Back to Journals » Infection and Drug Resistance » Volume 16
The Clinical Impact of Metagenomic Next-Generation Sequencing for the Diagnosis of Periprosthetic Joint Infection
Authors Li H, Niu E, Fu J, Huang Y, Gao Y, Chai W, Chen J
Received 7 May 2023
Accepted for publication 21 September 2023
Published 2 October 2023 Volume 2023:16 Pages 6521—6533
DOI https://doi.org/10.2147/IDR.S420325
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Hao Li,1,2 Erlong Niu,3 Jun Fu,2 Yinghao Huang,4 Yang Gao,2 Wei Chai,2,* Jiying Chen2,*
1Medical School of Chinese PLA, Chinese PLA General Hospital, Beijing, People’s Republic of China; 2Department of Orthopedic, Chinese PLA General Hospital, Beijing, People’s Republic of China; 3Department of Orthopedics, 305 Hospital of PLA, Beijing, People’s Republic of China; 4School of Computer and Information Technology, Beijing Jiaotong University, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jiying Chen; Wei Chai, Department of Orthopedic, Chinese PLA General Hospital, 28 Fuxing Road, Beijing, 100000, People’s Republic of China, Email [email protected]; [email protected]
Background: Synovial fluid metagenomic next-generation sequencing was introduced into the diagnosis of periprosthetic joint infection (PJI) in recent years. However, the clinical impact of mNGS remains unknown. Therefore, we performed a prospective cohort study to evaluate the clinical impact of mNGS for PJI diagnosis.
Materials and Methods: Between April 2019 and April 2021, a total of 201 patients with suspected PJI were recruited in a high-volume PJI revision center. All patients underwent joint aspiration before surgeries and the obtained synovial fluids were sent to tests for the diagnosis of PJI. Based on the clinical evaluation of these patients, the patients were categorized into three groups: Group A: the mNGS reports were not acted upon. Group B: mNGS confirmed the standard diagnostic tests of PJI and generated identical clinical impact compared to standard diagnostic tests. Group C: mNGS results guided clinical therapy. Then, the concordance between synovial mNGS and cultures was analyzed. After that, multivariate regressions were performed to explore the “targeted populations” of mNGS tests.
Results: A total of 107 patients were diagnosed with PJI based on the 2014 MSIS criteria and there were 33, 123, 45 patients in the group A, B, C respectively. The predictive factors of mNGS inducing clinical impact compared to standard diagnostic tests were negative culture results (adjusted OR: 5.88), previous history of joint infection (adjusted OR: 5.97), polymicrobial PJI revealed by culture (adjusted OR: 4.39) and PJI identified by MSIS criteria (adjusted OR: 17.06).
Conclusion: When standard diagnostic tests for PJI were performed, about 22% of synovial fluid mNGS tests can change the treatment protocols built on standard diagnostic tests and affect the clinical practice. Thus, the use of synovial fluid mNGS in some “target” populations is more valuable compared to others such as patients with previous joint infection, polymicrobial PJI, and culture-negative PJI.
Evidence Level: Level I.
Keywords: periprosthetic joint infection, metagenomic next-generation sequencing, clinical impact
Introduction
The total number of periprosthetic joint infection cases is increasing with the surging numbers of joint arthroplasties, internal fixation implantation and the use of immunosuppressive agents.1,2 Unfortunately, the identification of corresponding pathogens remains time-consuming and challenging because of inert bacteria, biofilm formation and prior antibiotic administration.3–8
Metagenomic next-generation sequencing (mNGS) is a promising unbiased approach for infection diagnosis and a broad spectrum of microorganisms (bacteria, fungi, and virus) can be identified by a single mNGS test.5,9,10 In recent years, the use of mNGS is emerging and several studies revealed that mNGS can detect pathogens from synovial fluid timely and precisely.5,11,12 However, some problems were encountered when mNGS was applied to clinical practice. Firstly, mNGS tests often revealed more than one pathogen compared to cultures but the clinical impact of these discordant pathogens remains controversial.9,13 Secondly, little is known about the real clinical impact of mNGS tests when applied into clinical practice, especially periprosthetic joint infection. Thirdly, the cost of the mNGS test is higher than any single standard test of periprosthetic joint infection (about 480$ in China). Considering its high cost and the clinical impact of mNGS, it may be not suitable to apply this technique in every patient with suspected periprosthetic joint infection.13
To our knowledge, there was still no comprehensive study that evaluated the clinical impact of synovial fluid mNGS and explored the scope of its application in the PJI field.12,14,15 To address the problems mentioned above and evaluate the real clinical impact of metagenomic next generation when applied to routine clinical practice for the diagnosis of periprosthetic joint infection, a prospective study was performed in a tertiary high-volume PJI revision center. This study was designed to 1) evaluate the clinical diagnostic performance and 2) explore the real clinical impact of mNGS on clinical practice. We hypothesize that the clinical impact generated from mNGS tests can vary in different populations when applied into clinical practice and adding mNGS into standard diagnostic tests is more likely to change the clinical decision in some “high-risk” populations. Based on the study, we try to answer the question: in which populations are mNGS tests more appropriate?
Materials and Methods
Study Design
The approval of the Institutional review board (ChiCTR1900025683) and the local Ethics Committee (S2018-209-1) were obtained prior to the commencement of this study and then, this single-center, prospective cohort study was performed in a tertiary joint center. In this study, the suspected periprosthetic joint infection patients with enough synovial fluid for mNGS and standard diagnostic tests (After standard diagnostic tests for PJI, there was sufficient synovial fluid (>0.5mL) to perform mNGS tests.) were recruited prospectively.
Based on the institutional protocol, infection was considered when a patient had one of the following signs or symptoms after total joint arthroplasty.
- Acute or persistent pain at rest, swelling, redness, or warmth around the joints;
- Elevated ESR or CRP level; or
- Implant failure within 5 years after total joint arthroplasty without any reasonable explanation.
All patients provided written informed consent before the mNGS tests. Between April 2019 and April 2021, a total of 201 patients were included in this study finally. The post-hoc power-analysis were performed and the corresponding β was calculated. The study has about 100% power, at an alpha of 0.05 and a prevalence of 29.8% (detected factor), to identify a >5.8 OR in the logistic regression.
For the diagnosis of periprosthetic joint infection, the index test was mNGS assay and the standard diagnostic tests of periprosthetic joint infection (including cultures, synovial fluid analysis and histological analysis) were based on the 2014 MSIS criteria (Table 1).
 |
Table 1 The 2014 MSIS Criteria Defined Infection Exists When |
Infection Definition and Clinical Evaluation
The clinical diagnosis and evaluation were performed by an experienced medical team (including orthopedic surgeons, clinical microbiologists, and treating physicians) according to the MSIS criteria (Table 1).16,17 In this study, PJI patients were confirmed by the MSIS criteria. And the non-PJI group was composed of patients who underwent one-stage aseptic revisions. The one-stage revisions did not fail because of any aseptic reasons such as loosening and mechanical complications within at least 6-month follow-up after one-stage revision.
After that, the clinical impact of the mNGS assay was evaluated according to the following criteria:
Group A: The mNGS reports were not acted upon and the treatment protocol was generated from standard diagnostic tests only. This group was defined according to the following criteria:
- mNGS results were deemed false or insignificant by the medical team.
- No additional pathogen was detected by mNGS compared to standard diagnostic tests.
Group B: The mNGS test confirmed the results of standard diagnostic tests and generated identical clinical impact compared to standard diagnostic tests.
Group C: Compared to cultures, mNGS detected new pathogens with high relevance to clinical manifestations, and treatment protocol was adjusted upon these “new” pathogens. Compared to standard diagnostic tests, mNGS adjusted treatment protocol that led to favorable clinical outcomes.
Any discrepancies in the assignment of diagnosis and treatment were resolved by communication within the medical team.
The Diagnostic Tests of Periprosthetic Joint Infection
In our institution, ESR and serum CRP was used to screen for PJI. Preoperative joint aspiration was performed in these patients to obtain synovial fluid and then the diagnostic workup was initiated. Preoperative joint aspiration was performed in these patients to obtain synovial fluid and then, the diagnostic workup was initiated. In this study, the joint aspiration was performed by two experienced surgeons to obtained synovial fluid for analysis according to methods described previously.18 And all synovial fluid was obtained before the management surgeries of periprosthetic joint infection. After joint aspiration, the collected synovial fluid was shipped for mNGS test, Leukocyte esterase (LE) test, synovial fluid analysis (WBC count, PMN%), and cultures (anaerobic bacterial culture, aerobic bacterial and fungal culture) within 3 hours. If PJI cannot be diagnosed based on the 2014 Musculoskeletal Infection Society (MSIS) criteria before joint revisions, intraoperative tissue histology was performed to further clarify the existence of infection when the joint was revised. Any patient with infection in whose joint more than 2 different organisms were isolated from the synovial fluid or deep-tissue cultures was considered to have polymicrobial periprosthetic joint infection.19
Sample Sequencing and Data Analysis
Volumes of at least 0.5 mL samples were collected from the subjects. To improve the efficiency of pathogen detection, the samples were firstly enriched into small solutions (~200 μL) centrifuged at 15,000 g for 10 min at 4 °C. Then 200 μL of enrichment solutions were used for nucleic acid extraction and purification with nucleic acid extraction kit combined with magnetic beads (Sagene, Guangzhou, CHINA) as directed by the manufacturer. And adsorption column method (Sagene, Guangzhou, China), and the metagenomic library was respectively constructed according to the protocol of the library construction Kit, Nextera XT (Illumina, USA), the extracted DNA was firstly broken into ~300 bp fragments and, followed by different index sequences added. The library size and quantification were analyzed using Agilent 2100 bioanalyzer system, and the accurate quantification was detected once again by qPCR (Bio-Rad CFX96, USA). After the libraries were mixed with equal amounts, high-throughput sequencing was performed on Illumina Nextseq 550 DX sequencing platform (sequencing strategy: PE150), an FDA-approved and CE-IVD-certified sequencer. Moreover, the negative control groups were set during sequencing in a bid to manage the DNA contamination during library construction and sequencing. In this study, we used saline as a negative control. Nucleic acid extraction, library construction and sequencing were performed parallelly for the same batch of samples, including negative control and clinical samples.
After the sequencing was finished, raw data was basically filtered by FastQC software, including removing the reads containing the sequencing adapters, the reads containing N more than 10%, and the low-quality reads containing less than 50% of low-quality bases (Q-value≤10). The remained reads were used for the next step analysis. Human related reads were removed by aligning with the human genome reference sequence (version: GRCh38), using BWA (http://bio-bwa.sourceforge.net/) software, and then kraken2/bracken was used for analysis to obtain the identification and quantitative results of pathogenic microorganisms, and finally generated a clinical test report, showing the accurate and reliable micro-test detection information. The microorganisms reference databases were downloaded from NCBI (ftp://ncbi.nlm.nih.gov/genomes/). And the depth and coverage of each species were calculated with the SoapCoverage software from the SOAP website (http://soap.genomics.org.cn/).
The criteria of data filtering are as follows: (1) genome coverage; (2) comparison of multiple databases, which meet the criteria and are considered reliable; (3) filtration of common polluting bacteria. In this study, we used saline as a negative control. Nucleic acid extraction, library construction and sequencing were performed parallelly for the same batch of samples, including negative control and clinical samples. Microorganisms detected in negative controls are often considered as environmentally polluting microorganisms, and when these microorganisms are detected in the same batch of clinical samples, they are usually considered as “background microorganisms” instead of “reported PJI pathogens”, unless their relative abundance is greater than 30% in the tested samples.
Results Interpretation
The interpretation criteria used to determine the results of the mNGS test were defined via reference to our laboratory reference and previous studies.20 1) For bacteria and fungi, the relative abundance was greater than 30% at the genus level excluding Mycobacterium tuberculosis; 2) Mycobacterium tuberculosis was considered positive, once it is satisfied that at least one read was aligned to the reference genome at species or genus level, and 3) When the pathogen was detected by the traditional detection method and the mNGS reads number was more than 50 at the same time, this pathogen can also be considered to be positively detected.21
Microbiological Cultures
The obtained synovial fluid (>1mL) was injected into a BacT/ALERT FA fastidious antimicrobial neutralization (FAN) bottle (BioMerieux) for anaerobic bacterial culture and a BacT/ALERT PF Pediatric FAN (BioMerieux) bottle for aerobic bacterial and fungal culture. Each bottle was incubated for 2 weeks, and VITEK-MS (BioMerieux) was used for microorganism identification if pathogens were detected.
For periprosthetic tissue culture, Sterile glass (Ballotini) beads and 5 mL of sterile saline were added, and vigorous shaking was used to disrupt tissue and release bacteria. One-millilitre aliquots were inoculated into: Robertson’s cooked meat broth or a chocolate agar plate. The Robertson’s cooked meat broth were incubated at 37°C anaerobically. The chocolate agar plate was incubated at 37°C in air. The broth was examined daily, and terminally subcultured at 5 days or if turbid, onto chocolate agar (at 37°C in air for 48 h) and Robertson’s cooked meat broth (at 37°C anaerobically for 48 h).
If a microorganism was revealed in either the aerobic bottle or anaerobic bottle, the pathogen was recorded as part of the preoperative aspiration results. Antibiotic sensitivity tests were performed by disk diffusion according to laboratory standard protocols. In addition, two to five different intraoperative periprosthetic tissues were also sent for microbiological cultures during revisions. Only when two different samples are positive, the pathogens were considered as positive culture.
Statistical Analysis
Continuous variables were presented as medians if the normal distribution was detected. Otherwise, they were summarized as means. Categorical variables were presented as rates. Chi-squared test, Fisher’s exact test and rank-sum test were used in different types of data to detect the significant difference. To further identify the “target population” of mNGS tests, group A and group B were merged into a new group. And univariate logistic regression and multivariate logistic regression were performed in the new group and group C to identify the predictive factors of mNGS inducing clinical impact which are different from the clinical impact generated from stand diagnostic tests. Statistical significance was considered when P value<0.05. All statistical analysis was performed on SPSS (IBM Inc; Version: 22.0) and GraphPad (GraphPad Inc; version: 7.0).
Results
Patient Characteristics
Between April 2019 and April 2021, a total of 201 patients were screened for prospective enrolment in this study. Finally, the synovial fluid from all 201 patients met the enrolment criteria and completed this study (Figure 1). Using the 2014 MSIS criteria (Table 1) as the “reference standard”, there were 107 patients in the infection group and 94 patients in the non-infection cohort. The mean age of the patients in the infection group was 63.5 years, compared to 67 years in the non-infection group (P=0.724). And 45 cases in the infection group (45/107) had a history of periprosthetic joint infection in the involved joint, compared to 13 cases in the non-infection group (13/94) (P=0.001). Besides, infection laboratory results revealed that the levels of serum CRP, ESR, D-dimer, synovial fluid WBC count, and PMN% were significantly higher in the infection group than that in the non-infection group (Figures 2 and 3). Moreover, the details about the characteristics of patients included in this study was shown in Table 2.
 |
Table 2 Patient Characteristics |
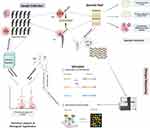 |
Figure 1 The summary of the study design. |
Performance of mNGS Relative to MSIS Criteria and the Overlap of Positivity Between mNGS and Culture for Different Pathogens
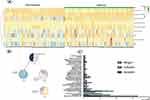
The synovial fluid from all 201 patients was analyzed by means of mNGS and the automated pipeline, as described in the methods. The mean turnaround time of mNGS was 48 hours. As shown in Figure 2, 79 patients (73.83%) were of positive mNGS results in the MSIS-confirmed infection group. And 86 patients (91.49%) were of negative mNGS results in the non-infection group. Among the microorganisms isolated, Staphylococcus epidermis (33) was the most commonly detected pathogen, followed by Staphylococcus aureus (18) and E. coli (16). Interestingly, Mycobacterium tuberculosis (MTB) (7) and nontuberculosis mycobacteria (NTM) (4) were also detected in this cohort. The details about the concordance between mNGS and the culture were shown in Figure 2. In general, the sensitivity, specificity, PPV, and NPV of synovial fluid mNGS compared to MSIS criteria was 73.83%, 91.49%, 90.80%, and 75.44%, respectively (Figure 4). Besides, the common microorganism detected in the negative controls was summarized in Supplementary Table 1.
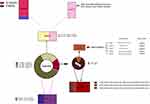 |
Figure 4 The clinical evaluation of mNGS results. |
In the comparison between synovial mNGS tests and cultures, mNGS showed promising value in detecting atypical pathogens such as MTB, NTB, and Virus (EB virus and HPV) and inert pathogens such as Staphylococcus epidermidis and Propionibacterium spp. Besides, Escherichia coli was also more likely to be detected by mNGS tests (Figure 2).
The Clinical Evaluation of mNGS Results
In group A (a total of 33 patients), the synovial fluid mNGS results were not acted upon. In 5 non-infection cases, the mNGS tests were deemed false by the medical team because there was not enough evidence to support the diagnosis of infection. In 28 infection cases, there were 5 mNGS clinical reports were deemed as false-positive because the reported pathogens were not considered clinically. And in 18 culture-negative infection cases, the mNGS clinical reports were deemed as false-negative because no microorganisms were reported by mNGS. And in 5 culture-positive cases, the mNGS reports were deemed as false-negative because the cultured pathogens were not reported by the mNGS (3 cases were missed because of the relatively low abundance and 2 cases were missed because of no detection of corresponding DNA). Besides, the mNGS tests were thought to be false-negative and clinically insignificant in 17 and 11 cases, respectively. In group B (123 patients), mNGS tests confirmed the orthogonal culture results and generated identical clinical impact. The mNGS tests further confirm and identify the pathogens revealed by synovial fluid cultures in a total of 37 (30%) patients. In contrast, the mNGS tests further confirm negative culture results and help rule out infection in a total of 86 (70%) patients. In group C (a total of 45 patients), the mNGS results were acted upon and showed valuable clinical impact compared to standard diagnostic tests. In group C, culture and mNGS were both positive in 16 patients but mNGS detected discordant pathogens compared to culture and the antibiotics were adjusted based on both culture and mNGS results. Antibiotics de-escalation was performed in 26 cases. In these cases, PJI was confirmed by the MSIS criteria but the culture results were negative. At the same time, the pathogens detected by mNGS in these culture-negative PJI cases were not considered as clinically significant, therefore the antibiotic therapy used in culture-negative PJI were de-escalated. And antibiotics escalation was performed in 19 cases because of additional pathogens detected by mNGS compared to culture and these pathogens were considered as clinically significant. Besides, in 2 cases, 2-stage septic revisions were performed because of positive mNGS tests. The details were shown in Figure 4, Table 3, and the information about the patients in group C (Supplementary Table 1) and group A (Supplementary Table 2).
 |
Table 3 Clinical Impact and Role of mNGS Result |
The Predictive Factors of mNGS Inducing Clinical Impact When mNGS Was Applied into Clinical Practice
To further explore the potential “target population” of mNGS, logistic regression was performed to evaluate the factors that may contribute to the clinical impact of mNGS potentially (Group A+B vs Group C). We found that predictive factors of mNGS inducing clinical impact compared to standard diagnostic tests were: patients with negative culture results (adjusted OR: 5.88; 95% CI: 2.08–16.60; P=0.001), previous history of periprosthetic joint infection (adjusted OR: 5.97; 95% CI: 2.25–15.85; P<0.0001), polymicrobial periprosthetic joint infection revealed by culture (adjusted OR: 4.39; 95% CI: 1.16–16.6; P=0.03) and periprosthetic joint infection identified by MSIS criteria (adjusted OR: 17.06; 95% CI: 5.31–54.78; P<0.0001) (Figure 5). The distribution of these potential predictive factors in each subgroup (A, B, and C) was further clarified in Table 3 and Figure 5.
Discussion
The use of mNGS in the diagnosis of periprosthetic joint infection is emerging but the clinical impact of mNGS remains unknown when applied to clinical practice. In this study, we evaluated the clinical impact of mNGS for diagnosing periprosthetic joint infection in a series of patients with suspected PJI, in parallel with conventional standard testing. To our knowledge, this is the first prospective study in which the clinical impact of mNGS was explored in a large cohort. In only about 22.4% of patients (group C), synovial fluid mNGS can change the established clinical treatment protocols based on the orthogonally current standard diagnostic test for periprosthetic joint infection when applied into common clinical practice. Given the current issues of cost and clinical impact, synovial fluid mNGS may not fit every patient with a suspected periprosthetic joint infection. But in selected populations such as patients with previous periprosthetic joint infection, polymicrobial periprosthetic joint infection and culture-negative periprosthetic joint infection, mNGS are more likely to change treatment decisions.
The use of synovial fluid mNGS tests in the diagnosis of periprosthetic joint infection was valuable and mNGS tests show some advantages over cultures in the identification of pathogens. Firstly, compared to the long turnaround time of cultures (2 weeks recommended by AAOS criteria), the relatively short turnaround time (24–48h) makes possible the rapid identification of pathogens and the adjustment of antibiotics in time.9,12,22 Secondly, the interaction between pathogens makes difficult the isolation of every pathogen in a single culture but the mNGS can detect these pathogens simultaneously without prior knowledge about these pathogens and the information about these pathogens can guide precise antibiotics administration.5,13 Thirdly, in culture-negative periprosthetic joint infection patients, mNGS can identify the pathogens and help clinicians further adjust antibiotics. As shown in Figure 2, mNGS showed promising value in detecting some pathogens such as MTB, NTM and other inert microorganisms. Hence, mNGS helped detect these atypical and inert pathogens when the culture result is inconclusive but infection is highly suspected.23
In group C, synovial fluid mNGS showed different clinical impacts compared to the standard diagnostic tests and the mNGS results were acted upon. In 4 cases where mNGS results were positive but there was not enough evidence to support the diagnosis of periprosthetic joint infection based on the 2014 MSIS criteria, prolonged antibiotics administration was adapted by the medical care team. In 22 patients with PJI where mNGS results were positive but the culture results were negative, the antibiotics were adjusted based on mNGS results. These patients often had a history of periprosthetic joint infection and corresponding mNGS tests were likely to be acted upon because the history of periprosthetic joint infection is a risk factor of PJI after total joint arthroplasty.
In this study, the promising fields of mNGS tests in patients with suspected periprosthetic joint infection were explored and we found that mNGS tests were more likely to be acted upon in some selected populations. Consistent with previous reports, some studies revealed that mNGS is a promising supplemental diagnostic tool in culture-negative PJI and patients with a high likelihood of PJI and our study further suggested that the mNGS results were also likely to be acted upon and affect clinical practice in these patients.1,2,23 Moreover, the patient with a previous history of joint infection and polymicrobial periprosthetic joint infection may also benefit from this test. In the patients with a previous history of joint infection (28 patients), the positive mNGS results were acted upon by the medical team because of the susception of latent periprosthetic joint infection. And in 5 patients with the previous infection, the culture results suggested new pathogens while mNGS also detected previously infected pathogens. Considering the previous history of periprosthetic joint infection and positive mNGS results, the surgery protocols changed in two patients (performing 2-staged arthroplasty instead of one stage arthroplasty) and antibiotics were adjusted based on both mNGS and standard diagnostic tests in these 28 patients. Similarly, the discordant pathogens reveal by mNGS are also likely to be acted upon in patients with polymicrobial periprosthetic joint infection. The discordant pathogens that were found by mNGS but were negative in cultures can be the result of the interaction between different pathogens.
Preestablished clinical thresholds for reporting a positive test for reporting the pathogens on synovial fluid mNGS were still intentionally conservative in order to improve the specificity of this test. In 8 of 201 cases, the pathogens were missed by mNGS owing to the low specific reads generated from sequencing. It highlighted that it would be appropriate to establish more liberal reporting threshold for high-priority pathogens than for organisms that are of unclear clinical significance such as environmental microorganisms. Modeled from the “tumor board” concept in oncology, the management team of periprosthetic joint infection can afford an opportunity to discuss the reported and supplementary results of mNGS in a clinical context, which can provide useful information to guide the selection of surgery and antibiotics beyond the straightforward reporting of a binary test result (ie “detected” or “not detected”) in mNGS.24
There are some limitations in this study. Firstly, our mNGS tests were delivered to a centralized mNGS laboratory rather than in-house labs, which may decrease the sensitivity of mNGS tests and turnaround time.5,12 Secondly, in this study, we used the 2014 MSIS criteria as the “reference standard” for PJI diagnosis because they were one of the most accepted criteria for PJI diagnosis and these criteria were used in our institution. Although some new criteria were proposed in recent years such as 2020 EBJIS criteria and 2018 ICM criteria and showed a high sensitivity for PJI diagnosis, these criteria still need further validations so these criteria were not adopted in this study. However, the difference in the “reference standard” can cause different sensitivity and specificity of mNGS tests. Therefore, the diagnostic performance of mNSG still needs exploring further when these “relatively new” criteria were used as the “reference standard”. Thirdly, this study was performed in a single tertiary transferal centre with high-volume PJI and the medical team was experienced in the management of advanced periprosthetic joint infection in China. The clinical evaluation of mNGS results was performed by an experienced medical team. However, in other centres, the mNGS results can be interpreted differently by clinicians, causing a different clinical impact and a multi-center study was still necessary to explore the suitable populations of mNGS tests. Finally, the clinical outcomes of corresponding patients were not evaluated comprehensively. The detection of microorganisms can suggest its correlation with the disease but the causal relationship between the microorganism and the disease can be difficult to discern. To some extent, the pathogens detected by mNGS can over-interpret their relationship with the diseases because of the existence of opportunistic pathogens. Moreover, the causal relationship between detected microorganisms and the disease and its role in the development of the disease indeed needs to be explored further. Our further study is necessary to evaluate whether the application of synovial mNGS can achieve better clinical outcomes in clinical practice. And in this study, only synovial fluid mNGS was used while some studies reported that the sensitivity of mNGS of ultrasonic fluid was higher than that of synovial fluid mNGS, this can be another limitation in this study and the clinical impact of ultrasonic fluid mNGS also need to be studies further.25
In conclusion, synovial fluid mNGS could emerge as an unbiased promising test to detect the pathogens in synovial fluid if the periprosthetic joint infection was highly suspected but it is not suitable for every patient and only about 22.4% of synovial fluid mNGS tests can change the treatment protocols built on the current standard diagnostic tests and affect clinical practice. Considering the clinical impact and the high cost of this test, not every mNGS test can change the clinical decision and the target populations can be selected. Therefore, we recommend the use of synovial fluid mNGS in appropriate populations such as patients with a history of periprosthetic joint infection, polymicrobial periprosthetic joint infection, and culture-negative periprosthetic joint infection.
Abbreviations
TJA, total joint arthroplasty; PJI, periprosthetic joint infection.
Data Sharing Statement
All data and materials were in full compliance with the journal’s policy. And the data were obtained in Department of Orthopedic Surgery, The First Medical Center, Chinese PLA General Hospital. The datasets used and during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This study was approved by the institutional review board of our hospitals (Chinese People’s Liberation Army General Hospital and PLA rocket force characteristics medical Center). Institutional review board approval was obtained prior to the commencement of this study and informed consents were obtained before revisions. And informed consent was obtained from the study participants prior to study commencement. This study was performed in accordance with the ethical standards in the 1964 Declaration of Helsinki.
Consent for Publication
We have obtained consent to publish from the participants.
Acknowledgments
The authors would like to thank Tao Deng for his participation in the data collection process of this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
National Key Research and Development Program of China (No.2020YFC2004900), General Program of China Postdoctoral Science Foundation (No.2020T130775), Youth Project of National Natural Science Foundation of China (No.82102585), and Military Medical Science and Technology Youth Training Project (No.21QNPY110).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Tarabichi M, Shohat N, Goswami K, et al. Diagnosis of periprosthetic joint infection: the potential of next-generation sequencing. J Bone Joint Surg Am. 2018;100(2):147–154. doi:10.2106/JBJS.17.00434
2. Tarabichi M, Shohat N, Goswami K, Parvizi J. Can next generation sequencing play a role in detecting pathogens in synovial fluid? Bone Joint J. 2018;100-b(2):127–133. doi:10.1302/0301-620X.100B2.BJJ-2017-0531.R2
3. An X, Wang J, Shi W, et al. The effect of passive smoking on early clinical outcomes after total knee arthroplasty among female patients. Risk Manag Healthc Policy. 2021;14:2407–2419. doi:10.2147/RMHP.S309893
4. Berbari EF, Marculescu C, Sia I, et al. Culture-negative prosthetic joint infection. Clin Infect Dis. 2007;45(9):1113–1119. doi:10.1086/522184
5. Chiu CY, Miller SA. Clinical metagenomics. Nat Rev Genet. 2019;20(6):341–355. doi:10.1038/s41576-019-0113-7
6. Kapadia BH, Berg RA, Daley JA, Fritz J, Bhave A, Mont MA. Periprosthetic joint infection. Lancet. 2016;387(10016):386–394. doi:10.1016/S0140-6736(14)61798-0
7. Malekzadeh D, Osmon DR, Lahr BD, Hanssen AD, Berbari EF. Prior use of antimicrobial therapy is a risk factor for culture-negative prosthetic joint infection. Clin Orthop Relat Res. 2010;468(8):2039–2045. doi:10.1007/s11999-010-1338-0
8. Palan J, Nolan C, Sarantos K, Westerman R, King R, Foguet P. Culture-negative periprosthetic joint infections. EFORT Open Rev. 2019;4(10):585–594. doi:10.1302/2058-5241.4.180067
9. Ivy MI, Thoendel MJ, Jeraldo PR, et al. Direct detection and identification of prosthetic joint infection pathogens in synovial fluid by metagenomic shotgun sequencing. J Clin Microbiol. 2018;56(9). doi:10.1128/JCM.00402-18
10. Thoendel M, Jeraldo P, Greenwood-Quaintance KE, et al. A novel prosthetic joint infection pathogen, mycoplasma salivarium, identified by metagenomic shotgun sequencing. Clin Infect Dis. 2017;65(2):332–335. doi:10.1093/cid/cix296
11. He R, Wang Q, Wang J, Tang J, Shen H, Zhang X. Better choice of the type of specimen used for untargeted metagenomic sequencing in the diagnosis of periprosthetic joint infections. Bone Joint J. 2021;103-b(5):923–930. doi:10.1302/0301-620X.103B5.BJJ-2020-0745.R1
12. Miao Q, Ma Y, Wang Q, et al. Microbiological diagnostic performance of metagenomic next-generation sequencing when applied to clinical practice. Clin Infect Dis. 2018;67(suppl_2):S231–S240. doi:10.1093/cid/ciy693
13. Wilson MR, Sample HA, Zorn KC, et al. Clinical metagenomic sequencing for diagnosis of meningitis and encephalitis. N Engl J Med. 2019;380(24):2327–2340. doi:10.1056/NEJMoa1803396
14. Huang Z, Li W, Lee GC, et al. Metagenomic next-generation sequencing of synovial fluid demonstrates high accuracy in prosthetic joint infection diagnostics: mNGS for diagnosing PJI. Bone Joint Res. 2020;9(7):440–449. doi:10.1302/2046-3758.97.BJR-2019-0325.R2
15. Torchia MT, Amakiri I, Werth P, Moschetti W. Characterization of native knee microorganisms using next-generation sequencing in patients undergoing primary total knee arthroplasty. Knee. 2020;27(3):1113–1119. doi:10.1016/j.knee.2019.12.013
16. Parvizi J, Gehrke T. Definition of periprosthetic joint infection. J Arthroplasty. 2014;29(7):1331. doi:10.1016/j.arth.2014.03.009
17. Parvizi J, Zmistowski B, Berbari EF, et al. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop Relat Res. 2011;469(11):2992–2994. doi:10.1007/s11999-011-2102-9
18. Li R, Li X, Ni M, Zheng QY, Zhang GQ, Chen JY. Anatomic landmark-guided hip aspiration in the diagnosis of periprosthetic joint infection. Orthopedics. 2021;44(1):e85–e90. doi:10.3928/01477447-20201007-04
19. Tan TL, Kheir MM, Tan DD, Parvizi J. Polymicrobial periprosthetic joint infections: outcome of treatment and identification of risk factors. J Bone Joint Surg Am. 2016;98(24):2082–2088. doi:10.2106/JBJS.15.01450
20. Zhao Z, Song J, Yang C, et al. Prevalence of fungal and bacterial co-infection in pulmonary fungal infections: a metagenomic next generation sequencing-based study. Front Cell Infect Microbiol. 2021;11:749905. doi:10.3389/fcimb.2021.749905
21. Li H, Gao H, Meng H, et al. Detection of pulmonary infectious pathogens from lung biopsy tissues by metagenomic next-generation sequencing. Front Cell Infect Microbiol. 2018;8:205. doi:10.3389/fcimb.2018.00205
22. Della Valle C, Parvizi J, Bauer TW, et al. American Academy of Orthopaedic Surgeons clinical practice guideline on: the diagnosis of periprosthetic joint infections of the hip and knee. J Bone Joint Surg Am. 2011;93(14):1355–1357. doi:10.2106/JBJS.9314ebo
23. Torchia MT, Austin DC, Kunkel ST, Dwyer KW, Moschetti WE. Next-generation sequencing vs culture-based methods for diagnosing periprosthetic joint infection after total knee arthroplasty: a cost-effectiveness analysis. J Arthroplasty. 2019;34(7):1333–1341. doi:10.1016/j.arth.2019.03.029
24. Li MM, Datto M, Duncavage EJ, et al. Standards and guidelines for the interpretation and reporting of sequence variants in cancer: a joint consensus recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists. J Mol Diagn. 2017;19(1):4–23. doi:10.1016/j.jmoldx.2016.10.002
25. Zhang C, Fang X, Huang Z, et al. Value of mNGS in sonication fluid for the diagnosis of periprosthetic joint infection. Arthroplasty. 2019;1(1):9. doi:10.1186/s42836-019-0006-4
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.