Back to Journals » The Application of Clinical Genetics » Volume 14
The Clinical Genetics of Hemophilia B (Factor IX Deficiency)
Authors Miller CH
Received 25 August 2021
Accepted for publication 26 October 2021
Published 23 November 2021 Volume 2021:14 Pages 445—454
DOI https://doi.org/10.2147/TACG.S288256
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Martin Maurer
Connie H Miller1,2
1Division of Blood Disorders, National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention, Atlanta, GA, USA; 2Synergy America, Inc., Duluth, GA, USA
Correspondence: Connie H Miller
Division of Blood Disorders, National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention, 1600 Clifton Road, MS D-02, Atlanta, GA, 30333, USA
Tel +1 501 408-6239
Email [email protected]
Abstract: Hemophilia B (HB) is a bleeding disorder caused by deficiency of or defect in blood coagulation factor IX (FIX) inherited in an X-linked manner. It results from one of over 1000 known pathogenic variants in the FIX gene, F9; missense and frameshift changes predominate. Although primarily males are affected with HB, heterozygous females may have excessive bleeding due to random or non-random X chromosome inactivation; in addition, homozygous, compound heterozygous, and hemizygous females have been reported. Somatic and germinal mosaicism for F9 variants has been observed. Development of antibodies to FIX treatment products (inhibitors) is rare and related to the type of causative variant present. Treatment is with products produced by recombinant DNA technology, and gene therapy is in clinical trials. Genetic counseling with up-to-date information is warranted for heterozygotes, potential heterozygotes, and men and women affected with HB.
Keywords: factor IX, hemophilia B, genetics, hematology
Introduction
Hemophilia B is a genetic disorder of impaired blood coagulation that causes excessive bleeding, particularly following trauma or medical procedures, which can be life-threatening if untreated. A hallmark of hemophilia is bleeding within joints and muscles that in the past often resulted in disability. Today the disorder is manageable by replacing the missing blood coagulation factor, factor IX (FIX), using recombinant drugs that are often administered by patients or family members to prevent or treat bleeding. Among the many known bleeding disorders, the term hemophilia is reserved for two X-linked disorders, hemophilia A (HA) and hemophilia B (HB), which are almost indistinguishable clinically.1 Since the gene for FIX, named F9, was cloned in 1982,2,3 more than 1000 variants causing HB have been reported.4,5 Genotype–phenotype associations have been made that clarify some of the basic characteristics of the disorder. The appearance of HB in females is also better understood based on developments in X-chromosome genetic testing.
History
Hemophilia is readily recognizable in historic literature because of its striking clinical features and inheritance pattern. Hemophilia was mentioned in Rabbinic writings of the 5th century.6 The first medical report was in 1803 by John C. Otto of Philadelphia.7 Yet, not until 1952 was it recognized that there are actually two forms of hemophilia with the same distinctive inheritance pattern: hemophilia A, or classic hemophilia, and hemophilia B, called Christmas disease. Christmas disease was named for the first patient in which it was described.8 When plasmas from this patient and six others were mixed with plasma from the majority of patients with hemophilia, their prolonged clotting was corrected, indicating that they did not have the same defect, an example of genetic complementation. The clotting factor deficient in hemophilia A was named factor VIII (FVIII) and that deficient in HB, once called Christmas factor, was named factor IX (FIX).9 The famous hemophilia family descended from Queen Victoria of England has been shown to have HB; their specific mutation in the F9 gene was identified in the recently discovered remains of the Russian royal family.10
Clinical Features
Hemophilia is typically suspected in an infant with intracranial hemorrhage or bleeding post circumcision; however, these occurred in only 19% and 27%, respectively, of 404 males with HA or HB who were attending US hemophilia treatment centers (HTCs) by age 2.11 The initial bleeding symptoms reported most often were iatrogenic (heel stick, intramuscular injection, venipuncture) and traumatic (oral mucosa, soft tissue, joint) episodes. Among this young group, 75% were diagnosed within the first month of life; however, 63% were diagnosed due to family history of hemophilia and only 35% due to bleeding. Some may have escaped bleeding because the diagnosis occurred prior to procedures, such as circumcision, particularly when family history was known. In children without a known family history of HB, diagnosis may be delayed until excessive bruising occurs during crawling or walking. Although males are affected much more often, females may have the disorder, as discussed below, and apparent female sex does not exclude the diagnosis.12
Both HA and HB will result in prolongation of the activated partial thromboplastin time (APTT), except in the milder forms. HB is diagnosed by measurement of FIX activity in plasma using a one-stage clot-based or chromogenic substrate assay. The results are expressed as % of normal, which is equivalent to units per deciliter (U/dL) or international units (IU) per deciliter when quantitated by comparison to an international plasma standard.13 HB is defined as severe if FIX activity is less than 1% (<1 U/dL), moderate if the level is 1–5% (1–5 U/dL), and mild if the level is greater than 5% but less than 40% (>5 to <40 U/dL), although some sources use <50% rather than <40% to define the hemophilia range.14 FIX activity may also be low in the following circumstances: normal neonates and premature infants; vitamin K deficiency at any life stage; inherited deficiency of vitamin K-dependent factors (an autosomal disorder); anticoagulant therapy with vitamin K antagonists, such as warfarin or coumadin; and liver disease. These conditions, however, will also result in a decrease in other vitamin K-dependent factors, such as factor VII, and result in prolongation of the prothrombin time in addition to the APTT. FIX may also be measured as FIX antigen by immunologic methods; this test will be normal in most acquired deficiencies but also is normal in some forms of HB and is not often available clinically.
Diagnosis of HB in the neonate may be difficult due to the low levels of FIX seen in unaffected infants. Levels do not reach the normal adult range until six months of age.15 In infants, severe disease can be detected by carefully controlled FIX assays, but mild or moderate levels may be difficult to interpret. Laboratories less experienced at measuring low FIX levels may prefer to use reference laboratories or referrals to specialized hemophilia treatment centers to avoid misdiagnosis. The analysis of the F9 gene for a causative variant can be used to confirm the diagnosis.
Severe HB may result in spontaneous bleeding into joints and muscles, which if untreated causes intense pain, immobility, and eventually permanent joint damage. Prompt treatment when bleeding occurs, termed episodic treatment, can reduce these effects; however, today’s standard of care for individuals with severe hemophilia is prophylactic treatment, providing frequent doses of factor to prevent bleeding episodes, which can reduce the long-term effects of the disease.16 In moderate HB, similar bleeding may occur; however, it is rarely spontaneous except in areas with previous damage. Bleeding in mild HB is most often the result of injury or medical or dental procedures. All forms will need pretreatment to normalize FIX levels for surgery or dental procedures. Treatment today is given by recombinant FIX products, some of which have been altered to prolong FIX half-life in the circulation and are referred to as extended half-life products.17 Treatment intervals are individually determined to maintain protective levels of FIX.18 In an international study conducted from 2015 to 2018, prophylactic treatment was received by 64% of severe patients, 8.7% of moderate patients, and no mild patients; severe patients averaged 57 infusions per year, moderate 12 infusions, and mild 5 infusions.19 In the United States, health care costs among patients with HB averaged 25 times those of matched controls, due primarily to the cost of factor replacement therapy.20 Some methods of gene therapy for HB are currently in clinical trials, as discussed below.21
It has long been suggested that HB is clinically less severe than HA.22,23 This perception may be partially due to the fact that the distribution of severity is different in the two disorders (Table 1). Among patients treated at US HTCs, those with severe disease make up 48.2% of HA patients but only 28.7% of those with HB.24 The differences go beyond frequencies, however. Studies have shown that patients with HB had fewer hemarthroses and less joint damage, fewer bleeding episodes, and lower rates of prophylactic treatment than those with HA, as reviewed by Castaman.1 The effects of the two diseases are also influenced by their mutation profiles.22 Severe HA results more often from null variants producing no gene product, while severe HB includes a higher proportion of missense variants.1 Because of this difference, HB patients are also less likely than those with HA to produce antibodies that interfere with the function of clotting factor replacement (inhibitors). In the United States, 2% of 3785 HB patients enrolled in a national surveillance program had inhibitors, including 4.9% of those with severe HB, 0.5% of those with moderate HB, and 0.1% of those with mild HB.25 HB patients with inhibitors, unlike those with HA, often develop anaphylaxis26 or nephrotic syndrome27 when treated with FIX-containing products. Patients with inhibitors to FIX usually require alternative treatments to achieve hemostasis, such as recombinant factor VIIa.28 The reduction of antibody response by frequent factor doses (ie, immune tolerance induction) is less successful in HB than in HA.29
 |
Table 1 Frequency of Hemophilia A and Hemophilia B Patients by Severity Among Patients Attending US Hemophilia Treatment Centers24 |
Epidemiology
HB is less common than HA. An international study30 found the prevalence of HA to be 17.1 per 100,000 males in the population, while the prevalence of HB was 3.8 males per 100,000; thus, HB affects 18% of people with hemophilia. The incidence, or prevalence at birth, was 23.2 per 100,000 males for HA and 4.7 per 100,000 males for HB, making the incidence of HB about one-fifth that for HA. Similar figures have been obtained from US HTCs, although the prevalence of HB in the United States was slightly higher, making up 24% of those with hemophilia.24 HB has been observed to be more common in some portions of the US;24 this is thought to be due to a founder effect, discussed below. When studied on a country-by-country basis, HB prevalence was found to vary among countries and to be higher in higher-income countries. It also appeared to increase over time. Both of these characteristics may be due to increased access to health care, longer survival, and better data collection.31
Inheritance
HA and HB both exhibit X-linked inheritance because the gene for FVIII, called F8, and the gene for FIX, called F9, are located on the long arm of the X chromosome. In the sex determination mechanism of humans, females have two X chromosomes, and males have one X chromosome and one Y chromosome. Because most genes on the X chromosome have a single allele on the X and no comparable allele on the Y chromosome, defects in X chromosome genes are apparent in the male, who is termed hemizygous and has the full phenotype of the disease. Females have two alleles for X chromosome genes, so a defect in a single allele may be compensated by a normal allele. The phenotype of such heterozygous females, however, is complicated by the normal process of X chromosome inactivation, discussed below, and more complex genetic situations, which rarely may produce hemophilic females.12 It is this X-linked inheritance pattern, which has been recognized for centuries, that is usually characterized by unaffected heterozygous females (carriers) transmitting hemophilia to one-half of their male offspring and heterozygosity to one-half of their daughters. Men with hemophilia transmit a hemophilia allele to all of their daughters and none of their sons, who get their Y chromosome only.
“Sporadic” cases of hemophilia appear when there is no known family history of the disease. They may be due to 1) a new hemophilia allele appearing by mutation in the egg or sperm cell producing the affected child, in which case the mother is not heterozygous; 2) a new mutation in the egg or sperm cell producing the mother, who is thus heterozygous; 3) a heterozygous mother whose family history was not known, due either to lack of information or the occurrence of few male births in the family; or 4) maternal mosaicism.32 Approximately one-third of births of males with HB are reported to be sporadic; however, many mothers are subsequently shown to be heterozygous or mosaic by testing or birth of further affected sons. In a study of 240 HB patients,33 149 (62%) were familial cases; of the 91 mothers with no family history, 42 (46%) subsequently had more affected offspring, proving themselves heterozygous or mosaic, and approximately 85% of the remaining 49 were found heterozygous by testing, indicating that overall, 92% of mothers of sporadic cases were actually heterozygous in reproductive cells. Cases born to truly non-heterozygous mothers are quite rare; and given the possibility of mosaicism, discussed below, non-heterozygosity cannot be proven with certainty in a sporadic case.32
Factor IX Gene and Protein
The FIX gene, F9, is located at Xq27.1 and includes 33.5 kilobases (kb) of DNA with 8 exons (Figure 1). The 2.7 kb FIX messenger RNA produces a single-chain precursor protein of 461 amino acids.34 The protein includes a signal peptide, a propeptide, a glutamic acid (Gla) domain, two epidermal growth factor-like (EGF) domains, a linking sequence, and the catalytic (protease) domain. Twelve Gla residues require vitamin K-dependent gamma-carboxylation. The first EGF domain undergoes beta-hydroxylation at residue Asp64, addition of di- or trisaccharide, and formation of three disulphide bonds. After the cleavage of a pre-pro leader, the mature protein has 415 amino acids.34 During activation, the cleavage of two peptide bonds releases an activation peptide of 35 amino acids. Activated factor IX (FIXa) consists of a light chain, including the Gla and EGF domains, and a heavy chain, the catalytic domain, and has serine protease activity.
FIX is produced in hepatocytes and is similar in structure to other vitamin K-dependent proteins, such as factors II, VII, and X.35 In the coagulation process, FIX is converted to FIXa by activated FVII and tissue factor through the extrinsic pathway or by activated factor XI through the intrinsic pathway. FIXa converts factor X to activated FX (FXa) in the presence of its cofactor FVIII. FXa then converts prothrombin to thrombin to facilitate the formation of the fibrin clot.
F9 Variants
Terminology for gene changes has evolved from the use of the terms “mutation” and “polymorphism” to a preference for the term “variant” with qualifiers indicating what is known about the ability of the change to cause disease. Standards and guidelines produced by the American College of Medical Genetics and Genomics and the Association for Molecular Pathology in 201536 have been applied specifically to bleeding disorders by Gomez et al.32 Under these guidelines, variants in the structure of the F9 gene that are disease-causing are termed pathogenic, while variants that do not affect FIX function and occur in the normal population are termed benign. Two online databases record reported F9 variants. The Factor IX Gene (F9) Variant Database at http://www.factorix.org includes both pathogenic and benign variants with individual patient data reported in the literature or submitted.4 The CDC Hemophilia B Mutation Project (CHBMP) at https://www.cdc.gov/ncbddd/hemophilia/champs.html lists variants reported to cause HB by their first literature report.5 Both include information on severity and inhibitor occurrence, when available. The Factor IX Gene (F9) Variant Database also includes structural models for missense variants. The variants listed in the Factor IX Gene (F9) Variant Database (Table 2A) are classified by DNA change. Point mutations make up 76.7% of disease-causing variants in F9, followed by deletions at 17.2%, with other variant types including fewer than 5% of variants. Table 2B shows the distribution of variant types currently reported in CHBMP, which are classified by their effect on the protein. Missense variants make up 58.1% of those reported, followed by frameshifts at 16.1%, splice site changes at 9.4%, and nonsense variants at 8.0%. Other variant types each make up fewer than 5% of variants observed.
 |
Table 2 Unique F9 Variants Reported to Cause Hemophilia B by Variant Type Classified by A. Type of Gene Change from Factor IX Gene (F9) Variant Database [http://www.factorix.org] and B. Type of Protein Change from CDC Hemophilia B Mutation Project [https://www.cdc.gov/ncbddd/hemophilia/champs.html |
Inhibitors to FIX occur primarily in patients with F9 deletions, frameshifts, and nonsense mutations, which result in truncated or absent FIX protein; only one missense and two splicing variants have been reported in patients with inhibitors.4
One form of HB is unique in that FIX levels are low in early life and rise at puberty: hemophilia B Leyden,37 which is caused by variants in the promoter region of F9. This apparent “cure” of HB with age was originally thought to be related to androgen receptors, but increases in FIX have been seen in some patients prior to puberty38 and in heterozygous women,39 suggesting a sex-independent mechanism.
Heterozygous Females (Carriers)
Females who are heterozygous for HB-causing F9 variants have a wide range of FIX levels (Figure 2) due to the phenomenon of X-chromosome inactivation (XCI), which in humans serves to inactivate one X chromosome in each somatic cell of a female and equalize the dosage of X chromosome genes between males and females. XCI occurs randomly early in development and results in clonal populations of cells with either the paternal or the maternal X inactive. This is not obvious unless a disease gene is involved. If the allele from one parent is defective, XCI, due to its random nature, can result in all X chromosomes with the defective allele being expressed or all of those with the normal allele being expressed, as shown at the extreme ends of the distribution in Figure 2, or any combination in between. This results in a substantial number of females heterozygous for HA or HB having factor levels below the hemostatic range of about 40%. These women and girls will have the bleeding symptoms typically seen in males with hemophilia of the same factor level, such as hemarthrosis and muscle bleeding, and should be considered to have hemophilia. Likewise, heterozygotes for HB may have FIX levels that are completely normal; such women are undetectable by measurement of FIX level alone. Thus, the ability to detect HB heterozygotes was limited prior to the availability of gene sequencing. Hemophilia heterozygotes have higher rates than non-heterozygotes of excessive bleeding with tooth extraction, surgery, and delivery, as recently summarized.12 Menorrhagia is often present but is not invariable.40,41 On a standardized bleeding assessment tool, the International Society on Thrombosis and Haemostasis Bleeding Assessment Tool, women heterozygous for HA or HB scored higher than controls in the categories of cutaneous, minor wound, oral cavity, menorrhagia, hemarthrosis, post dental, postsurgical, and postpartum bleeding, and there was a significant inverse correlation between factor level and bleeding score.42
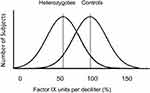 |
Figure 2 Distributions of factor IX activity in women heterozygous for variants causing hemophilia B (heterozygotes) and for women not having variants causing hemophilia (controls). Reproduced with permission from Miller CH, Bean CJ. Genetic causes of haemophilia in women and girls. Haemophilia. 2020;27(2):e164–e179. © Published 2020. This article is a U.S. Government work and is in the public domain in the USA.12 |
A female is considered an obligate heterozygote for HB if she is the mother of a HB son with another relative with HB, the mother of more than one HB son, or the daughter of a man with HB. Other women related to a male with HB through maternal ties are potential heterozygotes. Most HB heterozygotes within families transmitting HB can be detected by sequencing of the F9 gene, preferably after the disease-causing variant in the affected male has been identified. F9 deletions and duplications are not evident in the heterozygous state by sequencing and require Multiplex Ligation-Dependent Probe Amplification (MLPA®) or similar gene dosage analysis for detection.43 Each heterozygous or potentially heterozygous female is advised to have her factor IX level measured and bleeding history assessed at the time of genetic diagnosis and receive advice regarding her need for treatment and genetic counseling about available reproductive options and delivery planning, as discussed below.
Heterozygotes for HB are heterogeneous in their gene expression due to XCI; this does not usually affect detection of the heterozygous state by testing of DNA from blood cells. Rarely, however, individuals may have different genotypes in blood cells and reproductive cells and are mosaic, as discussed below. This occurrence makes it impossible to assure a woman with no family history of HB that she is not heterozygous for HB with complete certainty. This needs to be communicated when test results are discussed.
Females with Hemophilia B
Although females with severe or moderate HB are quite rare, women and girls made up 24% of individuals with mild HB seen for care at US HTCs.44 The genetic causes of hemophilia in those women and girls among whom a causative variant has been identified have recently been reviewed.12 They include homozygosity (two copies of the same hemophilia-causing variant), compound heterozygosity (two different hemophilia-causing variants in trans), hemizygosity (a single hemophilia-causing variant with no normal allele), and heterozygosity (one hemophilia-causing variant and one normal allele). Homozygosity was reported in a 9-year-old girl with FIX<1% who inherited a F9 variant, c.484C>T; p.Arg162*, from both a heterozygous mother and a father with severe HB who were related.45 Compound heterozygosity was seen in a girl with moderate HB (FIX 1%) whose mother was demonstrated to have somatic mosaicism, with a F9 variant present in 5% of leucocytes but 10–30% of buccal and urogenital cells and normal FIX, while a second mutation occurred de novo on her unaffected father’s X chromosome. Her sister received her mother’s variant only and had 7% FIX.46 Hemizygosity was identified in five females with HB: three had Turner syndrome (45,X), two of whom were mosaic for cell lines missing an X chromosome;40,47,48 and two had X-chromosome deletions that included their normal F9 alleles: 46,X,del(X)(q26.3q28)49 and 46,X,del(X)(q27).40 Heterozygosity with preferential inactivation of abnormal X chromosomes was seen in three families. Two involved X/autosome translocations with deletion of part of F9.50,51 In one case, maternal uniparental disomy of Xq27qter caused the X with the normal F9 allele to be inactivated.52 Presence of other disease genes on the X chromosome has been reported to cause preferential XCI leading to HA, but no such cases have been described in HB.12
As noted above, genetically proven heterozygotes with no overt X chromosome structural defect may have FIX levels diagnostic of HB. Eighteen of these have been studied in depth, with twelve demonstrated to have excessively skewed X-inactivation patterns, as reviewed.12 A pair of monozygotic twins had FIX levels of <1% and 15%,53 and a pair of dizygotic twins with 2% and 24% FIX showed 99:1 and 65:35 parental X activity, respectively.54 It has been suggested that undetected small structural changes in the X chromosome may account for some of these cases.54–56 Changes to the Xist gene resulting in familial skewed X inactivation have also been suggested for HA,57 and the same mechanism could apply to HB.
Evaluation of a female presenting with a low FIX level requires an initial assessment of personal and family history of bleeding; if no family history of HB is found, then further evaluation would include testing for other vitamin K-dependent factors (factors II, VII, and X) to rule out congenital or acquired combined deficiency and testing of FIX levels in both parents. Factor IX inhibitors causing acquired HB are quite rare but could be considered. F9 analysis for HB-causing variants should be conducted by sequencing and MLPA®, which is necessary to detect gene deletions and duplications in females. For those with FIX less than 5%, a karyotype with high-resolution examination of the X chromosome, paternity testing, and X inactivation studies are indicated. The differential diagnosis for a female with low FIX includes vitamin K deficiency, combined deficiency of vitamin K-dependent factors, acquired HB, and physiologic low level in a neonate.
Mutation and Mosaicism
Hemophilia was used in the first studies of the mutation rate of human genes.58,59 HB has been used to demonstrate a higher rate of mutation in spermatogenesis than oogenesis60,61 as well as the genetic concept of founder effect. Founder effect occurs when a disease-causing variant is present in one of the small number of founding members of an isolated population and thus is transmitted to a higher proportion of individuals than in other populations. Only three F9 variants were suggested to account for 25% of the HB patients in the United States.62 Two of these accounted for 19% of HB patients in Mexico, however with different haplotypes, suggesting independent origins.63 In Ireland, a different group of three HB variants made up 51% of HB cases, including an HB Leyden variant.64 No such effect was seen in Sweden65 or Germany,66 and it was suggested that “hot spots” in F9 prone to mutation might account for the higher prevalence of certain variants.
Mosaicism is the presence of cell lines with different genotypes appearing in various tissues in an individual.67 It may be somatic or germline or both; either can have implications for reproductive outcomes. Somatic mosaicism for HB has been reported among both males and females. Mosaicism in leukocyte DNA was found in 11% of 45 individuals studied with sensitive techniques.68 Only cases with extremely low levels of mosaicism are likely to affect tests based on DNA isolated from leukocytes. However, somatic mosaicism may cause misinterpretation of phenotype. In three cases, men thought to have normal or mildly decreased FIX were actually mosaic for HB variants that appeared as severe or moderate HB when fully expressed in their affected grandchildren.69–71 In a family study, the mother of a sporadic HB male and her four sisters had the same F9 haplotype but only the mother showed an HB-causing variant, which was also present in leukocyte DNA from the maternal grandmother on the haplotype inherited by all the sisters, suggesting that the grandmother had germline mosaicism.72 The birth of a second HB son when leukocyte DNA appeared normal has been reported.73 Additional testing of other tissues and with more sensitive methods has been advocated for mothers of sporadic cases;74 however, in some individuals, germline mutations may be present without variants in other tissues. This limits the ability to completely rule out heterozygosity by DNA analysis in blood and warrants a caveat in communication of test results.
Genetic Counseling and Reproductive Options
The development of sophisticated DNA analysis techniques has simplified the detection of heterozygotes in families transmitting HB yet has revealed the genetic complexity of the disease with its large number of pathogenic variants and potential for mosaicism and affected females. Genetic counseling for women undergoing genetic testing and all heterozygotes determined from pedigree analysis includes discussion of these concepts and measurement of FIX level to assess bleeding risk. Communication of this complex information warrants the use of a skilled genetic counselor. There are many reproductive options. Preimplantation genetic diagnosis has been performed for HB75 and is an option in some countries.76 Prenatal diagnosis by chorionic villus biopsy or amniocentesis is widely available and may be considered for delivery management; it may require pretreatment in women with low FIX levels. Non-invasive testing of fetal cells present in maternal plasma has been performed for HB as early as 10 weeks of pregnancy, although it has been more successful later in pregnancy.77 Men with HB also need genetic counseling, including education about the genetics of their disease, their risk of affected grandsons, and the possibility that their daughters may have bleeding symptoms.
Gene Therapy
Gene therapy for HB was first performed successfully in 2011 using an adeno-associated viral (AAV) vector directed to the liver, which is the site of synthesis of FIX. It was complicated by anti-AAV immune response and low levels of expression but achieved 2–6% levels of FIX over several years.78 Subsequent trials using the variant FIX-Padua, which has increased FIX production, and altered vectors have produced average FIX levels of 25–47%.21 Thus, levels in the upper range of mild HB or the normal range can be achieved, altering the clinical phenotype. Clinical trials, including two in Phase 3,79 are ongoing, as are those in animal models to answer questions surrounding the durability of the response, the need for immunosuppression, dose required, inhibitor risk, long-term liver effects, germline integration, and applicability to affected females.21 In addition, cost–benefit analyses of this expensive therapy are being carried out, and educational efforts directed to physicians and patients have been developed.79
Conclusions
Understanding of the basic genetics of HB has contributed to clinical care by providing information leading to improved treatment, better testing, and more accurate information for patients and families.
Acknowledgments
The findings and conclusions in this report are those of the author and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Disclosure
The author reports no conflicts of interest in this work.
References
1. Castaman G, Matino D. Hemophilia A and B: molecular and clinical similarities and differences. Haematologica. 2019;104(9):1702–1709. doi:10.3324/haematol.2019.221093
2. Choo KH, Gould KG, Rees DJG, Brownlee GG. Molecular cloning of the gene for human anti-haemophilic factor IX. Nature. 1982;299(5879):178–180. doi:10.1038/299178a0
3. Kurachi K, Davie EW. Isolation and characterization of a cDNA coding for human factor IX. Proc Natl Acad Sci USA. 1982;79(21I):6461–6464. doi:10.1073/pnas.79.21.6461
4. Rallapalli PM, Kemball-Cook G, Tuddenham EG, Gomez K, Perkins SJ. An interactive mutation database for human coagulation factor IX provides novel insights into the phenotypes and genetics of hemophilia B. J Thromb Haemost. 2013;11(7):1329–1340. doi:10.1111/jth.12276
5. Li T, Miller CH, Payne AB, Hooper WC. The CDC Hemophilia B mutation project mutation list: a new online resource. Mol Genet Genomic Med. 2013;1(4):238–245. doi:10.1002/mgg3.30
6. Rosner F. Hemophilia in the Talmud and rabbinic writings. Ann Intern Med. 1969;70(4):833–837. doi:10.7326/0003-4819-70-4-833
7. Hilgartner MW. Historical Annotation. An account of an hemorrhagic disposition existing in certain families. Haemophilia. 1997;3:154–157. doi:10.1046/j.1365-2516.1997.00050.x
8. Biggs R, Douglas AS, Macfarlane RG, et al. Christmas disease: a condition previously mistaken for haemophilia. Br Med J. 1952;2(4799):1378–1382. doi:10.1136/bmj.2.4799.1378
9. Wright IS. Nomenclature of blood clotting factors; acceptance by the International Committee on nomenclature of four factors, their characteristics and international number. Can Med Assoc J. 1958;80(8):659–661.
10. Rogaev EI, Grigorenko AP, Faskhutdinova G, Kittler ELW, Moliaka YK. Genotype analysis identifies the cause of the “royal disease.” Science. 2009;326(5954):817. doi:10.1126/science.1180660
11. Kulkarni R, Soucie JM, Lusher J, et al. Sites of initial bleeding episodes, mode of delivery and age of diagnosis in babies with haemophilia diagnosed before the age of 2 years: a report from the Centers for Disease Control and Prevention’s (CDC) Universal Data Collection (UDC) project. Haemophilia. 2009;15(6):1281–1290. doi:10.1111/j.1365-2516.2009.02074.x
12. Miller CH, Bean CJ. Genetic causes of haemophilia in women and girls. Haemophilia. 2021;27(2):e164–e179. doi:10.1111/hae.14186
13. Kitchen S, de Paula Careta F, de Lima Montalvão SA, et al. Laboratory diagnosis and monitoring. In: Srivastava A, Santagostino E, Dougall A, et al., editors. WFH Guidelines for the Management of Hemophilia, 3rd Edition. Haemophilia; 2020;26(Suppl 6):29–54. doi:10.1111/hae.14046
14. Blanchette VS, Key NS, Ljung LR, et al. Definitions in hemophilia: communication from the SSC of the ISTH. J Thromb Haemost. 2014;12:1935–1939. doi:10.1111/jth.12672
15. Andrew M, Paes B, Milner R, et al. Development of the human coagulation system in the full-term infant. Blood. 1987;70(1):165–172. doi:10.1182/blood.V70.1.165.165
16. Manco-Johnson MJ, Abshire TC, Shapiro AD, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357(6):535–544. doi:10.1056/NEJMoa067659
17. Chhabra A, Spurden D, Fogarty PF, et al. Real-world outcomes associated with standard half-life and extended half-life factor replacement products for treatment of haemophilia A and B. Blood Coagul Fibrinolysis. 2020;31(3):186–192. doi:10.1097/MBC.0000000000000885
18. Iorio A, Fischer K, Blanchette V, et al. Tailoring treatment of haemophilia B: accounting for the distribution and clearance of standard and extended half-life FIX concentrates. Thromb Haemost. 2017;117(6):1023–1030. doi:10.1160/TH16-12-0942
19. Shapiro AD, Ragni MV, Borhany M, et al. Natural history study of factor IX deficiency with focus on treatment and complications (B-Natural). Haemophilia. 2021;27(1):49–59. doi:10.1111/hae.14139
20. Buckner TW, Bocharova I, Hagan K, et al. Health care resource utilization and cost burden of hemophilia B in the United States. Blood Adv. 2021;5(7):1950–1962. doi:10.1182/bloodadvances.2020003424
21. Arruda VR, Weber J, Samelson-Jones BJ. Gene therapy for inherited bleeding disorders. Semin Thromb Haemost. 2021;47(2):161–173. doi:10.1055/s-0041-1722862
22. Mannucci PM, Franchini M. Is haemophilia B less severe than haemophilia A? Haemophilia. 2013;19(4):499–502. doi:10.1111/hae.12133
23. Quick AJ, Hussey CV. Hemophilia B (PTC deficiency, or Christmas disease). AMA Arch Intern Med. 1959;103(5):762–775. doi:10.1001/archinte.1959.00270050084014
24. Soucie JM, Miller CH, Dupervil B, Le B, Buckner TW. Occurrence rates of haemophilia among males in the United States based on surveillance conducted in specialized haemophilia treatment centres. Haemophilia. 2020;26(3):487–493. doi:10.1111/hae.13998
25. Puetz J, Soucie JM, Kempton CL, Monahan PE. Prevalent inhibitors in haemophilia B subjects enrolled in the Universal Data Collection database. Haemophilia. 2013;20(1):25–31. doi:10.1111/hae.12229
26. Warrier I, Ewenstein BM, Koerper MA, et al. Factor IX inhibitors and anaphylaxis in haemophilia B. Haemophilia. 1996;2(4):259–260. doi:10.1111/j.1365-2516.1996.tb00149.x
27. Ewenstein BM, Takemoto C, Warrier I, et al. Nephrotic syndrome as a complication of immune tolerance in hemophilia B. Blood. 1997;89(3):1115–1116. doi:10.1182/blood.V89.3.1115
28. Collins PW, Chalmers E, Hart D, et al. Diagnosis and management of acquired coagulation inhibitors: a guideline from UKHCDO. Br J Haematol. 2013;162(6):758–773. doi:10.1111/bjh.12463
29. DiMichele DM, Kroner BL. The North American Immune Tolerance Registry: practices, outcomes, outcome predictors. Thromb Haemost. 2002;87(1):52–57. doi:10.1055/s-0037-1612943
30. Iorio A, Stonebraker JS, Chambost H, et al. Establishing the prevalence and prevalence at birth of hemophilia in males a meta-analytic approach using national registries. Ann Intern Med. 2019;171(8):542–546. doi:10.7326/M19-1208
31. Stonebraker JS, Bolton-Maggs PHB, Soucie JM, Walker I, Brooker M. A study of variations in the reported haemophilia B prevalence around the world. Haemophilia. 2012;18(3):e91–e94. doi:10.1111/j.1365-2516.2011.02588.x
32. Gomez K, Laffan M, Keeney S, Sutherland M, Curry N, Lunt P. Recommendations for the clinical interpretation of genetic variants and presentation of results to patients with inherited bleeding disorders. A UK Haemophilia Centre Doctors’ Organisation Good Practice Paper. Haemophilia. 2019;25(1):116–126. doi:10.1111/hae.13637
33. Kasper CK, Lin JC. Prevalence of sporadic and familial haemophilia. Haemophilia. 2007;13(1):90–92. doi:10.1111/j.1365-2516.2006.01397.x
34. Yoshitake S, Schach BG, Foster DC, Davie EW, Kurachi K. Complete nucleotide sequences of the gene for human factor IX (antihemophilic factor B). Biochemistry. 1985;24(14):3736–3750. doi:10.1021/bi00335a049
35. Roberts HR, Cederbaum AI. The liver and blood coagulation: physiology and pathology. Gastroenterology. 1972;63(2):297–320. doi:10.1016/S0016-5085(19)33318-9
36. Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–424. doi:10.1038/gim.2015.30
37. Briet E, Bertina RM, van Tilburg NH, Veltkamp JJ. Hemophilia B Leyden: a sex-linked hereditary disorder that improves after puberty. N Engl J Med. 1982;306(13):788–790. doi:10.1056/NEJM198204013061306
38. Ahmed SZ, O’Rourke M, Jenkins V, Regan I, Nolan B. Progressive increase in FIX level in males with haemophilia B Leyden and c.35G>A mutation in early childhood not related to androgen effect. Br J Haematol. 2020;189(6):e262–e265. doi:10.1111/bjh.16688
39. Lavin M, Jenkins PV, Healy ML, Byrne M, O’Connell NM, O’Donnell JS. Age-related factor IX correction in symptomatic female carriers with haemophilia B Leyden. Haemophilia. 2015;21(6):e498–e500. doi:10.1111/hae.12761
40. DiMichele DM, Gibb C, Lefkowitz JM, Ni Q, Gerber LM, Ganguly A. Severe and moderate haemophilia A and B in US females. Haemophilia. 2014;20(2):e136–e143. doi:10.1111/hae.12364
41. Chaudhury A, Sidonio R
42. James PD, Mahlangu J, Bidlingmaier C, et al. Evaluation of the utility of the ISTH-BAT in haemophilia carriers: a multinational study. Haemophilia. 2016;22(6):912–918. doi:10.1111/hae.13089
43. Payne AB, Bean CJ, Hooper WC, Miller CH. Utility of multiplex ligation-dependent probe amplification (MLPA) for hemophilia mutation screening. J Thromb Haemost. 2012;10(9):1951–1954. doi:10.1111/j.1538-7836.2012.04843.x
44. Miller CH, Soucie JM, Byams VR, et al. Women and girls with haemophilia receiving care at specialized haemophilia treatment centres in the United States. Haemophilia. 2021. doi:10.1111/hae.14403
45. Karimipoor M, Kokabee L, Kamali E, Karizi SZ, Zeinali S. Molecular analysis of factor IX gene in an Iranian female with severe hemophilia B. Acta Haematol. 2008;119(3):151–153. doi:10.1159/000128043
46. Costa JM, Vidaud D, Laurendeau I, et al. Somatic mosaicism and compound heterozygosity in female hemophilia B. Blood. 2000;96(4):1585–1587. doi:10.1182/blood.V96.4.1585.h8001585_1585_1587
47. Kelsey G, Monagle P, Barnes C. Delayed diagnosis of congenital factor IX deficiency (Christmas disease) in a girl with Turner’s syndrome. Clin Lab Haematol. 2006;28(5):355–356. doi:10.1111/j.1365-2257.2006.00810.x
48. Chan DWK, Lam JCM. Young girl with bruising: finding the X factor. J Paediatr Child Health. 2019;55(4):465–467. doi:10.1111/jpc.14282
49. Stoof SCM, Kersseboom R, de Vries FAT, Kruip MJHA, Kievit AJA, Leebeek FWG. Hemophilia B in a female with intellectual disability caused by a deletion of Xq26.3q28 encompassing the F9. Mol Genet Genomic Med. 2018;6(6):1220–1224. doi:10.1002/mgg3.425
50. Janczar S, Babol-Pokora K, Jatczak-Pawlik I, et al. Puzzling outcome of the nationwide genetic survey of severe/moderate female haemophilia B in Poland. Haemophilia. 2019;25(6):e373–e376. doi:10.1111/hae.13854
51. Krepischi-Santos ACV, Carneiro JDA, Svartman M, Bendit I, Odone-Filho V, Vianna-Morgante AM. Deletion of the factor IX gene as a result of translocation t(X;1) in a girl affected by haemophilia B. Br J Haematol. 2001;113(3):616–620. doi:10.1046/j.1365-2141.2001.02786.x
52. Sellner LN, Price PJ. Segmental isodisomy and skewed X-inactivation resulting in haemophilia B in a female. Br J Haematol. 2005;131(3):410–411. doi:10.1111/j.1365-2141.2005.05780.x
53. Schröder W, Wulff K, Wollina K, Herrmann FH. Haemophilia B in female twins caused by a point mutation in one factor IX gene and nonrandom inactivation patterns of the X-chromosomes. Thromb Haemost. 1997;78(5):1347–1351. doi:10.1055/s-0038-1665409
54. Okumura K, Fujimori Y, Takagi A, et al. Skewed X chromosome inactivation in fraternal female twins results in moderately severe and mild haemophilia B. Haemophilia. 2008;14(5):1088–1093. doi:10.1111/j.1365-2516.2008.01786.x
55. Mason JA, Aung HT, Nandini A, et al. Demonstration of a novel Xp22.2 microdeletion as the cause of familial extreme skewing of X-inactivation utilizing case-parent trio SNP microarray analysis. Mol Genet Genomic Med. 2018;6(3):357–369. doi:10.1002/mgg3.378
56. Mason JA, Robertson JD. Extreme skewing of X-inactivation: rethinking severe haemophilia in women and girls. Haemophilia. 2019;25(4):e286–e287. doi:10.1111/hae.13755
57. Bicocchi MP, Migeon BR, Pasino M, et al. Familial nonrandom inactivation linked to the X inactivation centre in heterozygotes manifesting haemophilia A. Eur J Hum Genet. 2005;13(5):635–640. doi:10.1038/sj.ejhg.5201386
58. Haldane JBS. On the rate of spontaneous mutation of a human gene. J Genet. 1935;31:317–326. doi:10.1007/BF02982403
59. Haldane JB. The mutation rate of the gene for haemophilia, and its segregation ratios in males and females. Ann Eugen. 1947;13(4):262–271. doi:10.1111/j.1469-1809.1946.tb02367.x
60. Green PM, Saad S, Lewis CM, Giannelli F. Mutation rates in humans. I. Overall and sex-specific rates obtained from a population study of hemophilia B. Am J Hum Genet. 1999;65(6):1572–1579. doi:10.1086/302651
61. Ketterling RP, Vielhaber E, Bottema CDK, et al. Germ-line origins of mutation in families with hemophilia B: the sex ratio varies with the type of mutation. Am J Hum Genet. 1993;52(1):152–166.
62. Ketterling RP, Bottema CDK, PhillipsJA III, Sommer SS. Evidence that descendants of three founders constitute about 25% of hemophilia B in the United States. Genomics. 1991;10(4):1093–1096. doi:10.1016/0888-7543(91)90207-U
63. Thorland EC, Weinshenker BG, Liu JZ, et al. Molecular epidemiology of factor IX germline mutations in Mexican Hispanics: pattern of mutation and potential founder effects. Thromb Haemost. 1995;74(6):1416–1422. doi:10.1055/s-0038-1649957
64. Jenkins VV, Egan H, Keenan C, et al. Mutation analysis of haemophilia B in the Irish population: increased prevalence caused by founder effect. Haemophilia. 2008;14(4):717–722. doi:10.1111/j.1365-2516.2008.01765.x
65. Ljung R, Petrini P, Tengborn L, Sjörin E. Haemophilia B mutations in Sweden: a population-based study of mutational heterogeneity. Br J Haematol. 2001;113(1):81–86. doi:10.1046/j.1365-2141.2001.02759.x
66. Knobloch O, Zoll B, Zerres K, Brackmann HH, Olek K, Ludwig M. Recurrent mutations in the factor IX gene: founder effect or repeat de novo events - Investigation of the German haemophilia B population and review of de novo mutations. Hum Genet. 1993;92(1):40–48. doi:10.1007/BF00216143
67. Youssoufian H, Pyeritz RE. Mechanisms and consequences of somatic mosaicism in humans. Nat Rev Genet. 2002;3(10):748–758. doi:10.1038/nrg906
68. Ketterling RP, Vielhaber E, Li X, et al. Germline origins in the human F9 gene: frequent G:C→A:T mosaicism and increased mutations with advanced maternal age. Hum Genet. 1999;105(6):629–640. doi:10.1007/s004390051155
69. Cutler JA, Mitchell MJ, Smith MP, Savidge GF. Germline mosaicism resulting in the transmission of severe hemophilia B from a grandfather with a mild deficiency. Am J Med Genet. 2004;129A(1):13–15. doi:10.1002/ajmg.a.30162
70. Kasper CK, Buzin CH. Mosaics and haemophilia. Haemophilia. 2009;15(6):1181–1186. doi:10.1111/j.1365-2516.2009.02003.x
71. Taylor SAM, Deugau KV, Lillicrap DP. Somatic mosaicism and female-to-female transmission in a kindred with hemophilia B (factor IX deficiency). Proc Natl Acad Sci USA. 1991;88(1):39–42. doi:10.1073/pnas.88.1.39
72. Sommer SS, Knöll A, Greenberg CR, Ketterling RP. Germline mosaicism in a female who seemed to be a carrier by sequence analysis. Hum Mol Genet. 1995;4(11):2181–2182. doi:10.1093/hmg/4.11.2181
73. Kim HJ, Lee KO, Yoo KY, Kim SH, Kim HJ. Maternal low-level somatic mosaicism of Cys155Tyr of F9 in severe hemophilia B. Blood Coagul Fibrinolysis. 2015;26(8):866–868. doi:10.1097/MBC.0000000000000234
74. Lannoy N, Hermans C. Genetic mosaicism in haemophilia: a practical review to help evaluate the risk of transmitting the disease. Haemophilia. 2020;26(3):375–383. doi:10.1111/hae.13975
75. Perez J, Lucena E, Hughes M, Lizcano L, Kasper CK. Preimplantation genetic diagnosis–a historical annotation: first PGD baby turns fifteen. Haemophilia. 2011;17(Suppl 1):18–19. doi:10.1111/j.1365-2516.2011.02560.x
76. Peyvandi F, Garagiola I, Mortarino M. Prenatal diagnosis and preimplantation genetic diagnosis: novel technologies and state of the art of PGD in different regions of the world. Haemophilia. 2011;17(Suppl 1):14–17. doi:10.1111/j.1365-2516.2011.02559.x
77. Hudecova I, Jiang P, Davies J, Lo YMD, Kadir RA, Chiu RWK. Noninvasive detection of F8 int22h-related inversions and sequence variants in maternal plasma of hemophilia carriers. Blood. 2017;130(3):340–347. doi:10.1182/blood-2016-12-755017
78. Nathwani AC. Gene therapy for hemophilia. Hematology Am Soc Hematol Educ Program. 2019;2019(1):1–8. doi:10.1182/hematology.2019000007
79. Pipe SW. Delivering on the promise of gene therapy for haemophilia. Haemophilia. 2021;27(S3):114–121. doi:10.1111/hae.14027
80. UCL. Factor IX Gene (F9) Variant Database. Available from: http://www.factorix.org/statistics.html.php. Accessed November 07, 2021.
81. Centers for Disease Control and Prevention. CHBMP F9 Database. Available from: https://www.cdc.gov/ncbddd/hemophilia/champs.html. Accessed October 19, 2021.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

