Back to Journals » Open Access Rheumatology: Research and Reviews » Volume 14
The Clinical Presentation and Factors Associated with Disease Severity of Rheumatoid Arthritis in Uganda: A Cross-Sectional Study
Authors Ochola B, Nankabirwa J, Buwembo W , Kaddumukasa M , Mayanja-Kizza H
Received 7 February 2022
Accepted for publication 19 April 2022
Published 3 May 2022 Volume 2022:14 Pages 75—86
DOI https://doi.org/10.2147/OARRR.S361454
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Chuan-Ju Liu
Ben Ochola,1 Joaniter Nankabirwa,1 William Buwembo,2 Mark Kaddumukasa,1 Harriet Mayanja-Kizza1
1Department of Medicine, School of Medicine, College of Health Sciences, Makerere University, Kampala, Uganda; 2Department of Anatomy, School of Biomedical Sciences, College of Health Sciences, Makerere University, Kampala, Uganda
Correspondence: Ben Ochola, Email [email protected]
Background: Rheumatoid arthritis (RA) is a chronic, debilitating disease that leads to joint destruction and disability if left untreated. Few studies on RA have been conducted in Uganda, and there is limited information on disease severity and associated factors. This study sought to characterize the clinical presentation and to determine disease severity and the factors associated with disease severity among participants with RA in Uganda.
Methods: Between August 2018 and February 2019, patients presenting to the rheumatology outpatient clinic in Mulago National Referral Hospital were enrolled into the study using a cross-sectional design. Participants’ demographics and clinical characteristics were collected using a study questionnaire, and laboratory results were extracted from their charts. The patients’ functionality was assessed using the Modified Health Assessment Questionnaire and their disease severity was assessed using the RA Disease Activity Score based on 28-joint count (DAS28).
Results: A total of 170 participants were enrolled, of whom 81.2% were female. Nearly two-thirds (111/170) were classified as having severe disease. Having a functional status score of > 0.5 (adjusted odds ratio 1.7, 95% confidence interval 1.4– 2.2, p< 0.001) was significantly associated with severe disease.
Conclusion: In this population, the majority of the patients seen at the rheumatology outpatient clinic had severe disease, suggesting that patients may be presenting late, with limited early detection of the disease. Impaired functional status was associated with increased disease severity and may be used by clinicians to highlight disease severity when it is not possible to assess the RA DAS28.
Keywords: rheumatoid arthritis, disease severity, Uganda
Background
Rheumatoid arthritis (RA) is a symmetrical, persistent and destructive chronic inflammatory disease of connective tissue that predominantly affects the synovial membrane of freely movable joints, causing pain and deformity.1 RA has been noted as a major cause of inflammatory disability. Early detection of disease and initiation of disease-modifying drugs can reduce the progression of disease to severe states.2 Earlier studies showed that patients with RA in Uganda tend to have a relatively mild disease compared to those in other countries. However, more recent observations suggest that patients currently seen at Mulago rheumatology clinic present late and with severe debilitating disease. Although a number of reports have noted this observation, no study has documented the current RA disease profile among patients presenting to the rheumatology clinics in Uganda. Understanding the clinical profile of RA is needed to guide its early identification and initiation of treatment in order to reduce the long-term effects of the disease.
We describe the clinical presentation, disease severity and factors associated with severe RA disease severity among patients attending the rheumatology clinic at Mulago National Referral Hospital (MNRH), Kampala, Uganda. Results from this study will be critical in formulating appropriate treatment guidelines for our population.
Methods
This study aimed to characterize the clinical presentation of RA and to describe the factors associated with disease severity among patients with RA in Uganda.
The study was approved by the Makerere University College of Health Sciences School of Medicine research and ethics committee (# REC REF 2018-109).
Study Design and Setting
This was a cross-sectional study conducted among patients with confirmed RA attending the rheumatology outpatient clinic of MNRH, Kampala, Uganda, between August 2018 and February 2019. Participants were consecutively recruited from the clinic. The clinic has over 2000 registered patients who attend for different purposes, including clinical monitoring, drug refills, and disease and drug toxicity monitoring.
The clinic serves as a referral clinic for health units serving MNRH. The clinic is the largest in the country and serves as a national referral center for all rheumatoid patients across the country. The clinic is run by an attending rheumatologist.
Study Population
All patients attending the rheumatology adult clinic between August 2018 and February 2019 were eligible for inclusion in the study if they: 1) had a confirmed diagnosis of RA based on the 2010 American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) classification criteria; 2) were aged 18 years and above, and 3) provided written informed consent to participate in the study. Patients were excluded if they had mixed connective tissue disease and undifferentiated arthritis.
Data Collection
Using a structured pretested questionnaire (developed for this study), socio-demographic data, including age, sex, marital status, place of origin, level of education, and alcohol plus smoking history was collected. In addition, clinical data on key symptoms for RA, including joint pain and swelling, morning stiffness, fever and weight loss pattern, duration of disease since diagnosis, current medication, history of family members with similar illness, and presence of any other chronic illnesses, were collected. A focused clinical examination was conducted identifying relevant clinical signs, the pattern of joint involvement, extra-articular manifestations and complications of the disease.
Laboratory data for rheumatoid factor (RF), anti-cyclic citrullinated peptide (anti-CCP), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR) levels, and platelet and hemoglobin count were collected during the study. RF was reported qualitatively as positive or negative, while anti-CCP was considered positive if the antibody titers were >7 IU/mL. CRP was measured using the AQT90 immunoassay analyzer, and categorized as elevated or normal, with <3 mg/L indicating normal levels for whole blood. The ESR was considered elevated for individuals aged >50 years if it was >30 mm/h for females and >20 mm/h for males. For those aged ≤50 years, the ESR was considered elevated at >20 mm/h in females and >15 mm/h in males.3
Hemoglobin levels were classified according to the World Health Organization (WHO) guidelines as: mild anemia 11.0–13.0 g/dL (male) and 11.0–12 g/dL (female); moderate anemia 8.0–11.0 g/dL (both sexes); or severe anemia <8.0 g/dL (both sexes). The platelet count was considered normal between 150 and 450×109 cells/mL.3
Body mass index (BMI) was calculated using the formula BMI= Weight (kg)/height2 (m). A BMI <18.5 kg/m2 was considered underweight, 18.5–24.9 kg/m2 was considered normal and 25–29.9 kg/m2 was considered overweight.4
Performance disability was assessed using the Modified Health Assessment Questionnaire (MHAQ), validated for all populations with RA. The MHAQ total score ranges between 0.0 and 3.0. The MHAQ scores are categorized as mild (MHAQ <1.3), moderate (1.3< MHAQ <1.8) and severe (MHAQ >1.8).5 The frequencies of the participants’ scores were determined with the average score being 0.5, and this was used to categorize each participant’s scores as either above or below. The questionnaire includes questions on the ease with which participants performed daily activities, which assesses patient disability, and questions regarding perceived patient satisfaction with the same activities of daily living, along with perceived change in degree of difficulty. Fatigue was assessed using the Bristol Rheumatoid Arthritis Fatigue Numerical Rating Scales (BRAF-NRS), with participants scoring a total average score of 5 or more considered to be fatigued.6
Disease severity was assessed using the RA Disease Activity Score based on 28-joint count (DAS28), which has been validated for classifying disease.7 The DAS28 is calculated using the formula DAS28 = 0.56 × √(Tender joint count 28) + 0.28 × √(Swollen joint count 28) + 0.7 × In (Erythrocyte sedimentation rate) + 0.014 × (Global health).8 A low DAS28 (<2.6) means disease remission while a high score (>5.1) means severe disease. A score ≥2.6 but <3.2 means low disease and ≥3.2 but ≤5.1 signifies moderate disease.
Data Analysis
Data were entered into EpiData, and exported into STATA version 14.0 (STATA Corp., Texas, USA) for analysis. Continuous data were summarized as means with standard deviation for normally distributed data and medians with interquartile ranges (IQRs) for non-normally distributed data. Categorical data were summarized as frequencies and percentages.
RF was summarized as categorical data because qualitative data were collected. Participants with severe disease were determined as a proportion, defined as the number with DAS28 >5.1 divided by the total number of participants and expressed with its 95% confidence interval (CI). Logistic regression was performed to determine factors associated with severe disease among the study participants. The exploratory variables included the socio- demographic factors, clinical characteristics and laboratory characteristics. The outcome variable was having severe disease, defined as DAS28 >5.1. In multivariable analysis, all the factors in the bivariate analysis were considered in the model. Confounding factors, including educational level and BMI, were left in the final model despite having a p-value >0.05. In addition, variables such as anti-CCP levels, RF, use of conventional medicine and disease duration were left in the model owing to biological plausibility.
Results
Baseline Characteristics of the Study Population
In total, 175 participants were identified as having confirmed RA; however, two were excluded for being aged below 18 years and three for having mixed connective tissue disease. A total of 170 participants were therefore enrolled in this study. The median age of the participants was 43.5 (IQR 32–55) years and the majority of the participants were females, 138 (81.2%). Nearly 42% (n=71) had received education to primary level, while 34.1% (n=58) and 21.1% (n=36) had secondary and tertiary level education, respectively. Only 8.8% (n=15) reported a history of smoking and 31.2% (n=53) reported using alcohol. Only 7% of the study participants reported an immediate family member with a similar illness. The majority of the study participants were from the central region, while 20.6% (35/170), 9.4% and 9.4% were from the western, eastern and northern regions, respectively.
Of the 170 participants, 56.5% (n=96) had a coexisting comorbid illness, with hypertension being the commonest (39.6%).
The majority of the participants were receiving conventional disease-modifying anti-rheumatic drugs (DMARDs), 69% (118) for their RA. Among those on DMARDs, 95% (116/118) were taking only one DMARD, 2.5% (3/118) were on dual therapy and none was on triple therapy. Three study participants had received biologics. The majority of the study participants, 81% (134/170), were receiving oral prednisolone at a median dose of 10 mg daily (range 5–20 mg). Table 1 shows the demographic and clinical characteristics of the study population.
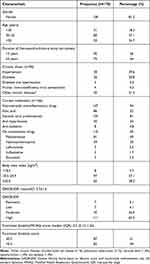 |
Table 1 Socio-Demographic Characteristics of the Study Participants |
Laboratory Characteristics
Nearly 90% (147/164) of the study participants had a positive RF test. The median (IQR) anti-CCP titers were 154 (26.5–580) IU/mL and the median (IQR) ESR was 42 (29–70) mm/h. Among those who had a CRP test carried out, 92.2% (71/77) had elevated levels. Table 2 provides details of the laboratory characteristics of the study participants.
 |
Table 2 Laboratory Characteristics of the Study Participants |
Clinical Characterization of Rheumatoid Arthritis
Small joints (metacarpophalangeal [MCP] and proximal interphalangeal [PIP]) of the hands were the most commonly involved joints among the study participants. About six percent (10/170) had subcutaneous nodules on the extensor surfaces while 15.3% (26/170) had associated deformities such as swan neck deformity, ulnar deviation and subluxation at the wrist joint. About 62.9% (107/170) of the study participants presented with morning stiffness greater than 30 minutes, while 5.9% (10/170) and 5.9% presented with sicca symptoms and fatigue, respectively. Table 3 shows the pattern of joint involvement.
 |
Table 3 Clinical Characteristics of Rheumatoid Arthritis by Joint Involvement |
Disease Severity of Study Participants
Sixty five percent (111/170) of the study participants had a high DAS28 signifying severe disease and only 4% (7/170) had disease remission based on the DAS28. Figure 1 shows the percentages of disease severity based on the DAS28 across the study participants.
 |
Figure 1 Disease Activity Scores based on 28-joint count and erythrocyte sedimentation rate (DAS28-ESR) among the study participants. |
Factors Associated with Severe Disease
Socio-Demographic and Clinical Factors Associated with Disease Severity
None of the socio-demographic factors was associated with disease severity. The presence of a coexisting chronic illness, functional disability >0.5, duration of RA and an elevated ESR were independently associated with disease severity in bivariate analysis, with p-values of 0.031, <0.001, <0.001 and 0.048, respectively. Educational level attained, presence of a family member with similar illness and use of conventional medicine were not associated with disease severity, with p-values of 0.083, 0.905 and 0.159, respectively. Other factors, such as the use of conventional DMARDs (odds ratio [OR] 0.59, 95% CI 0.3–1.2) and elevated anti-CCP titers (OR 1.2, 95% CI 0.5–3.0) were not associated with disease severity, as shown in Table 4.
 |
Table 4 Clinical and Laboratory Factors Associated with Disease Severity |
Multivariate Analysis for Factors Associated with Severe Disease
The study participants with a functional disability score of >0.5 had higher odds of developing severe disease (adjusted odds ratio [aOR] 1.7, 95% CI 1.4−2.2, p<0.001). The duration of RA at the time of recruitment was also associated with higher odds of developing severe disease (aOR 0.9, 95% CI 0.8−0.9, p=0.004). Educational level attained, history of cigarette smoking, high titers of anti-CCP and ESR were not associated with severe disease. Table 5 summarizes the multivariate analysis of factors associated with severe disease.
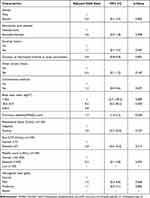 |
Table 5 Multivariable Logistic Analysis for Factors Associated with Disease Severity |
Discussion
This study set out to characterize the clinical presentation of RA and to determine the socio-demographic, clinical and laboratory factors associated with disease severity in RA. We report that the disease commonly involved the small joints of the hands and that more than half of the study participants had severe RA based on the DAS28-ESR. In our study, a poor functional status score (>0.5) was associated with severe disease.
In our study, the average age of participants was 43.5 years, lower than their European counterparts, whose average age was 55 years,12 which could be explained by the generally young population in sub-Saharan Africa, with Uganda having a median age of 16.7 years.9
Participants predominantly presented with joint pain and swelling, which commonly involved the small joints of the hands, namely the wrist, MCP and PIP joints, but multiple joint involvement was also noticed.
This is because inflammation of the synovia extends to involve the articular cartilage.10,11 These findings are consistent with findings from an earlier study on RA in Uganda by Kanyerezi and Lutalo, which demonstrated that small joints were the most commonly involved in the disease.12 These findings show that the majority of the patients in our setting present with early disease, as demonstrated by Fleming et al in a study on the pattern of joint involvement in early RA.13
Late manifestations of disease in the form of skeletal deformities were observed in 21% of the study participants. An earlier Ugandan study demonstrated a higher percentage of late manifestations of the disease.12 For this study, we used the 2010 ACR/EULAR criteria for classification of the disease, whereas the earlier studies used the Rome diagnostic criteria, which distinguished established RA patients with complications such as subcutaneous nodules.14 Improvements in diagnostics and medical training have probably also made it easier to improve early detection, early diagnosis and institution of treatment during early RA, before the onset of complications. In the previous Ugandan study, radiological changes in late disease were observed in 46 of the 76 participants,12 and in 51 of 76 participants in a Kenyan study.15 Subcutaneous nodules over the extensor surface of the hand, seen in 6% of our study participants, are a common feature in late RA, as reported in several other studies; they were identified in 9% of the participants in Uganda,12 4.8% of participants in Burkina Faso16 and 32% of participants in Kenya.15 Our findings on morning stiffness (62% of participants) are comparable to other study findings in African17 and white populations,18 which demonstrated morning stiffness to be a major finding in RA. Sicca symptoms (decreased tears, dry mouth, thirsty sensation), reported by 12% of the participants in this study, are a prevalent extra-articular manifestation of RA.19 They have been described as one of the most frequent extra-articular manifestations observed in Africans with RA, as described by Ndongo et al in a West African study, which found sicca symptoms in almost 40% of the population with RA,17 whereas a large cohort study found sicca symptoms to be less prevalent among white populations.20 As in other African studies, fatigue was not a major symptom, with only 6% of participants in this study having it. A study in Nigeria21 described fatigue in less than 10% of participants. However, it has been a major finding in white populations; a British population study found 41% of patients with RA to have fatigue.22
The findings of this study indicate that patients with RA in our setting have severe disease. We found that more than half (65.3%) of our study participants had severe disease based on the DAS28. Our study was conducted at the national referral hospital with the only attending rheumatologist in the country. It is possible that only severe cases are referred to this clinic, and this may contribute to the high percentage of participants with severe disease that we enrolled into the study. There may also be challenges and delays in making the diagnosis of early RA and initiating treatment, and hence patients present late with severe disease. Unfortunately, our study did not determine the delay in initiating treatment, but it is probably way beyond the recommended window of opportunity. Studies that have described delays in initiating methotrexate from the onset of symptoms in high-income countries varied from 125 to 205 days.23–25 There are limited data from sub-Saharan Africa regarding delay in diagnosis and initiation of treatment with DMARDs. Earlier research reported milder forms of the disease with low morbidity and low seropositivity in Uganda,12 but later studies from African settings indicate severe disease with high rates of seropositivity and extra-articular manifestations.17 The prevalence of RA remission in our setting was low, with only 4.1% of the study participants achieving remission. This could be attributed to health-are system challenges in RA diagnosis and treatment, limited rheumatological services countrywide and the challenges of maintaining regular supplies of DMARDs, as these are purchased out of pocket by the patients. These findings may be attributed to improvements in the diagnosis and detection of RA in populations. In one study on the severity of RA in sub-Saharan Africa, 71% of cases had severe disease,26 and in another population-based study conducted in Congo,27 the mean disease severity as assessed by DAS28 was high. Disease severity findings in western populations are similar to our study findings, with the majority of patients having severe disease. In the ESPOIR (Étude et Suivi des POlyarthrites Indifférenciées Récentes) cohort, most participants had severe disease; however, they had a higher remission rate, which may be attributed to better access to care and the availability of better drugs.28,29
Participants with a functional status score >0.5, as determined by the MHAQ, had higher (20-fold) odds of developing severe disease. A high functional status score has been related to the pain and swelling from the inflammatory process of RA that create difficulty in the execution of daily activities.30 This finding is similar to what was seen in the West African study by Ndongo et al17 and in the Mexican study by Glave-Testino et al.31 A longer duration of RA was associated with individuals having severe disease. This might be explained by late presentation for care, delayed initiation of DMARDs, longer DMARD exposure and patients not responding to treatment as well as those with early disease. This may also emphasize the window of opportunity for effective treatment of RA during the early stages of the disease.32,33
Conclusions
Few patients with RA achieved remission in our settings and those having impaired functional status were associated with having severe RA disease. Additional studies with more patients and including predictors of response may be needed to elucidate this subject further.
Study Limitations
In this study, we did not perform quantitative measurements of RF despite its relative importance in determining its association with severe disease. Less than half of the participants had a CRP carried out; this could have been used as an alternative way to determine disease severity. CRP has been more standardized compared to ESR for the assessment of severity in the DAS28. The small sample size numbers for some of the study parameters may have influenced the robustness of the study; hence, a well-designed study may be needed to further explore this issue. There may have been a referral bias in determining the severity of disease as patients with severe disease are likely to be referred to MNRH. Disease severity was assessed once at the point of initial contact; this did not give us the opportunity to assess the interaction of disease with disease-specific drugs.
Study Strength
Standardized tools were used to determine disease severity and functional disability.
Abbreviations
RA, rheumatoid arthritis; MNRH, Mulago National Referral Hospital; ACR, American College of Rheumatology; EULAR, European League Against Rheumatism; RF, rheumatoid factor; anti-CCP, anti-cyclic citrullinated peptide; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; BMI, body mass index; MHAQ, Modified Health Assessment Questionnaire; DAS28, Disease Activity Score based on 28-joint count; IQR, interquartile range; CI, confidence interval; DMARD, disease-modifying anti-rheumatic drug; MCP, metacarpopharyngeal; PIP, proximal interphalangeal; odds ratio; aOR, adjusted odds ratio.
Data Sharing Statement
The original data set used and analyzed during the study is available from the corresponding author on reasonable request.
Ethical Approval and Consent to Participate
The study complied with the Declaration of Helsinki and was approved by the Makerere University College of Health Sciences School of Medicine research and ethics committee (# REC REF 2018-109). Participants were asked to give their written consent, and their identities were protected by identifying them using study numbers.
Consent for Publication
Publication was approved by all participants, who gave their consent to participate in the study.
Acknowledgments
We would like to thank all patients, especially those who accepted the invitation to participate in this study. We are also grateful to the Mulago National Referral Hospital outpatient staff, who made the working environment conducive to the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The corresponding author declares that there was no funding received for the study. Mark Kaddumukasa receives support from the National Institutes Health (K43TW010401) NINDS and Fogarty International Center (FIC). The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Disclosure
The authors declare that they have no competing interests.
References
1. Smolen JS, Landewe RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79(6):685–699. doi:10.1136/annrheumdis-2019-216655
2. van Dongen H, van Aken J, Lard LR, et al. Efficacy of methotrexate treatment in patients with probable rheumatoid arthritis: a double-blind, randomized, placebo-controlled trial. Arthritis Rheum. 2007;56(5):1424–1432. doi:10.1002/art.22525
3. World Health Organization. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity; 2011.
4. World Health Organization. Obesity and overweight. Fact Sheet No 3112013; 2021.
5. Pincus TSJ, Soraci SA
6. Hewlett S, Kirwan J, Bode C, et al. The revised Bristol Rheumatoid Arthritis Fatigue measures and the rheumatoid arthritis impact of disease scale: validation in six countries. Rheumatology. 2018;57(2):300–308. doi:10.1093/rheumatology/kex370
7. Prevoo ML, van ‘t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38(1):44–48. doi:10.1002/art.1780380107
8. Fransen J, Van Riel P. The Disease Activity Score and the EULAR response criteria. Clin Exp Rheumatol. 2005;23(5):S93.
9. Uganda Bureau of Statistics (UBOS), 2018. Uganda National Household Survey 2016/2017. Kampala, Uganda, UBOS. Available from: https://www.ubos.org/wp-content/uploads/publications/03_20182016_UNHS_FINAL_REPORT.pdf. Accessed April 29, 2022.
10. Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423(6937):356–361. doi:10.1038/nature01661
11. Smolen JS, Aletaha D, Koeller M, Weisman MH, Emery P. New therapies for treatment of rheumatoid arthritis. Lancet. 2007;370(9602):1861–1874. doi:10.1016/S0140-6736(07)60784-3
12. Kanyerezi BR, Lutalo SK. Some aspects of rheumatoid disease in Uganda. East Afr Med J. 1980;57(1):39–43.
13. Fleming A, Benn RT, Corbett M, Wood PH. Early rheumatoid disease. II. Patterns of joint involvement. Ann Rheum Dis. 1976;35(4):361–364. doi:10.1136/ard.35.4.361
14. Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–2581. doi:10.1002/art.27584
15. Bagg LR, Hansen DP, Lewis C, Houba V. Rheumatoid arthritis in Kenya. I. Clinical observations. Ann Rheum Dis. 1979;38(1):23–25. doi:10.1136/ard.38.1.23
16. Ouedraogo DD, Singbo J, Diallo O, Sawadogo SA, Tieno H, Drabo YJ. Rheumatoid arthritis in Burkina Faso: clinical and serological profiles. Clin Rheumatol. 2011;30(12):1617–1621. doi:10.1007/s10067-011-1831-1
17. Ndongo S, Lekpa FK, Ka MM, Ndiaye N, Diop TM. Presentation and severity of rheumatoid arthritis at diagnosis in Senegal. Rheumatology. 2009;48(9):1111–1113. doi:10.1093/rheumatology/kep178
18. Yazici Y, Pincus T, Kautiainen H, Sokka T. Morning stiffness in patients with early rheumatoid arthritis is associated more strongly with functional disability than with joint swelling and erythrocyte sedimentation rate. J Rheumatol. 2004;31(9):1723–1726.
19. Cojocaru M, Cojocaru IM, Silosi I, Vrabie CD, Tanasescu R. Extra-articular Manifestations in Rheumatoid Arthritis. Maedica. 2010;5(4):286–291.
20. Richman NC, Yazdany J, Graf J, Chernitskiy V, Imboden JB. Extraarticular manifestations of rheumatoid arthritis in a multiethnic cohort of predominantly Hispanic and Asian patients. Medicine. 2013;92(2):92–97. doi:10.1097/MD.0b013e318289ce01
21. Ohagwu KOH, Oba R, Adelowo O. Pattern of rheumatoid arthritis in Nigeria; study of patients from a teaching hospital. Afr j Rheumatol. 2017;5(2):45–49.
22. Wolfe F, Hawley DJ, Wilson K. The prevalence and meaning of fatigue in rheumatic disease. J Rheumatol. 1996;23(8):1407–1417.
23. Versteeg GA, Steunebrink LMM, Vonkeman HE, Ten Klooster PM, van der Bijl AE, van de Laar M. Long-term disease and patient-reported outcomes of a continuous treat-to-target approach in patients with early rheumatoid arthritis in daily clinical practice. Clin Rheumatol. 2018;37(5):1189–1197. doi:10.1007/s10067-017-3962-5
24. Kimsey L, Weissman JS, Patel A, Drew A, Koehlmoos T, Sparks JA. Delay in initiation of DMARD or anti-inflammatory therapy in patients newly diagnosed with rheumatoid arthritis: an analysis of United States Military Health System TRICARE beneficiaries. Semin Arthritis Rheum. 2019;48(5):821–827. doi:10.1016/j.semarthrit.2018.07.003
25. Benaglio F, Fornaro M, Montecucco C, et al. Methotrexate in Italian patients wiTh Rheumatoid Arthritis (MITRA study): an observational study about the use of methotrexate in early RA patients and the adherence to the EULAR 2013 recommendations. A project of the Italian Society for Rheumatology. Clin Exp Rheumatol. 2021;39(5):1077–1084.
26. Niasse M, Kane BS, Ndiaye AA, et al. Severity of the rheumatoid arthritis in Sub-Saharan Africa: study of 403 senegalese observations. Open J Intern Med. 2017;7(04):151. doi:10.4236/ojim.2017.74016
27. Malemba JJ, Mbuyi-Muamba JM, Mukaya J, Bossuyt X, Verschueren P, Westhovens R. The epidemiology of rheumatoid arthritis in Kinshasa, Democratic Republic Of Congo—a population-based study. Rheumatology. 2012;51(9):1644–1647. doi:10.1093/rheumatology/kes092
28. Combe B, Landewé R, Lukas C, et al. EULAR recommendations for the management of early arthritis: report of a task force of the European Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis. 2007;66(1):34–45. doi:10.1136/ard.2005.044354
29. Lukas C, Combe B, Ravaud P, Sibilia J, Landew R, Van Der Heijde D. Favorable effect of very early disease‐modifying antirheumatic drug treatment on radiographic progression in early inflammatory arthritis: data from the Étude et Suivi des Polyarthrites IndifféRenciées récentes (study and followup of early undifferentiated polyarthritis). Arthritis Rheum. 2011;63(7):1804–1811.
30. Altinkesen E, Gelecek N. Functional status and quality of life in patients with early and late stage rheumatoid arthritis. Fiz Rehabil. 2011;22(2):93–99.
31. Glave-Testino C, Cardiel M, Arce-Salinas A, Alarcon-Segovia D. Factors associated with disease severity in Mexican patients with rheumatoid arthritis. Clin Exp Rheumatol. 1994;12(6):589–594.
32. Naeem F, Khan SEA, Saeed MA, Farman S. Diagnostic and therapeutic delay in Rheumatoid Arthritis patients: impact on disease outcome. Pak J Med Sci. 2021;37(4):1001–1007. doi:10.12669/pjms.37.4.3471
33. Anderson JJ, Wells G, Verhoeven AC, Felson DT. Factors predicting response to treatment in rheumatoid arthritis: the importance of disease duration. Arthritis Rheum. 2000;43(1):22–29. doi:10.1002/1529-0131(200001)43:1<22::AID-ANR4>3.0.CO;2-9
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
