systemic inflammation response index in cancer patients: a pooled analysis of 19 cohort studies. Dis Markers. 2020;2020:8854267. PMID: 32934755; PMCID: PMC7479458. doi:10.1155/2020/8854267
26. Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17(7):796–808. PMID: 21738161; PMCID: PMC3137275. doi:10.1038/nm.2399
27. Kumar AD, Boehme AK, Siegler JE, et al. Leukocytosis in patients with neurologic deterioration after acute ischemic stroke is associated with poor outcomes. J Stroke Cerebrovasc Dis. 2013;22(7):111–117. doi:10.1016/j.jstrokecerebrovasdis.2012.08.008
28. Buck BH, Liebeskind DS, Saver JL, et al. Early neutrophilia is associated with volume of ischemic tissue in acute stroke. Stroke. 2008;39(2):355–360. PMID: 18162626. doi: 10.1161/STROKEAHA.107.490128
29. Kim J, Song TJ, Park JH, et al. Different prognostic value of white blood cell subtypes in patients with acute cerebral infarction. Atherosclerosis. 2012;222(2):464–467. PMID: 22460048. doi: 10.1016/j.atherosclerosis.2012.02.042
30. Rangasamy S, McGuire PG, Franco Nitta C, et al. Chemokine mediated monocyte trafficking into the retina: role of inflammation in alteration of the blood-retinal barrier in diabetic retinopathy. PLoS One. 2014;9(10):e108508. PMID: 25329075; PMCID: PMC4203688. doi:10.1371/journal.pone.0108508
31. Arnold IC, Mathisen S, Schulthess J, et al. CD11c(+) monocyte/macrophages promote chronic Helicobacter hepaticus-induced intestinal inflammation through the production of IL-23. Mucosal Immunol. 2016;9(2):352–363. PMID: 26242598; PMCID: PMC4650208. doi: 10.1038/mi.2015.65
32. Anselmo AC, Gilbert JB, Kumar S, et al. Monocyte-mediated delivery of polymeric backpacks to inflamed tissues: a generalized strategy to deliver drugs to treat inflammation. J Control Release. 2015;199:29–36. PMID: 25481443. doi:10.1016/j.jconrel.2014.11.027
33. Rodrigues SF, Granger DN. Blood cells and endothelial barrier function. Tissue Barriers. 2015;3(1–2):e978720. PMID: 25838983; PMCID: PMC4372023. doi:10.4161/21688370.2014.978720
34. Viedt C, Orth SR. Monocyte chemoattractant protein-1 (MCP-1) in the kidney: does it more than simply attract monocytes? Nephrol Dial Transplant. 2002;17(12):2043–2047. PMID: 12454208. doi:10.1093/ndt/17.12.2043
35. Schwartz M, Moalem G. Beneficial immune activity after CNS injury: prospects for vaccination. J Neuroimmunol. 2001;113(2):185–192. PMID: 11164901. doi:10.1016/s0165-5728(00)00447-1
36. Ren X, Akiyoshi K, Dziennis S, et al. Regulatory B cells limit CNS inflammation and neurologic deficits in murine experimental stroke. J Neurosci. 2011;31(23):8556–8563. PMID: 21653859; PMCID: PMC3111929. doi:10.1523/JNEUROSCI.1623-11.2011
37. Liesz A, Zhou W, Na SY, et al. Boosting regulatory T cells limits neuroinflammation in permanent cortical stroke. J Neurosci. 2013;33(44):17350–17362. PMID: 24174668; PMCID: PMC6618366. doi:10.1523/JNEUROSCI.4901-12.2013
38. Urbanowicz T, Michalak M, Olasińska-Wiśniewska A, et al. Neutrophil counts, Neutrophil-to-Lymphocyte Ratio, and Systemic Inflammatory Response Index (SIRI) predict mortality after off-pump coronary artery bypass surgery. Cells. 2022;11(7):1124. PMID: 35406687; PMCID: PMC8997598. doi:10.3390/cells11071124
39. Eissa M, Shaarawy S, Abdellateif MS. The role of different inflammatory indices in the diagnosis of COVID-19. Int J Gen Med. 2021;14:7843–7853. PMID: 34795505; PMCID: PMC8593597. doi:10.2147/IJGM.S337488
40. Dirican N, Dirican A, Anar C, et al. A new inflammatory prognostic index, based on C-reactive protein, the Neutrophil to Lymphocyte Ratio and serum albumin is useful for predicting prognosis in non-small cell lung cancer cases. Asian Pac J Cancer Prev. 2016;17(12):5101–5106. PMID: 28122441; PMCID: PMC5454643. doi:10.22034/APJCP.2016.17.12.5101
41. Xinhe S, Jun Y. Correlation between C-reactive protein and fibrinogen levels and acute ischemic stroke. Gansu Sci Technol. 2017;33(12):106–108.
42. Kitagawa K, Hosomi N, Nagai Y, et al. Cumulative effects of LDL cholesterol and CRP Levels on recurrent stroke and TIA. J Atheroscler Thromb. 2019;26(5):432–441. PMID: 30318492; PMCID: PMC6514170. doi: 10.5551/jat.45989
43. Horne BD, Anderson JL, John JM, et al. Which white blood cell subtypes predict increased cardiovascular risk? J Am Coll Cardiol. 2005;45(10):1638–1643. PMID: 15893180. doi: 10.1016/j.jacc.2005.02.054
44. Cho KH, Jeong MH, Ahmed K, et al. Value of early risk stratification using hemoglobin level and neutrophil-to-lymphocyte ratio in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am J Cardiol. 2011;107(6):849–856. PMID: 21247535. doi: 10.1016/j.amjcard.2010.10.067
45. Tamhane UU, Aneja S, Montgomery D, et al. Association between admission neutrophil to lymphocyte ratio and outcomes in patients with acute coronary syndrome. Am J Cardiol. 2008;102(6):653–657. PMID: 18773982. doi: 10.1016/j.amjcard.2008.05.006
46. Pieszko K, Hiczkiewicz J, Budzianowski P, et al. Predicting long-term mortality after acute coronary syndrome using machine learning techniques and hematological markers. Dis Markers. 2019;2019:9056402–9056409. PMID: 30838085; PMCID: PMC6374871. doi:10.1155/2019/9056402
47. Suh B, Shin DW, Kwon HM, et al. Elevated neutrophil to lymphocyte ratio and ischemic stroke risk in generally healthy adults. PLoS One. 2017;12(8):e0183706. PMID: 28829826; PMCID: PMC5567907. doi:10.1371/journal.pone.0183706
48. Xue J, Huang W, Chen X, et al. Neutrophil-to-Lymphocyte ratio is a prognostic marker in acute ischemic stroke. J Stroke Cerebrovasc Dis. 2017;26(3):650–657. PMID: 27955949. doi: 10.1016/j.jstrokecerebrovasdis.2016.11.010
49. Yu S, Arima H, Bertmar C, et al. Neutrophil to lymphocyte ratio and early clinical outcomes in patients with acute ischemic stroke. J Neurol Sci. 2018;387:115–118. PMID: 29571846. doi:10.1016/j.jns.2018.02.002
50. Brooks SD, Spears C, Cummings C, et al. Admission neutrophil-lymphocyte ratio predicts 90 day outcome after endovascular stroke therapy. J Neurointerv Surg. 2014;6(8):578–583. PMID: 24122003; PMCID: PMC4373618. doi: 10.1136/neurintsurg-2013-010780
51. Yaming S. Predictive value of NLR and MHR on clinical outcome of patients with acute ischemic stroke. Suzhou University; 2018.
52. Acharya P, Jakobleff WA, Forest SJ, et al. Fibrinogen albumin ratio and ischemic stroke during venoarterial extracorporeal membrane oxygenation. ASAIO J. 2020;66(3):277–282. PMID: 30973402; PMCID: PMC7666805. doi:10.1097/MAT.0000000000000992
53. Babu MS, Kaul S, Dadheech S, Rajeshwar K, Jyothy A, Munshi A. Serum albumin levels in ischemic stroke and its subtypes: correlation with clinical outcome. Nutrition. 2013;29(6):872–875. PMID: 23422540. doi:10.1016/j.nut.2012.12.015
54. Pascoe MC, Skoog I, Blomstrand C, et al. Albumin and depression in elderly stroke survivors: an observational cohort study. Psychiatry Res. 2015;230(2):658–663. PMID: 26520562. doi: 10.1016/j.psychres.2015.10.023
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.