Back to Journals » International Journal of General Medicine » Volume 17
The Atherogenic Index of Plasma Predicts Carotid in-Stent Restenosis: Development and Validation of a Nomogram
Authors Zhou Y, Ma Y, Qian D, Zhou Z, Li B, Chai E
Received 4 November 2023
Accepted for publication 16 January 2024
Published 25 January 2024 Volume 2024:17 Pages 263—274
DOI https://doi.org/10.2147/IJGM.S447008
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Yuriy Sirenko
Yu Zhou,1– 3,* Yong Ma,2– 4,* Dongliang Qian,2,3,* Zhou Zhou,5,6,* Bin Li,2,3,* Erqing Chai1– 3
1First Clinical Medical College, Lanzhou University, Lanzhou, People’s Republic of China; 2Cerebrovascular Disease Center, Gansu Provincial Hospital, Lanzhou, People’s Republic of China; 3Key Laboratory of Cerebrovascular Diseases, Lanzhou, People’s Republic of China; 4Clinical Medicine College, Ningxia Medical University, Yinchuan, People’s Republic of China; 5First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, Tianjin, People’s Republic of China; 6National Clinical Research Center for Chinese Medicine Acupuncture and Moxibustion, Tianjin, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Erqing Chai, First Clinical Medical College, Lanzhou University, No. 204, Donggang West Road, Chengguan District, Lanzhou City, 730000, People’s Republic of China, Tel +86 13893439044, Email [email protected]
Purpose: To explore the predictive value of atherogenic index of plasma(AIP) for carotid in-stent restenosis(ISR).
Methods: Patients who underwent carotid artery stenting (CAS) in hospital from January 2016 to January 2021 were retrospectively enrolled. They were randomly divided into training and validation sets. Based on the results of carotid digital subtraction angiography (DSA) during the follow-up period, the patients were divided into ISR group and non-ISR group. The differences of AIP and lipid levels between the two groups were compared. The independent risk factors of ISR and the predictive value of AIP for ISR were analyzed. A nomogram was developed based on the independent risk factors, and the receiver operating characteristic (ROC) curve, the calibration curve and the decision curve analysis were conducted to assess the predictive ability and clinical practicability of the nomogram in both the training set and validation sets.
Results: A total of 361 patients were enrolled, including 98 in the ISR group and 263 in the non-ISR group. In the training set, AIP was significantly higher in the ISR group than in the non-ISR group (P < 0.05) and was independently associated with ISR (OR= 10.912, 95% CI: 2.520– 47.248). When AIP was 0.10, it had the highest predictive value for ISR, with a sensitivity of 72. 1% and a specificity of 75.0%. Additionally, hypertension, residual stenosis, symptomatic stenosis and Hcy were also independent risk factors for ISR. The nomogram showed good discrimination performance and clinical practicability in both the training set (AUC = 0.827) and the validation set (AUC = 0.880).
Conclusion: AIP was an independent risk factor for ISR and was closely related to ISR. The nomogram developed by AIP and other variables had good predictive ability and clinical practicability for ISR.
Keywords: atherogenic index of plasma, carotid stenosis, carotid in-stent restenosis, neurointervention, nomogram
Introduction
Carotid artery stenosis is a primary cause of ischemic stroke, accounting for 10–20% of all cases.1 Ischemic stroke is characterized by its high prevalence, disability rate, and mortality.2 Currently, surgical treatments for carotid stenosis primarily include carotid endarterectomy (CEA) and carotid artery stenting (CAS).3 An increasing body of clinical evidence suggests that CAS and CEA exhibit equivalent efficacy and safety, with some experts even favoring CAS to a certain extent.4–7 Studies have shown that 3–30% of patients with carotid artery stenosis develop in-stent restenosis (ISR) after CAS, which is a common complication.8 It not only impacts patients’ long-term prognosis and quality of life but also increases the risk of recurrent ischemic strokes, resulting in significant economic burdens and family strain. Therefore, it holds great significance to identify the risk factors associated with ISR after CAS and provide early interventions for CAS.
The Atherogenic Index of Plasma (AIP), proposed by Dobiasova M et al, is calculated based on triglyceride (TG) and high-density lipoprotein cholesterol (HDL-C) levels and the formula is lg(TG/HDL-C)9. It has been suggested that AIP is the sole lipid parameter independently associated with symptomatic carotid stenosis.10 M. Gentile et al also demonstrated an association between elevated AIP and increased Intima Media Thickness (IMT).11 Therefore, it is reasonable to speculate that elevated AIP may be related to ISR. However, the association between these two factors is seldom reported. Hence, this study aimed to investigate the relationship between AIP and ISR and to develop and validate a nomogram for predicting ISR risk, which will aid clinicians in early ISR prevention and improve patient prognosis.
Methods
Population
Patients who underwent CAS between January 2016 and January 2021 were included in this study.
Inclusion Criteria
- Carotid stenosis or occlusion diagnosed by digital subtraction angiography(DSA).
- Age>18.
- Undergo CAS.
- Complete DSA follow-up for a duration exceeding 6 months.
- Complete clinical data.
Exclusion Criteria
- Patients with non-atherosclerotic diseases, such as internal carotid artery congenital vascular stenosis, internal carotid artery rupture, internal carotid artery dissection, internal carotid artery aneurysm, etc., were excluded, even if they had undergone CAS treatment.
- Together with severe organ dysfunction, such as liver or kidney impairment.
- Together with cerebral hemorrhage and other hemorrhagic diseases.
- Disorders of consciousness and mental consciousness.
- Incomplete clinical data.
- Loss to follow-up.
Finally, a total of 361 patients participated in the study and were randomly divided into the training set and the validation set in a 7:3 ratio (Figure 1). This study was approved by the Clinical Research Ethics Committee of Gansu Provincial Hospital without any opinions, and all patients were given written informed consent to participate in this study. This study fully followed the principles outlined in the Declaration of Helsinki.
 |
Figure 1 Schematic of patient’s inclusion process and flow chart with the study. |
Observation Indicators
We collected patient information from electronic and paper medical records, as well as imaging data. This included general information such as gender, age, stent diameter (6mm,7mm,8mm,9mm), stent length (30mm,40mm), residual stenosis degree (<10%, 10–30%, 30–40%), and past medical history (smoking, hypertension, atrial fibrillation, diabetes mellitus(DM), symptomatic stenosis). Additionally, we recorded the levels of key biomarker at admission, which included low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglycerides (TG), total cholesterol (TC), homocysteine (Hcy), white blood cell count (WBC), red blood cell count (RBC), hemoglobin (Hg), hematocrit (HCT), and Atherogenic Index of Plasma (AIP). LDL-C, HDL-C, TG, TC and Hcy were measured by AU5831 Automatic Biochemical analyzer (Beckman Coulter). WBC, RBC, Hg, HCT were measured by BC-7500 Hematology Analyzer (Beckman Coulter). AIP = lg(TG/HDL-C).
Carotid Artery Stenting Procedure
Prior to CAS, patients received a daily regimen of 100mg aspirin and 75mg clopidogrel for at least 3 days. If CAS was scheduled within 72 hours, an additional 300mg of clopidogrel was administered 4 hours before the procedure. Patients with symptomatic carotid stenosis, coronary or peripheral artery disease, hypercholesterolemia, or ulcerated plaques were given 20mg atorvastatin or 10mg rosuvastatin daily for at least 3 days before CAS in addition to aspirin and clopidogrel and continued these statins afterward unless statin intolerance occurred. The procedure began by inserting an 8F sheath into the femoral artery to obtain vascular access, followed by administering a weight-adjusted dose of heparin (70IU/kg) to reduce the risk of thromboembolic events. After positioning the 8F guide catheter near the stenosis, a distal embolic protection device was placed through the stenotic lesion. If it was a subtotal occlusive stenosis, predilation was usually performed, followed by placement and deployment of a self-expanding stent of appropriate size, if required. Postdilation was typically conducted for residual stenosis degree exceeding 40%, followed by a final angiography to assess residual stenosis and removal of the protection device. Following surgery, patients received dual antiplatelet therapy (aspirin and clopidogrel) for a minimum of 90 days. Afterward, they continued with monotherapy, typically aspirin, for life.
Follow-Up and Grouping
Patients underwent one DSA imaging follow-up session at 6–12 months after discharge. Subsequent DSA imaging follow-ups were scheduled based on patient symptoms and the results of the initial follow-up. The follow-up period concluded in December 2022. ISR was defined as the presence of more than 50% stenosis in the treated region. According to the follow-up results of DSA, the enrolled patients were divided into the ISR group and the no-ISR group. DSA was performed with the Artis zee III ceiling digital subtraction angiography machine (SIEMENS AG).
Statistical Analysis
The statistical analysis was performed by using SPSS 26.0 and R software (version 4.3.0). Continuous variables with normal distribution were expressed as mean ± standard deviation, while non-normally distributed variables were expressed as the interquartile range. Categorical variables were presented as frequency (%).
Univariate analysis was used to screen potential risk factors for ISR. To determine the independent risk factors for ISR, the variables with P < 0.05 in the univariate analysis were included in the multivariate logistic regression model. Then, the predictive nomogram was developed based on the independent risk factors by using the “rms” package in R software. The receiver operating characteristic (ROC) curve, the calibration curve and the decision curve analysis(DCA) were conducted to assess the predictive ability and clinical practicability of the nomogram in both the training set and validation sets. The statistical significance for all variables was set at P < 0.05 (two-sided tests), and the regression coefficients reported 95% confidence intervals (CI).
Result
Clinical characteristics of patients in the training and validation set
A total of 361 patients were included in the analysis, including 253 patients in the training set and 108 patients in the validation set. ISR was observed in 61 patients (24.11%) in the training cohort and 37 patients (34.26%) in the validation cohort. Patients characteristics of the training and validation sets were no significant differences in Table 1 (P >0.05).
 |
Table 1 Baseline and Clinical Characteristics of Patients in the Training Set and Validation Set |
Baseline characteristics of ISR group and no-ISR group in the training set
Descriptive analysis showed that there were significant differences in hypertension(P=0.003), DM(p=0.021), smoking(P=0.032), symptomatic stenosis(P=0.001), Hcy(p<0.001), TG(P<0.001), HDL(P=0.022), AIP(P<0.001) and residual stenosis(P=0.002) between the two groups; Table 2
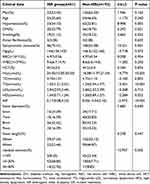 |
Table 2 Baseline Characteristics of ISR Group and Non-ISR Group in the Training Set |
Identifying the Independent Risk Factors for ISR
All the potential risk factors (P < 0.05) in the univariate regression analysis were included in the multivariate regression model. Multivariate logistic regression analysis revealed that hypertension(OR=2.340, 95% CI: 1.157–4.730, P=0.018), symptomatic stenosis(OR=3.256, 95% CI: 1.516–6.993, P =0.002), Hcy (OR=1.094, 95% CI:1.051–1.138, P<0.001), AIP (OR =10.912, 95% CI: 2.520–47.248, P=0.001) and residual stenosis(P=0.006) were independent risk predictors of ISR (Table 3).
 |
Table 3 Univariate and Multivariate Logistic Regression Analyses of ISR in the Training Set |
The Predictive Value of AIP for ISR
ROC curve analysis demonstrated that AIP exhibited a good predictive accuracy for prognosis, as illustrated in Figure 2. The area under the curve (AUC) for AIP was 75.4% (95% CI: 0.692–0.816). The optimal predictive value of AIP for ISR was 0.10, with a sensitivity of 72.1% and a specificity of 75.0%.
 |
Figure 2 ROC curve for the predictive value of AIP for ISR. |
The Predictive Nomogram Development
The nomogram was developed for predicting the risk of ISR based on the results from the multivariate logistic model, which included five variables (Figure 3). A vertical line was drawn up to the ”Point” axis to calculate the score of each variable, and the total score was summarized by the preliminary scores. The total score was located on the “Total Points” axis, and then, the predicted risk of ISR could be located on the bottom axis.
The Performance of the Nomogram
The calibration curve of the nomogram for the probability of ISR demonstrated a good agreement between prediction and observation for both sets (Figure 4). The Hosmer-Lemeshow H-test indicated that the model did not depart from perfect fit, which had non-statistical significance in the training set (P = 0.2418) and validation set (P = 0.6765).
The AUC for the nomogram was 0.827 (95% CI: 0.770–0.884) in the training set (Figure 5A) and was confirmed to be 0.880 (95% CI: 0.813–0.946) through internal validation in the validation set (Figure 5B), which demonstrated that the nomogram had a greater discriminatory performance. In addition, the discrimination ability of the nomogram calculated by the AUC was superior to the other risk factors in the training set: hypertension (0.606, 95% CI: 0.535–0.678), symptomatic stenosis (0.617, 95% CI: 0.552–0.682), residual stenosis (0.623, 95% CI: 0.559–0.688), Hcy (0.703, 95% CI: 0.621–0.785), and AIP (0.754, 95% CI: 0.692–0.816)(Figure 5A).
 |
Figure 5 The receiver operating characteristic (ROC) curve of the nomogram in the training set and the validation set. (A) ROC in the training set; (B) ROC in the validation set. |
Clinical Use
Moreover, the decision curve analysis (DCA) was used to assess the clinical validity of the nomogram, which indicated the predictive nomogram to be clinically useful (Figure 6).
 |
Figure 6 The DCA of the nomogram in the training set and validation set. (A) DCA in the training set; (B) DCA in the validation set. |
Discussion
The etiology of ISR after CAS is not attributed to a single factor but results from the interaction and convergence of various factors. Currently, numerous studies have examined ISR risk factors, including age, gender, diabetes, hypertension, hyperlipidemia, and biological markers, yet the findings remain diverse.12–16 The specific mechanisms underlying ISR, as well as prevention and mitigation measures, still require further research for resolution. In this study, the incidence of ISR was 27.15%, which was generally in line with previous research where ISR incidence ranged from 3% to 30%.8 The main findings were as follows: (1) Multivariate logistic regression analysis revealed that AIP was both an independent risk factor for ISR and a standalone predictor of ISR. (2) The nomogram, constructed using independent risk factors like AIP, demonstrated an excellent predictive capabilities for ISR.
The migration and proliferation of smooth muscle cells follow the secondary injury to the vascular endothelium after CAS stimulates the release of inflammatory mediators, facilitating the development of vascular neointima and ultimately contributing to ISR. Lipid metabolism disorder plays a crucial role in this process.17–19 Studies have demonstrated that AIP is considered an effective indicator for predicting atherosclerosis, providing a comprehensive reflection of the interaction among various plasma lipoprotein metabolism factors.20 The increased risk of carotid ISR in patients with elevated AIP may be attributed to the following factors: Firstly, AIP is primarily used to estimate the size of small, dense LDL (sdLDL) particles, a subcomponent of LDL known for its proinflammatory and pro-atherogenic properties. The higher AIP corresponds to smaller sdLDL particle sizes, which is characterized by slower blood clearance, susceptibility to oxidative modification, easy penetration of the endothelial barrier, and ready uptake by macrophage scavenger receptors9,21.These properties collectively promote smooth muscle cell migration and proliferation, leading to neointimal formation and ISR development.22 Secondly, M2 macrophages possess a transmembrane glycoprotein called mannose receptor type C1 (MRC1) on their cell membrane, which contributes to their high phagocytic capabilities. sdLDL has the ability to increase the expression of M2 macrophages, consequently promoting the phagocytosis of lipoproteins by these macrophages, resulting in foam cell formation. Furthermore, under the influence of sdLDL, M2 macrophages induce the expression of pro-inflammatory factors like histamine, leading to the release of inflammatory mediators such as IL-13, IL-10, IL-6, and IL-4. This, in turn, causes increased vascular permeability. Therefore, the combination of M2 macrophages’ heightened phagocytic capacity and their proinflammatory response can exacerbate the inflammatory cascade, ultimately leading to the formation of carotid neointima and the development of carotid ISR.23 Finally, studies have shown that AIP is positively correlated with various metabolic disorders, such as diabetes, insulin resistance, and hyperuricemia, which are closely related to ISR.24–28 Therefore, optimizing lipid management based on AIP may be a promising approach to prevent ISR, but this needs validation in further prospective studies.
Holmberg et al discovered that when blood pressure fluctuates or remains consistently high, it accelerated shear forces on the vessel wall, which led to increased cell proliferation, macrophage infiltration, and collagen deposition following mechanical injury, and thought that high blood pressure may contribute to the increased incidence of ISR.29 Furthermore, elevated levels of vasoactive substances such as catecholamines and renin-angiotensin in the plasma of hypertensive patients result in increased tension in vascular smooth muscle cells (SMCs). Prolonged tension elevation can lead to damage and metabolic disorders in SMCs. In the presence of various SMC growth factors working synergistically, this promotes the proliferation of vascular SMCs, ultimately leading to the development of ISR. Homocysteine (Hcy) has been identified as a risk factor for atherosclerosis.30 Elevated levels of Hcy can directly or indirectly cause injury or apoptosis in vascular endothelial cells, subsequently promoting the proliferation of arterial smooth muscle cells. This process also involves the oxidation of low-density lipoprotein cholesterol, a reduction in high-density lipoprotein cholesterol, induction of vascular wall thickening, and the formation of in-stent plaque. These mechanisms are likely the underlying factors in Hcy’s promotion of ISR.31
A multicenter, randomized, controlled trial conducted in Canada included 1321 patients with symptomatic carotid stenosis and 1181 patients with asymptomatic carotid stenosis. The results indicated that the incidence of perioperative stroke or death in patients with symptomatic stenosis was significantly higher than that in patients with asymptomatic stenosis (6.0% vs 2.5%).32 Another Meta-analysis, comprising 206 studies and a total of 54,713 patients, also suggested that symptomatic stenosis was an independent risk factor for complications (stroke or death) within 30 days after CAS (OR: 1.86; 95% CI: 1.61–2.14).33 Overall, patients with symptomatic stenosis had an increased risk of complications after CAS compared with patients with asymptomatic stenosis. On the one hand, carotid lesions in patients with symptomatic stenosis often have a pathological basis of plaque ulceration and intraplaque thrombosis, making them more prone to vascular stenosis. On the other hand, patients with symptomatic stenosis exhibit elevated levels of local or systemic inflammatory markers compared to those with asymptomatic stenosis. Inflammation has a significant role in ISR development, with the vascular injury site around the stent seeing infiltration by numerous mononuclear macrophages and T lymphocytes. This infiltration leads to the production of various growth factors, chemokines, and cytokines, further promoting white blood cell infiltration and triggering a local inflammatory response. This cascade results in neointimal hyperplasia, vascular wall remodeling, and ultimately, ISR.34–36
Consistent with previous findings, residual stenosis was an independent risk factor for ISR.37,38 Cosottirni et al showed that for every 1% increase in residual stenosis after CAS, the risk of ISR increased by 1.09-fold.39 This association is likely because higher degrees of residual stenosis signify inadequate lesion treatment. Furthermore, elevated residual stenosis levels can trigger hemodynamic abnormalities, and then cause platelet aggregation, leading to the occurrence of ISR. Therefore, releasing the stent as fully as possible can reduce residual stenosis. At the same time, post-balloon dilation of the carotid artery can reduce the residual stenosis and reduce the risk of ISR. However, it should also be noted that excessive pursuit of no residual stenosis will increase the perioperative risk and the possibility of long-term restenosis. A lower degree of residual stenosis may indicate that the balloon or stent causes more mechanical damage to the vascular endothelium, which may lead to a higher risk of ISR.40
The reasons why variables such as smoking history, diabetes history, atrial fibrillation history, stent length, or diameter were not found to be associated with ISR in this study may be as follows: 1.Due to the limitations of this study sample and single-center study, although there were numerical differences in the same factors between different groups, such numerical differences had not shown statistical significance. 2.We provided post-operative health education to guide patients in actively controlling risk factors that could lead to recurrent stroke, which might also have had an impact on controlling ISR-related risk factors to some extent.
Limitations
This study had several limitations. Firstly, it was a single-center retrospective study, lacking external validation. Secondly, the sample size and potential unforeseen confounding factors may impact the results. Finally, categorical variables, such as hypertension, DM, smoking and so on, were not further classified, potentially affecting the outcomes. Furthermore, the study did not involve dynamic monitoring of clinical data, which could have provided more accurate predictive value for ISR.
Conclusion
This study revealed an association between AIP levels and carotid ISR, and AIP had a certain value in predicting carotid ISR. Furthermore, we constructed a nomogram based on five independent risk factors to predict ISR, which was beneficial for clinicians in identifying high-risk ISR groups, stratifying and managing the prognosis of CAS patients, and maximizing the benefits of CAS treatment.
Data Sharing Statement
The datasets used or analysed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This study was approved by the Clinical Research Ethics Committee of Gansu Provincial Hospital, and all methods were performed in accordance with the relevant guidelines and regulations. All patients were informed and agreed to participate in this study.
Funding
This work was supported by the Natural Science Foundation of Gansu Province (23JRRA1308) and the Natural Science Foundation of Gansu Province(20JR10RA384).
Disclosure
The authors declare no competing interests.
References
1. White H, Boden-Albala B, Wang C., et al. Ischemic stroke subtype incidence among Whites, Blacks, and Hispanics: the northern Manhattan study. Circulation. 2005;111(10):1327–1331. doi:10.1161/01.Cir.0000157736.19739.D0
2. Wu S, Wu B, Liu M, et al. Stroke in China: advances and challenges in epidemiology, prevention, and management. Lancet Neurol. 2019; 18,394–405.doi:10.1016/s1474-4422(18)30500-3
3. Texakalidis P, Giannopoulos S, K JA, et al. Carotid artery endarterectomy versus carotid artery stenting for restenosis after carotid artery endarterectomy: a systematic review and meta-analysis. World Neurosurg. 2018;115:421–429.e421. doi:10.1016/j.wneu.2018.02.196
4. H BL, Dobson J, L FR, et al. Long-term outcomes after stenting versus endarterectomy for treatment of symptomatic carotid stenosis: the International Carotid Stenting Study (ICSS) randomised trial. Lancet. 2015;385:529–538. doi:10.1016/s0140-6736(14)61184-3
5. G BT, Howard G, S RG, et al. Long-term results of stenting versus endarterectomy for carotid-artery stenosis. N Engl J Med. 2016;374:1021–1031. doi:10.1056/NEJMoa1505215
6. S GH, Yadav JS, Fayad P, et al. Long-term results of carotid stenting versus endarterectomy in high-risk patients. N Engl J Med. 2008;358:1572–1579. doi:10.1056/NEJMoa0708028
7. Katano H, Mase M, Nishikawa Y, Yamada K. Surgical treatment for carotid stenoses with highly calcified plaques. J Stroke Cerebrovasc Dis. 2014;23:148–154. doi:10.1016/j.jstrokecerebrovasdis.2012.11.019
8. Dharmakidari S, Bhattacharya P, Chaturvedi S. Carotid artery stenosis: medical therapy, surgery, and stenting. Curr Neurol Neurosci Rep. 2017;17:77. doi:10.1007/s11910-017-0786-2
9. Dobiásová M, Frohlich J. The plasma parameter log (TG/HDL-C) as an atherogenic index: correlation with lipoprotein particle size and esterification rate in apoB-lipoprotein-depleted plasma (FER(HDL)). Clin Biochem. 2001;34:583–588. doi:10.1016/s0009-9120(01)00263-6
10. Garg R, Knox N, Prasad S, Zinzuwadia S, Rech MA. The atherogenic index of plasma is independently associated with symptomatic carotid artery stenosis. J Stroke Cerebrovasc Dis. 2020;29,105351. doi:10.1016/j.jstrokecerebrovasdis.2020.105351
11. Gentile M, Iannuzzo G, Simeon V, et al. Association between atherogenic index of plasma and carotid intima-media thickness in a cohort of Mediterranean women. Acta Cardiol. 2021;76:987–992. doi:10.1080/00015385.2020.1858537
12. Kwon BJ, Jung C, Sheen SH, Cho JH, Han MH. CT angiography of stented carotid arteries: comparison with Doppler ultrasonography. J Endovasc Ther. 2007;14:489–497. doi:10.1177/152660280701400409
13. Barros P, Felgueiras H, Pinheiro D, Guerra M, Gama V, Veloso M. Restenosis after carotid artery stenting using a specific designed ultrasonographic protocol. J Stroke Cerebrovasc Dis. 2014;23:1416–1420. doi:10.1016/j.jstrokecerebrovasdis.2013.12.002
14. Wasser K, Schnaudigel S, Wohlfahrt J, Psychogios MN, Knauth M, Gröschel K. Inflammation and in-stent restenosis: the role of serum markers and stent characteristics in carotid artery stenting. PLoS One. 2011;6:e22683. doi:10.1371/journal.pone.0022683
15. Shinozaki N, Ogata N, Ikari Y. Plaque protrusion detected by intravascular ultrasound during carotid artery stenting. J Stroke Cerebrovasc Dis. 2014;23:2622–2625. doi:10.1016/j.jstrokecerebrovasdis.2014.06.007
16. Bao X, Zhou G, Xu W, Liu X, Ye Z, Jiang F. Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio: novel markers for the diagnosis and prognosis in patients with restenosis following CAS. Biomarker Med. 2020;14(4):271–282. doi:10.2217/bmm-2019-0155
17. Dai Z, Xu G. Restenosis after carotid artery stenting. Vascular. 2017;25(6):576–586. doi:10.1177/1708538117706273
18. Vanags LZ, Wong NKP, Nicholls SJ, Bursill CA. High-density lipoproteins and apolipoprotein A-I improve stent biocompatibility. Arterioscler Thromb Vasc Biol. 2018;38:1691–1701. doi:10.1161/atvbaha.118.310788
19. Kang Z, Cao Y, Li L, Zhang G. The association between apolipoprotein e gene polymorphism and in-stent restenosis after extracranial and intracranial artery stenting. J Stroke Cerebrovasc Dis. 2021;30:105424. doi:10.1016/j.jstrokecerebrovasdis.2020.105424
20. Kammar-García A, López-Moreno P, E -H-HM, Ortíz-Bueno AM, C M-M-ML. Atherogenic index of plasma as a marker of cardiovascular risk factors in mexicans aged 18 to 22 years. Proc. 2020;34:22–27. doi:10.1080/08998280.2020.1799479
21. L JJ, W ZH, X CY, et al. Association of small dense low-density lipoprotein with cardiovascular outcome in patients with coronary artery disease and diabetes: a prospective, observational cohort study. Cardiovasc Diabetol. 2020;19:45. doi:10.1186/s12933-020-01015-6
22. Ivanova EA, Myasoedova VA, Melnichenko AA, Grechko AV, Orekhov AN. Small dense low-density lipoprotein as biomarker for atherosclerotic diseases. Oxid Med Cell Longev. 2017;2017:1273042. doi:10.1155/2017/1273042
23. Yarnazari A, Hassanpour P, Hosseini-Fard SR, Amirfarhangi A, Najafi M. The sdLDL reduces MRC1 expression level and secretion of histamin e in differentiated m2-macrophages from patients with coronary artery stenosis. Cardiovasc Hematol Disord. 2017;17:28–32. doi:10.2174/1871529x17666170106095554
24. D YC, Shen Y, Lu L, et al. Visit-to-visit HbA(1c) variability is associated with in-stent restenosis in patients with type 2 diabetes after percutaneous coronary intervention. Cardiovasc Diabetol. 2020;19(133). doi:10.1186/s12933-020-01111-7
25. Turak O, Canpolat U, Özcan F, et al. Usefulness of preprocedural serum uric acid level to predict restenosis of bare metal stents. Am J Cardiol. 2014;113:197–202. doi:10.1016/j.amjcard.2013.09.004
26. P ZL, T XW, Wang L, et al. Influence of insulin resistance on in-stent restenosis in patients undergoing coronary drug-eluting stent implantation after long-term angiographic follow-up. Coron Artery Dis. 2015;26(1):5–10. doi:10.1097/mca.0000000000000170
27. Zhou K, Qin Z, Tian J, Cui K, Yan Y, Lyu S. The atherogenic index of plasma: a powerful and reliable predictor for coronary artery disease in patients with type 2 diabetes. Angiology. 2021;72(10):934–941. doi:10.1177/00033197211012129
28. Zhu XW, Deng FY, Lei SF. Meta-analysis of atherogenic index of plasma and other lipid parameters in relation to risk of type 2 diabetes mellitus. Prim Care Diabetes. 2015;9(1):60–67. doi:10.1016/j.pcd.2014.03.007
29. Holmberg J, Bhattachariya A, Alajbegovic A, et al. Loss of vascular myogenic tone in miR-143/145 knockout mice is associated with hypertension-induced vascular lesions in small mesenteric arteries. Arterioscler Thromb Vasc Biol. 2018;38(2):414–424. doi:10.1161/atvbaha.117.310499
30. McCully KS. Homocysteine and the pathogenesis of atherosclerosis. Expert Rev Clin Pharmacol. 2015;8(2):211–219. doi:10.1586/17512433.2015.1010516
31. Cheng G, J CF, Wang Y, et al. Factors influencing stent restenosis after percutaneous coronary intervention in patients with coronary heart disease: a clinical trial based on 1-year follow-up. Med Sci Monit. 2019;25:240–247. doi:10.12659/msm.908692
32. L SF, Mackey A, M CW, et al. Safety of stenting and endarterectomy by symptomatic status in the Carotid Revascularization Endarterectomy Versus Stenting Trial (CREST). Stroke. 2011;42:675–680. doi:10.1161/strokeaha.110.610212
33. Touzé E, Trinquart L, Chatellier G, Mas JL. Systematic review of the perioperative risks of stroke or death after carotid angioplasty and stenting. Stroke. 2009;40:e683–693. doi:10.1161/strokeaha.109.562041
34. D RR, Seiler C, Wustmann K, et al. Tumour necrosis factor-alpha and interleukin-6 release during primary percutaneous coronary intervention for acute myocardial infarction is related to coronary collateral flow. Coron Artery Dis. 2005;16:147–152. doi:10.1097/00019501-200505000-00003
35. Lee SY, Hong MK, Jang Y. Formation and transformation of neointima after drug-eluting stent implantation: insights from optical coherence tomographic studies. Korean Circ J. 2017;47:823–832. doi:10.4070/kcj.2017.0157
36. Aoki J, Tanabe K. Mechanisms of drug-eluting stent restenosis. Cardiovasc Interv Ther. 2021;36:23–29. doi:10.1007/s12928-020-00734-7
37. Ösken A, Akdeniz E, Keskin M, et al. Estimated glomerular filtration rate as a predictor of restenosis after carotid stenting using first-generation stents. Angiology. 2021;72:762–769. doi:10.1177/00033197211014684
38. Yamashita K, Kokuzawa J, Kuroda T, Murase S, Kumagai M, Kaku Y. In-stent hypodense area at two weeks following carotid artery stenting predicts neointimal hyperplasia after two years. Neuroradiol J. 2018;31:280–287. doi:10.1177/1971400917727006
39. Cosottini M, C MM, Bencivelli W, et al. In stent restenosis predictors after carotid artery stenting. Stroke Res Treat. 2010;2010:1–6. doi:10.4061/2010/864724
40. Peng G, Zhang Y, Miao Z. Incidence and risk factors of in-stent restenosis for symptomatic intracranial atherosclerotic stenosis: a systematic review and meta-Analysis. AJNR Am J Neuroradiol. 2020;41:1447–1452. doi:10.3174/ajnr.A6689
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


