Back to Journals » Clinical Interventions in Aging » Volume 17
The Awareness and Attitude of Physicians to Older Adult Routine Vaccination Scheme
Authors Ates Bulut E , Badak SO , Aksoy H, Fadiloglu A , Isik AT
Received 14 July 2022
Accepted for publication 22 October 2022
Published 31 October 2022 Volume 2022:17 Pages 1581—1588
DOI https://doi.org/10.2147/CIA.S382311
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Maddalena Illario
Esra Ates Bulut,1 Suade Ozlem Badak,2 Huseyin Aksoy,3 Ayse Fadiloglu,4 Ahmet Turan Isik4
1Division of Geriatric Medicine, Department of Internal Medicine, Adana City Training and Research Hospital, Adana, Turkey; 2Division of Rheumatology, Department of Internal Medicine, Adana City Training and Research Hospital, Adana, Turkey; 3Department of Family Medicine, Adana City Training and Research Hospital, Adana, Turkey; 4Division of Geriatric Medicine, Department of Internal Medicine, Dokuz Eylul University, School of Medicine, Izmir, Turkey
Correspondence: Ahmet Turan Isik, Email [email protected]; [email protected]
Purpose: Immunization is one of the main components of preventive medicine measures. Influenza, pneumococcal, tetanus, and shingles vaccines are recommended for older adults routinely. This study aimed to show the knowledge and attitudes of the physicians to older adults’ vaccination schemes.
Patients and Methods: An electronic self-reported questionnaire was sent to physicians between March and July 2021 in Turkey. Sociodemographic characteristics, professional experience, area of expertise, and practice setting of the participants were recorded. As multiple-choice questions; the routinely recommended vaccines, and vaccines suggested in their daily practice before and after the COVID-19 pandemic were enquired.
Results: A total of 435 participants were included in the study. 43.9% of the patients were primary family physicians, and 36.8% were internists. 63.4% of the participants had reported reviewing the National Vaccination Scheme. 94.5% of the medical doctors indicated that they had recommended any vaccination to their patients. 20.9% of the practitioners could select four or five of the routinely recommended vaccines. Reviewing the National Adult Vaccination Scheme and being an internist were positively related to predicting the recommended vaccines. The recommendation rates of influenza and pneumococcal conjugate (PCV13) were seen at 88% and 78%, respectively. Except for PCV13, recommendation rates of other routine vaccines were decreased after the pandemic.
Conclusion: Awareness of routine vaccination schedules should be improved among health-care professionals, and reminders for immunization should be provided periodically in each health-care setting.
Keywords: older adults, attitudes to vaccination, vaccine hesitancy, influenza, herpes zoster, preventive care
Introduction
The older population is increasing rapidly due to increasing life expectancy and decreasing birth rates worldwide. By 2050, it is predicted that the number of older adults in the world will increase by three times to 2 billion and will exceed 20% of the population.1 Because chronic diseases are common in older patients, it is expected that countries’ health expenditures will greatly increase in the future.2 With the aging population, the importance of preventive health-care services for older adults has become more evident in recent years.
Physiological changes are seen in both innate and acquired immune systems with aging which is called immunosenescence. A decrease in bone marrow hematopoietic stem cell tissue and hematopoietic stem cell proliferation occurs.3 The decrease in all these humoral and cellular immune system functions and the formation of a relative immune deficiency have increased susceptibility to infectious pathogens and weak vaccine responses in older adults.4 Frailty results in more immune dysregulation than age-related changes and causes vulnerability to infectious diseases.5 After 65, the risk of death from influenza and pneumococcal disease increases markedly. Respiratory failure due to influenza is 10–30 times more common in the elderly than in the young.6,7 It has been shown that the annual cost of vaccine-preventable diseases in the USA is 9–26 million dollars, and 80% of this amount is used in the treatment of unvaccinated people.8 The annual cost for individuals aged 65 and over for four vaccine-preventable diseases (pneumonia, pertussis, shingles, and influenza) was at least $15 million.9 Although some countries have started to develop elderly vaccination policies, adult vaccination rates are still low in many countries, including developed countries.10 The effectiveness and necessity of preventive health-care measures and notable vaccination were also underlined during the COVID-19 pandemic.11,12
The Centers for Disease Control and Prevention recommends inactivated or recombinant influenza, Pneumococcal polysaccharide (PPSV23), Pneumococcal conjugate (PCV13) (if previously not administered), Tetanus, diphtheria, pertussis (Tdap or Td), and recombinant herpes zoster (RZV) vaccines in older adults.13 Only 69% of older adults have covered the suggested pneumococcal vaccines in 2017 in the United States.14 Many factors are thought to influence the vaccination decision of the patients, including sociocultural determinants, awareness, reaching correct information, and misperceptions. Physicians’ negative attitudes, inadequate knowledge of vaccination, or the neglect of preventive health-care measures may aggravate patients’ vaccine hesitancy.15 Therefore, the study aimed to show the awareness of older adults’ vaccination schedules among specialists and the rate of recommendation of the defined vaccines to the patients in daily practice.
Methods
Study Population and the Questionnaire
An electronic survey link was delivered to the medical doctors via email, social media, and text message. The survey was created with Google Forms, and participants were obligated to select one or multiple choices for each question to complete and send the form. The list of potential participants was reached by the hospital medical doctor groups, local primary care physician federations, and local medical societies. The responders could forward the invitation to their colleagues. Medical doctors who are willing to fulfill the questionnaire completed the form online. Informed consent was obtained from the participants. The study was conducted between March and July 2021 in Turkey.
The participants were classified according to their organizations, such as primary, secondary, or tertiary health-care services. Sociodemographic characteristics including sex, age, professional experience, area of expertise, and whether being a resident or specialist were recorded. A questionnaire containing eleven questions except for descriptive information about the participants was constructed via Google Forms. When designing the form, the Centers for Disease Control (CDC) Recommended Adult Immunization Schedule13 and National Adult Vaccination Scheme16 were accepted for the routine vaccination recommendations in older adults. The recommended vaccinations for the elderly in both guidelines were five vaccines, including inactivated or recombinant influenza, PPSV23, PCV13, Tdap or Td, and RZV vaccines. All the vaccines except for RZV are available in Turkey.
In the survey, the routinely recommended vaccines in the guideline, and vaccines suggested in their daily practice before and after the COVID-19 pandemic were enquired. Additionally, it was questioned who should advise the vaccines (pharmacists, primary care physicians, internists, pulmonologists, or Infectious diseases doctors), whether the physician had examined patients’ vaccination history, prescribed any vaccines, and informed patients about the COVID-19 vaccine. The questionnaire form was included as a Supplementary Questionnaire.
Compliance with Ethical Standards
All procedures performed in the study were in accordance with the ethical standards of the national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Ethical approval for this study was obtained from Cukurova University local ethics committee. Informed consent was obtained from all participants.
Study Size
Estimating 153,100 medical doctors serving in the country, it was decided to include at least 384 participants in the study to make a generalization, at the level of 5% acceptable error and 95% confidence.
Statistical Analysis
Descriptive statistics are shown as the number of cases and percentage (%) for nominal variables. Continuous variables with normal distribution were compared with the independent-samples t-test, and the Mann–Whitney U-test was used in the case of non-normal distribution. Logistic regression and multinominal logistic regression analysis were performed to show the association between predicting at least 4 of 5 routinely recommended vaccines and the variables including age, sex, practice setting, specialty, and experience. The effect of the pandemic on the vaccination suggestion was defined with McNemar’s test. The data analysis was carried out in SPSS for Windows 25 package program (SPSS Inc., Chicago, IL, USA). p < 0.05 was considered statistically significant.
Results
A total of 435 participants were included in the study. 43.9% (191) of the patients were primary family physicians, and 36.8% (160) were internists. 63.4% of the participants had reported reviewing the National Vaccination Scheme. 94.5% (411) of the medical doctors indicated that they had recommended vaccination to their patients. 87.8% of the doctors recommended COVID-19 vaccination to their patients. 33.8% of the participants had not questioned the vaccination history of the patients. Doctors who work in a secondary or tertiary healthcare service had not reviewed the “National Adult Vaccination Scheme”, were younger, had less experience, and had not questioned vaccination history. According to the assessment of vaccination history, participant characteristics are summarized in Table 1.
 |
Table 1 Characteristics of the Physicians According to the Assessment of Vaccination History |
When the routine vaccination scheme was asked to the participants, 17.5% (76), 6.0% (26), 9.0% (39), 9.2% (38), and 28.0% (122) of them incorrectly selected Haemophilus influenzae type b (Hib), Varicella, Measles, mumps, rubella (MMR), Hepatitis A (HepA) and Hepatitis B (HepB), respectively. Figure 1 shows the percentages of the incorrectly predicted as recommended vaccines.
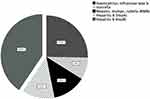 |
Figure 1 Incorrectly Predicted as Routinely Recommended Vaccines. |
20.9% (91) of the practitioners correctly selected four or five of the routinely recommended vaccines. Logistic and multinomial regression analysis results show that reviewing the National Adult Vaccination Scheme and being an internist were related to predicting four or five of the recommended vaccines (Table 2).
 |
Table 2 Predicting Four or Five of the Suggested Vaccinations in Older Adults |
The recommendation rate of influenza and PCV 13 was seen at 88% and 78%, respectively. PPSV23, RZV and Tdap or Td were lower. Additionally, except for PCV13, recommendation rates of other routine vaccines were decreased after the pandemic. The change in the recommendation rates is shown in Table 3.
 |
Table 3 Recommendation of Vaccinations Before and After the Pandemic |
Discussion
The study, which aims to assess the awareness and attitude of the physicians to older vaccination, showed that 95% of participants recommended vaccination to their patients, and 63% reviewed the vaccination recommendations. Almost one out of five physicians correctly selected routinely recommended vaccinations. Hib and HepB vaccines were commonly incorrectly presumed to be in the routine vaccination scheme. Recommendations rates were lower for PPSV23, RZV, Tdap, or Td. Moreover, attention given to vaccination was shifted to the COVID-19 vaccine during the pandemic except for PCV13.
Vaccine-preventable diseases are still related to unwanted severe diseases and mortality among vulnerable older adults. Although the efficacy of influenza vaccines is reduced in older people compared to adults, annual influenza vaccination is suggested by the World Health Organization (WHO) as an effective strategy for invasive disease prevention.17 A total of 24,663 invasive pneumococcal diseases were reported, and the rates were highest in older adults (18.7 per 100,000 population) in 2018. Among the cases, 73% were covered by serotypes included in the PPSV23, and 29% were serotyped in the PCV13.18 In this context, because of the PCV13 inclusion in routine childhood vaccination programs, PCV13 serotype disease was reduced. The Advisory Committee on Immunization Practices (ACIP) recommended discontinuing for routine use of PCV13 among adults ≥65.13 However, the decision should be given personally, especially to those who had not been administered a dose before.
Influenza and pneumococcal vaccines, especially PCV13 are generally better known and suggested to older patients than other vaccines. Reimbursement for annual inactivated influenza vaccine and PCV13 once is available for adults over 65 years in Turkey. A survey study conducted with 620 general internal medicine and family physicians reported that 98% of the participants always recommend influenza vaccines to older adults. Standing orders and electronic alerts were commonly used in the practice-based immunization.19 In addition, Internal medicine specialists were reported to be higher rates of assessing, recommending, and stocking for most vaccine types, notably susceptible adults under risk with comorbidities.20 Compatible with the literature, in the present study, influenza, and PCV13 were the most recommended vaccines, and primary physicians and internists suggest and review the vaccination history frequently. Vaccination review was most commonly made in primary care. A study conducted in Australia supporting our results reported that most hospital doctors agreed that competing priorities make a challenge to vaccinations.21
Nevertheless, coverage among adults for influenza vaccination during the 2017−18 season was 46.1%, and coverage for pneumococcal at least one dose of PPSV23 or PCV13 in older adults was 69.0% in the United States.22 Vaccination coverage remains below goal. Vaccine hesitancy is the major limitation to reaching the vaccination goals. The reasons for vaccine hesitancy are generally multifactorial including age, gender, education level, economic status, patients’ health conditions, awareness of preventive measures, adequate and accurate information, and accessibility to health services.23 Among the factors, it is reported that the strongest predictor for pneumococcal vaccine uptake was being offered by a health-care professional.24
Herpes zoster (HZ) causes mainly undesirable complications chronic neuropathic pain of postherpetic neuralgia. Incidence of HZ is reported to be 5.23 to 10.9/1000 person-years in ≥50 years of age.25 Two doses of RZV provide effective prevention regardless of a prior episode of shingles and are also safe in immunocompromised persons. RZV vaccine coverage remains suboptimal in the United States (34.5% among those aged ≥60).22 Lower rates of RZV vaccination may be explained by the unavailability of the vaccine in the country, lack of information about the vaccine, less priority given by health-care providers, exclusion of vaccine from reimbursement, and concerns about the vaccine.26 Accordingly, RZV was the lowest recommended in the present study.
Td or Tdap decennial booster is suggested for previously vaccinated adults. Tdap coverage was reported at 31.2% in adults aged ≥19 years.22 In this study, Tdap or Td is the less recommended vaccine following RZV, and it has been shown in a previous study that the vaccination rate is low among health-care workers.27 Tdap recommendations to pregnant women were also reported as %61 among gynecologists, and 15% of the participants did not know the vaccine was on the routine immunization schedule.28 The booster doses are frequently not considered in older adults except for wound management.
The present study highlights the low rates of immunization recommendations from physicians and even lower rates after the pandemic. Vaccination, an important public health policy, was observed to be given lower priority in our clinical routine. Multiple approaches were shown to be effective to achieve high vaccination coverage rates.29 To help address gaps in the success of health measures and improve performance on preventive services, on-The-job training activities for health-care providers, reminder software programs for the evaluation of older patients in primary care, promotion of communication, and removal of fears and concerns about vaccines,30 co-administration of suitable vaccines (eg, PPV23 and influenza)31 may be effective strategies. Additionally, admission to the outpatient setting or hospitalization could also be assumed as an opportunity to offer the indicated vaccines and inform about the immunization options for the patients who have obstacles to access healthcare. Doctor–patient relationship should be constructed with trust, and empathy to convince the patient to the therapy.32 Since health-care professionals’ attitude was shown to be more convincing on vaccination, greater implementation of the routine vaccination recommendations by all health-care providers across the clinical specialties could improve adult vaccination rates. Preventive medicine practices should be included more frequently in medical school and post-graduation education curricula.
The study has many strengths, including reaching physicians in different specialties, practice settings, and regions of the country, and reviewing all the vaccination schemes for older adults. To the best of our knowledge, this is the first study evaluating physicians’ attitudes and knowledge on older adults’ vaccination schemes. On the other hand, it has some limitations. Many physicians were included in different practice settings and specialties, and comparisons were performed in these groups. However, we cannot define physicians’ awareness from different country regions. Additionally, the representativeness of survey respondents could not be assessed, and the results presented may not be generalized. The accuracy of the answers cannot be controlled in survey studies since participants are free to express their responses. Moreover, because of limited studies focusing on vaccination coverage rates in Turkey, we could not compare the rates between different countries.
Conclusion
Awareness of routine vaccination schedules should be improved among health-care professionals, and reminders for immunization should be provided periodically in each health-care setting. New strategies should be implemented to enhance physicians’ attitudes to vaccination coverage in older adults.
Data Sharing Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
Authors thank the administrators of the Geriatric Science Association and the Adana Association of Family Physicians for their help in recruiting participants.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ellen ME, Panisset U, Araujo de Carvalho I, Goodwin J, Beard J. A knowledge translation framework on ageing and health. Health Policy. 2017;121(3):282–291. doi:10.1016/j.healthpol.2016.12.009
2. Doherty TM, Connolly MP, Del Giudice G, et al. Vaccination programs for older adults in an era of demographic change. Eur Geriatr Med. 2018;9(3):289–300. doi:10.1007/s41999-018-0040-8
3. Sadighi Akha AA. Aging and the immune system: an overview. J Immunol Methods. 2018;463:21–26. doi:10.1016/j.jim.2018.08.005
4. Crooke SN, Ovsyannikova IG, Poland GA, Kennedy RB. Immunosenescence and human vaccine immune responses. Immun Ageing. 2019;16:25. doi:10.1186/s12979-019-0164-9
5. Palermo S. Covid-19 pandemic: maximizing future vaccination treatments considering aging and frailty. Front Med. 2020;7. doi:10.3389/fmed.2020.558835
6. Smetana J, Chlibek R, Shaw J, Splino M, Prymula R. Influenza vaccination in the elderly. Hum Vaccin Immunother. 2018;14(3):540–549. doi:10.1080/21645515.2017.1343226
7. Gatwood J, Shuvo S, Hohmeier KC, et al. Pneumococcal vaccination in older adults: an initial analysis of social determinants of health and vaccine uptake. Vaccine. 2020;38(35):5607–5617. doi:10.1016/j.vaccine.2020.06.077
8. Ozawa S, Portnoy A, Getaneh H, et al. Modeling the economic burden of adult vaccine-preventable diseases in the United States. Health Aff. 2016;35(11):2124–2132. doi:10.1377/hlthaff.2016.0462
9. McLaughlin JM, McGinnis JJ, Tan L, Mercatante A, Fortuna J. Estimated human and economic burden of four major adult vaccine-preventable diseases in the United States, 2013. J Prim Prev. 2015;36(4):259–273. doi:10.1007/s10935-015-0394-3
10. Williams WW, Lu PJ, O’Halloran A, et al. Surveillance of vaccination coverage among adult populations - United States, 2015. Morb Mortal Wkly Rep Surveill Summ. 2017;66(11):1–28. doi:10.15585/mmwr.ss6611a1
11. Gallant AJ, Nicholls LAB, Rasmussen S, Cogan N, Young D, Williams L. Changes in attitudes to vaccination as a result of the COVID-19 pandemic: a longitudinal study of older adults in the UK. PLoS One. 2021;16(12):e0261844. doi:10.1371/journal.pone.0261844
12. Pedote PD, Termite S, Gigliobianco A, Lopalco PL, Bianchi FP. Influenza vaccination and health outcomes in COVID-19 patients: a retrospective cohort study. Vaccines. 2021;9(4). doi:10.3390/vaccines9040358
13. Freedman MS, Bernstein H, Ault KA. Recommended adult immunization schedule, United States, 2021. Ann Intern Med. 2021;174(3):374–384. doi:10.7326/M20-8080
14. Centers for Disease Control and Prevention. Vaccination coverage among adults in the United States, national health interview survey; 2017. Available from: https://www.cdc.gov/vaccines/imz-managers/coverage/adultvaxview/pubs-resources/NHIS-2017.ht.
15. Ozisik L, Calik Basaran N, Oz SG, Sain Guven G, Durusu Tanriover M. Perceptions and attitudes of patients about adult vaccination and their vaccination status: still a long way to go? Med Sci Monit. 2017;23:3178–3184. doi:10.12659/msm.901856
16. Turkish Infectious Diseases and Clinical Microbiology Association. Adult immunization working group. Adult Immunization Guide; 2019. Available from: https://www.ekmud.org.tr/rehberler/1-ekmud-rehberleri;.
17. Ciabattini A, Nardini C, Santoro F, Garagnani P, Franceschi C, Medaglini D. Vaccination in the elderly: the challenge of immune changes with aging. Semin Immunol. 2018;40:83–94. doi:10.1016/j.smim.2018.10.010
18. European Centre for Disease Prevention and Control. Invasive pneumococcal disease. Annual epidemiological report for 2018. Stockholm: ECDC; 2020. Available from: https://www.ecdc.europa.eu/en/publications-data/invasive-pneumococcal-disease-annual-epidemiological-report-2018.
19. Cataldi JR, O’Leary ST, Lindley MC, et al. Survey of adult influenza vaccination practices and perspectives among US primary care providers (2016–2017 influenza season). J Gen Intern Med. 2019;34(10):2167–2175. doi:10.1007/s11606-019-05164-7
20. Lutz CS, Kim DK, Black CL, et al. Clinicians’ and pharmacists’ reported implementation of vaccination practices for adults. Am J Prev Med. 2018;55(3):308–318. doi:10.1016/j.amepre.2018.05.011
21. Ridda I, Lindley IR, Gao Z, McIntyre P, Macintyre CR. Differences in attitudes, beliefs and knowledge of hospital health care workers and community doctors to vaccination of older people. Vaccine. 2008;26(44):5633–5640. doi:10.1016/j.vaccine.2008.07.070
22. Lu PJ, Hung MC, Srivastav A, et al. Surveillance of vaccination coverage among adult populations -United States, 2018. Morb Mortal Wkly Rep Surveill Summ. 2021;70(3):1–26. doi:10.15585/mmwr.ss7003a1
23. Ventola CL. Immunization in the United States: recommendations, barriers, and measures to improve compliance: part 2: adult vaccinations. P t. 2016;41(8):492–506.
24. Nicholls LAB, Gallant AJ, Cogan N, Rasmussen S, Young D, Williams L. Older adults’ vaccine hesitancy: psychosocial factors associated with influenza, pneumococcal, and shingles vaccine uptake. Vaccine. 2021;39(26):3520–3527. doi:10.1016/j.vaccine.2021.04.062
25. Harbecke R, Cohen JI, Oxman MN. Herpes Zoster Vaccines. J Infect Dis. 2021;224(12Suppl 2):S429–s442. doi:10.1093/infdis/jiab387
26. Ackerson B, Qian L, Sy LS, et al. Completion of the two-dose recombinant zoster vaccine series in adults 50 years and older. Vaccine. 2021;39(6):926–932. doi:10.1016/j.vaccine.2020.12.076
27. Randi BA, Miyaji KT, Lara AN, et al. Low tetanus-diphtheria-acellular pertussis (Tdap) vaccine coverage among healthcare workers in a quaternary university hospital in São Paulo, Brazil: need for continuous surveillance and implementation of active strategies. Braz J Infect Dis. 2019;23(4):231–236. doi:10.1016/j.bjid.2019.06.007
28. Mazzilli S, Tavoschi L, Lopalco PL. Knowledge, attitudes and practices concerning pertussis maternal immunization in a sample of Italian gynaecologists. Hum Vaccin Immunother. 2021;17(6):1681–1685. doi:10.1080/21645515.2020.1833580
29. Bianchi FP, Tafuri S. Vaccination of elderly people affected by chronic diseases: a challenge for public health. Vaccines. 2022;10(5). doi:10.3390/vaccines10050641
30. Sato K, Kondo N, Murata C, Shobugawa Y, Saito K, Kondo K. Association of pneumococcal and influenza vaccination with patient-physician communication in older adults: a nationwide cross-sectional study from the JAGES 2016. J Epidemiol. 2021;32:401–407. doi:10.2188/jea.JE20200505
31. Triglav TK, Poljak M. Vaccination indications and limits in the elderly. Acta Dermatovenerol Alp Panon Adriat. 2013;22(3):65–70.
32. Benedetti F. Placebo and the new physiology of the doctor-patient relationship. Physiol Rev. 2013;93(3):1207–1246. doi:10.1152/physrev.00043.2012
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
