Back to Journals » Infection and Drug Resistance » Volume 11
The changes of expressive levels of IL-17A, STAT3, and RORγt in different invasive pulmonary aspergillosis mice
Authors Xu LN, Xu RA, Zhang D, Su SS , Xu HY, Wu Q, Li YP
Received 3 May 2018
Accepted for publication 14 June 2018
Published 27 August 2018 Volume 2018:11 Pages 1321—1328
DOI https://doi.org/10.2147/IDR.S172949
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Lingna Xu,1,* Ren-ai Xu,2,* Dan Zhang,1 Shanshan Su,1 Hanyan Xu,1 Qing Wu,3 Yuping Li1
1Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, China; 2Department of Pharmacy, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, China; 3Department of Microbiology Laboratory, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, China
*These authors contributed equally to this work
Background: T helper 17 (Th17) lymphocytes play an important role in Aspergillus adaptive immune response against Aspergillus fumigatus, but there is little attention focused on the different types of immunosuppressive models in which invasive pulmonary aspergillosis (IPA) develops. In addition, the expression levels of signal transducer and activator of transcription 3 (STAT3)/retinoic acid-related orphan nuclear receptor gamma (RORγt)/interleukin (IL)-17A signaling pathway, which is involved in the regulation of Th17 cells, as well as whether there are differences between two types of IPA mice models, remain unknown.
Materials and methods: Six to eight weeks old female BALB/c mice were treated with cortisone acetate or cyclophosphamide to establish the immunosuppressive mice models, and then, A. fumigatus inoculum was injected to form the IPA groups and sterile saline was injected to form the control groups. Flow cytometry was performed to measure the proportion of Th17 cells in CD4+ T cells in the peripheral blood, spleen, and lung of the mice. The expression of IL-17A, RORγt, and STAT3 mRNA was detected by real-time polymerase chain reaction. Concentrations of IL-6 in the plasma and bronchoalveolar lavage fluid were measured by enzyme-linked immunosorbent assay.
Results: The proportion of Th17 in the peripheral blood and lung tissue in neutropenic IPA mice showed a more significant increase than in non-neutropenic IPA mice (P<0.01). The IL-6 protein also showed the same trend in plasma and bronchoalveolar lavage fluid (P<0.01). Compared with the control groups, the expression of IL-17A at mRNA level in the lung was significantly increased, while RORγt/STAT3 mRNA was significantly decreased in the IPA groups (P<0.01).
Conclusion: The expression of RORγt and STAT3 mRNA in the lung tissue in both groups was significantly decreased. IL-17 may play a negative role in the defense against Aspergillus through uprating IL-6.
Keywords: non-neutropenic invasive pulmonary aspergillosis, neutropenic invasive pulmonary aspergillosis, Th17, IL-17A, RORγt, STAT3
Introduction
Aspergillus fumigatus is the material cause of invasive pulmonary aspergillosis (IPA), which is related to a high fatality rate even with common antifungal therapy. Patients with prolonged neutropenia, allogeneic hematopoietic stem cell transplant, solid organ transplant, inherited or acquired immunodeficiency, or corticosteroid use are known to have a high risk of IPA.1 However, recently, numerous studies found that patients with severe pulmonary dysfunction or COPD are also susceptible to IPA in the absence of neutropenia.2 Monocytes, macrophages, and neutrophils constitute the innate immunity defense against A. fumigatus. It is well known that T helper 17 (Th17) cells are involved in the recruitment, activation, and migration of neutrophil granulocytes to the site of a fungal infection, playing a positive role against A. fumigatus.3,4 However, several papers reported that Th17 cells do not have a role in the defense against Aspergillus or may even have a damaging effect.5,6 Despite this, Th17 lymphocytes do play a role in the adaptive immune response against A. fumigatus.7
The differentiation and immune function of Th17 cells are regulated by many molecules, of which interleukin (IL)-6 and transforming growth factor-β play a crucial role through the JAK/signal transducer and activator of transcription 3 (STAT3) signaling pathway.8 Retinoic acid-related orphan nuclear receptor γt (RORγt) is an essential transcription factor for Th17 differentiation. The activation of RORγt is regulated by a series of regulatory factors under normal conditions. It was confirmed that STAT3 is one of the most important RORγt regulatory factors.9
Experimental mice models have been developed for research of the role of innate immunity in IPA. However, there is little attention focused on the different immunosuppressive models in which IPA occurs. Moreover, the expression levels of STAT3/RORγt/IL-17 signaling pathway in the IPA mice model, as well as whether there are differences between two types of IPA mice models, are not yet clear. Therefore, in this study, we attempt to establish two patterns of IPA mice models treated with cortisone acetate and cyclophosphamide, respectively, to investigate the changes in STAT3, RORγt, and IL-17A expression in pulmonary, as well as the IL-6 protein concentration in blood and bronchoalveolar lavage fluid (BALF).
Materials and methods
Fungal isolates
A clinical A. fumigatus isolate from a pulmonary aspergillosis patient treated at the First Affiliated Hospital of Wenzhou Medical University (Wenzhou, China), which was provided by the microbiology laboratory was used in our study. A. fumigatus was grown on Sabouraud dextrose agar plates for 5 days at 37°C to prepare the inoculum, and then, conidia were collected by flushing the plates with 10 mL sterile saline within 0.1% Tween 80 and then filtering through four layers of sterile gauze. After the inoculum was adjusted to the required concentration (1×107/mL) using a hemocytometer, the conidial suspension was stored at 4°C.
Source of mice
Six to eight weeks old female BALB/c mice weighing 18–22 g (Slaker lab, Shanghai, China) were used in this study. All procedures involving mice were approved by the institutional animal use and care committee of Wenzhou Medical University, according to the National Institutes of Health guidelines for animal housing and care.
Mouse model of IPA
After 1 week of adaptive feeding, the mice were randomly divided into five groups of 12 mice as experiment or control groups. Mice in group A were treated with cortisone acetate (Aladdin, Shanghai, China) at 500 mg/kg subcutaneously every other day, starting on Day 4 relative to infection and finishing on Day +3, and served as the non-neutropenic IPA model.10 Mice in group B were treated with cyclophosphamide (Baxter Oncology GmbH, Halle, Germany) administered intraperitoneally twice at 150 mg/kg on Day 4 and Day –1 and served as the neutropenic IPA model.11 Mice in groups C and D were only treated with cortisone acetate or A. fumigatus at the same dose as in group A, respectively. Mice in group E were saline-treated immunocompetent mice without immunosuppression and infection. All animals were anesthetized by intraperitoneal injection of pentobarbital (Aladdin) on Day 0 and then intratracheally injected with 30 µL conidial suspension (groups A, B, and D) or sterile saline (groups C and E). Then, 250 mg of tetracycline (Aladdin) was dissolved in 1 L of sterile water to prevent bacterial infections. In the survival studies, six mice from each group were monitored for survival.
After 72 hours of infection, the mice were sacrificed after they were anaesthetized and their peripheral blood, BALF, lung, and spleen were harvested. The IPA model was identified through the lung tissue histopathologic analysis and fungal culture. The sections were stained with HE for tissue examination and periodic acid-Schiff and periodic Schiff-methenamine silver stain for fungus detection.
Peripheral blood and BALF
Plasma was collected and stored at –80°C after centrifuging the anticoagulant peripheral blood at 3,000 rpm for 5 minutes, and the setting blood cells were used for flow cytometry after hemolysis with Lysing Buffer (BD Biosciences, San Jose, CA, USA). The chest of mice was exposed to ligate the left main bronchus, and then, its right lung was washed out with 0.5 mL cold PBS twice, aspirating three times each. Then, the supernatant was collected and stored at –80°C after centrifuging at 3,000 rpm for 10 minutes at 4°C.
Flow cytometry
The lungs and spleens of mice were carefully removed, and a single cell suspension was generated using a 200-mesh sieve. Then the lymphocytes were separated with a mouse lymphocyte separation solution (TBD science, Tianjin, China). Cells from lungs, spleens, and blood were all incubated at 37°C in RM1640 (Thermo Fisher Scientific, Waltham, MA, USA) containing Phorbol 12-myristate 13-acetate/ionomycin mixture and Brefeldin A/monensin mixture (Multisciences, Hangzhou, China). The stimulated cells were washed and incubated with fluorescein isothiocyanate Rat Anti-Mouse CD4 (BD Biosciences). For intracellular staining, Cytofix/Cytoperm Soln Kit (BD Biosciences) was used and the cells were stained with PE Rat Anti-Mouse IL-17A (BD Biosciences), after washing with Wash/Perm solution. Isotype controls (BD Biosciences) were used to determine gates for positive antibody responses. Th17 proportion was analyzed using a flow cytometer (BD Biosciences).
Quantitative real-time polymerase chain reaction (qPCR)
The IL-17A, RORγt, and STAT3 mRNA expression in the lung tissue and pulmonary A. fumigatus fungal burden were detected by qPCR. The lung tissues of mice were homogenized in liquid nitrogen, and total RNA was extracted by TRIzol Reagent (Thermo Fisher Scientific) following the manufacturer’s instructions. Samples with an OD260/280 ratio of 1.8:2.0 were used to generate cDNA by reverse transcription.
A total of 3 µg of RNA was reverse-transcribed to cDNA with RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific). qPCR for IL-17A, RORγt, and STAT3 mRNA was performed on Bio-Rad CFX 96 (Bio-Rad Laboratories Inc., Hercules, CA, USA) using the ChamQ Universal SYBR qPCR Master Mix (Vazyme, Nanjing, China) as the detecting probe. The products were also compared with GAPDH as the loading control. The primer sequences used are shown in Table 1.
Cytokine quantification by enzyme-linked immunosorbent assay
Cytokine production in plasma and BALF was measured by enzyme-linked immunosorbent assay according to the manufacturer’s directions (Multisciences).
Statistical analysis
All measurement data are shown as mean and standard errors of the mean in this study, and statistical analysis was performed using an unpaired t-test or one-way analysis of variance (GraphPad Prism Software). A P-value <0.05 was considered significant.
Results
Survival
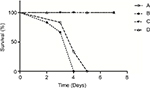
The survival curves (Figure 1) were established in various models using the same concentration of inoculum (107 conidia/mouse). Infected immunocompetent mice all survived, whereas 100% of non-neutropenic IPA mice died between Days +3 and +5. Infection of neutropenic mice resulted in 100% mortality between Days +2 and +4.
Lung injury and fungal culture
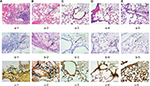
The differences in pulmonary injury were assessed by histological examination performed on lung samples collected 72 hours after infection of mice in different groups (Figure 2). The lungs of non-neutropenic IPA mice showed hemorrhagic necrosis pneumonia with neutrophil infiltration. Destruction of bronchi and alveoli was also evident (Figure 2Aa-1). A small amount of hyphae of A. fumigatus was observed paratracheal (Figure 2Ab-1, Ac-1). In contrast, for neutropenic mice, we also observed unsystematic pneumonia without inflammatory exudate including polymorphonuclear neutrophil or other cells in the alveoli with hemorrhagic necrosis (Figure 2Ba-2). Alveoli and vessels were invaded by a mass of hyphae of A. fumigatus (Figure 2Bb-2, Bc-2).
Mice in both IPA groups showed visible A. fumigatus in the lung tissue culture, while in those in immunocompetent infected group, it could be rarely seen.
Th17 proportion in peripheral blood, spleen, and lung
The Th17 cells (CD4+, IL-17+)12 proportion in the peripheral blood, spleen, and lung tissue of both patterns of IPA mice was significantly increased compared with that in saline-treated immunocompetent mice. There was no difference between the different control groups. The increase in peripheral blood and lungs of neutropenic IPA mice was more significant than that in non-neutropenic ones (P<0.01; Figure 3)
qPCR analysis of the expression of IL-17, RORγt, STAT3 mRNA, and pulmonary fungal burden in the lung tissue
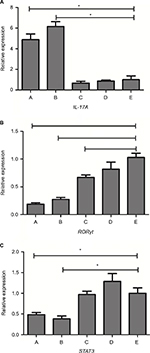
The expression level of these genes was given as the ratio with respect to saline-treated immunocompetent mice (Figure 4). Levels of GAPDH were analyzed as the control and showed no statistical differences. Overall, there was a generalized reduction in RORγt expression in immunosuppressive mice vs immunocompetent mice, and more significant reduction was seen in IPA groups. Both IPA mice showed a significant reduction in STAT3/RORγt expression and increases in IL-17A expression in response to infection, compared with saline controls (P<0.01). There was no significant difference between the two patterns of IPA mice, as well as the three control groups.
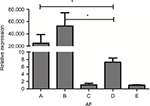
We used a real-time PCR that targets a previously described 67 bp segment of a 28S ribosomal RNA coding DNA to detect Aspergillus DNA.13 It can be seen that the A. fumigatus fungal burden in both IPA mice were significantly higher than in the immunocompetent infected mice (P<0.01). Neutropenic IPA mice showed higher fungal burden compared with the non-neutropenic IPA mice (Figure 5).
IL-6 concentration in BALF and plasma
Compared with the saline-treated immunocompetent mice, IL-6 concentrations in the plasma and BALF of neutropenic mice showed a more significant increase than in non-neutropenic mice (P<0.01). Immunocompetent infected mice showed a slight increase of IL-6 in BALF and no obvious change in plasma (Figure 6).
Discussion
Th17 cells can specifically secrete IL-17 at high levels, which was reported by Harrington et al for the first time in 2005.14 The differentiation of naïve T cells is not only regulated by cytokines from the environment but also by their inherent procedure. As a novel type of T lymphocytes, Th17 cells have been shown to be regulated positively or negatively by many cytokines. However, the specific regulation mechanism of their lineage differentiation has not been well understood.
In this study, we demonstrated that the STAT3/RORγt expression level was significantly decreased in immunosuppressive infected mice compared with that in saline immunocompetent mice. However, the proportions of Th17 cells and the expression level of IL-17 were significantly increased, which was consistent with the results of Armstrong et al.15
JAK/STAT pathway plays an important role in the differentiation of Th17 cells. Among the STATs, STAT3 is the most active protein which can be activated by numerous cytokines including growth hormone, IL-6 family cytokines, and so on. IL-6 had been shown activating the STAT3/RORγt signaling pathway, which can induce Th17 cells secreting IL-6, IL-17 and other cytokines.8 Previous studies confirmed that RORγt is an indispensable specific transcription factor for IL-17A synthesis in Th17 cells.9,16 The above results of our study indicated that STAT3/ROR signaling pathway was obviously inhibited. On the contrary, the proportions of Th17 cells and the expression level of IL-17 were significantly increased.
Therefore, two aspects of this problem have to be discussed. One possibility is that the result may suggest that RORγt is not that essential a regulatory factor to Th17. Yang et al found that RORγt deficiency did not absolutely forbid Th17 cytokine expression. They reported that Th17 cytokine was also regulated by another related nuclear receptor RORα, but it is still unclear whether RORγt or RORα directly participates in the transcriptional regulation of IL-17A gene.17 Another possibility is that Th17 immunity is, indeed, inhibited in IPA mice. All the CD4+ T cells were inhibited in the immunosuppressed mice, resulting in reduction in the absolute number of Th17 cells, though its proportion increased. Furthermore, the high expression of IL-17A in the lung tissue is not exclusively produced by Th17 cells. Studies have shown that despite the fact that most of the recent literature describes IL-17 as a T cell-secreted cytokine, much of the IL-17 released during an inflammatory response is produced by innate immune cells.18
Moreover, IL-6 was significantly increased in the plasma and BALF of IPA mice in our study, which is in agreement with the study of Heldt et al based on clinical patients with IPA.19 IL-6, a proinflammatory cytokine, is not only a promoter of STAT3/RORγt signaling pathway but also a key downstream target of IL-17.20 Wang et al demonstrated that IL-17 can induce IL-6 production to promote tumor growth.21
Comparing the non-neutropenic IPA mice and the neutropenic IPA mice, the latter had more severe disease with the same inoculum, manifesting as more severe lung injury, higher pulmonary fungal burden, as well as higher IL-6 protein levels and Th17 proportion in the lung and blood. So, we considered that through uprating IL-6, IL-17 may play a negative role in the defense against Aspergillus. This is consistent with the results of a study showing that the cytokine IL-17A can promote the growth of A. fumigatus in vivo and in vitro.6
Unlike autoimmune diseases, researchers have not reached an agreement on the immune function of Th17 cells in defense of A. fumigatus at present, which may perform a two-way effect of promoting infection15 and resisting infection22 in different immune status. Further studies to clarify the role of Th17 cells in IPA hosts are necessary.
There were limitations in our study. We detected the STAT3/RORγt/IL-17A mRNA based on the entire lung tissue which contained various cells, such as epithelial cells, endothelial cells, lymphocytes, and many primary immune cells, such as macrophages. Detection of STAT3/RORγt signaling pathway expression and IL-17A mRNA by extracting total RNA from monocytes in the lung may be the better way of specifically evaluating Th17 functional changes in two types of IPA mice.
Conclusion
Our study demonstrated that the STAT3/RORγt expression level was significantly decreased in immunosuppressive infected mice compared with the saline immunocompetent mice. However, the proportions of Th17 cells and the expression level of IL-17 were significantly increased. We considered that IL-17 may play a negative role in the defense against Aspergillus through uprating IL-6.
Acknowledgments
The authors are grateful to the teachers in central laboratory for their invaluable support and input. This study was supported by a grant from the Zhejiang Provincial Natural Science Foundation of China (LY15H010007).
Disclosure
The authors report no conflicts of interest in this work.
References
Patterson TF, Thompson GR, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the infectious diseases society of America. Clin Infect Dis. 2016;63(4):e1–e60. | ||
Dai Z, Zhao H, Cai S, Lv Y, Tong W. Invasive pulmonary aspergillosis in non-neutropenic patients with and without underlying disease: a single-centre retrospective analysis of 52 subjects. Respirology. 2013;18(2):323–331. | ||
Deepe GS, Gibbons RS. Interleukins 17 and 23 influence the host response to Histoplasma capsulatum. J Infect Dis. 2009;200(1):142–151. | ||
Taylor PR, Leal SM, Sun Y, Pearlman E. Aspergillus and Fusarium corneal infections are regulated by Th17 cells and IL-17-producing neutrophils. J Immunol. 2014;192(7):3319–3327. | ||
Zelante T, Bozza S, de Luca A, et al. Th17 cells in the setting of Aspergillus infection and pathology. Med Mycol. 2009;47(Suppl 1):S162–S169. | ||
Zelante T, Iannitti RG, de Luca A, et al. Sensing of mammalian IL-17A regulates fungal adaptation and virulence. Nat Commun. 2012;3:683. | ||
Dewi IMW, van de Veerdonk FL, Gresnigt MS. The multifaceted role of T-helper responses in host defense against Aspergillus fumigatus. J Fungi. 2017;3(4):E55. | ||
Egwuagu CE. STAT3 in CD4+ T helper cell differentiation and inflammatory diseases. Cytokine. 2009;47(3):149–156. | ||
Wan CK, Andraski AB, Spolski R, et al. Opposing roles of STAT1 and STAT3 in IL-21 function in CD4+ T cells. Proc Natl Acad Sci U S A. 2015;112(30):9394–9399. | ||
Ejzykowicz DE, Solis NV, Gravelat FN, et al. Role of Aspergillus fumigatus DvrA in host cell interactions and virulence. Eukaryot Cell. 2010;9(10):1432–1440. | ||
Yang J, Lu Q, Liu W, Wan Z, Wang X, Li R. Cyclophosphamide reduces dectin-1 expression in the lungs of naive and Aspergillus fumigatus-infected mice. Med Mycol. 2010;48(2):303–309. | ||
Chai LY, van de Veerdonk F, Marijnissen RJ, et al. Anti-Aspergillus human host defence relies on type 1 T helper (Th1), rather than type 17 T helper (Th17), cellular immunity. Immunology. 2010;130(1):46–54. | ||
Imbert S, Gauthier L, Joly I, et al. Aspergillus PCR in serum for the diagnosis, follow-up and prognosis of invasive aspergillosis in neutropenic and nonneutropenic patients. Clin Microbiol Infect. 2016;22(6):562.e1–565.e1. | ||
Harrington LE, Hatton RD, Mangan PR, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6(11):1123–1132. | ||
Armstrong-James DP, Turnbull SA, Teo I, et al. Impaired interferon-gamma responses, increased interleukin-17 expression, and a tumor necrosis factor-alpha transcriptional program in invasive aspergillosis. J Infect Dis. 2009;200(8):1341–1351. | ||
Ivanov II, Mckenzie BS, Zhou L, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126(6):1121–1133. | ||
Yang XO, Pappu BP, Nurieva R, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28(1):29–39. | ||
Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010;10(7):479–489. | ||
Heldt S, Eigl S, Prattes J, et al. Levels of interleukin (IL)-6 and IL-8 are elevated in serum and bronchoalveolar lavage fluid of haematological patients with invasive pulmonary aspergillosis. Mycoses. 2017;60(12):818–825. | ||
Ogura H, Murakami M, Okuyama Y, et al. Interleukin-17 promotes autoimmunity by triggering a positive-feedback loop via interleukin-6 induction. Immunity. 2008;29(4):628–636. | ||
Wang L, Yi T, Kortylewski M, Pardoll DM, Zeng D, Yu H. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med. 2009;206(7):1457–1464. | ||
Werner JL, Metz AE, Horn D, et al. Requisite role for the dectin-1 beta-glucan receptor in pulmonary defense against Aspergillus fumigatus. J Immunol. 2009;182(8):4938–4946. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.