Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 18
The Associations Between Serum Vitamins and Carotenoids with Chronic Obstructive Pulmonary Disease: Results from the NHANES
Authors Zheng L, Yu X, Xia Z, Guo Y, Dai Y
Received 9 September 2023
Accepted for publication 25 November 2023
Published 13 December 2023 Volume 2023:18 Pages 2985—2997
DOI https://doi.org/10.2147/COPD.S432995
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Min Zhang
Lei Zheng,1 Xiaofei Yu,1 Zehai Xia,1 Yehao Guo,2 Yifan Dai1
1Respiratory Department, The Affiliated Hospital of Hangzhou Normal University, Hangzhou City, Zhejiang Province, People’s Republic of China; 2Postgraduate Training Base Department, Wenzhou Medical University, Wenzhou City, Zhejiang Province, People’s Republic of China
Correspondence: Yifan Dai, Department of Respiratory and Critical Care Medicine, Hangzhou Normal University, No. 126, Wenzhou Street, Gongshu District, Hangzhou, Zhejiang Province, People’s Republic of China, Tel +86-571-13208000079, Email [email protected]
Purpose: Vitamins and carotenoids are essential in preventing and treating chronic obstructive pulmonary disease (COPD). This study investigated the associations between serum vitamins, carotenoids, and COPD in adults aged ≥ 40 years in the United States.
Methods: We selected 3487 participants aged ≥ 40 from the NHANES (2017– 2018) and used demographic analysis, sensitivity tests, and different weighted multivariate regression models to investigate the relationship between serum vitamins, carotenoids, and COPD.
Results: Subjects in the highest tertile of serum vitamin C, vitamin E (α-tocopherol), α-carotene, trans-β-carotene, and cis-β-carotene had a 50%, 35%, 51%, 54%, and 51% lower risk of COPD than those in the lowest tertile (P for trend: P=0.0005, < 0.0001, 0.0054, 0.0066, and 0.0049). Unfortunately, no significant correlation was found for serum vitamin D levels.
Conclusion: Our analysis of nationally representative data from 3487 participants showed that serum levels of vitamin C, vitamin E (α-tocopherol), α-carotene, and β-carotene were negatively associated with the incidence of COPD in adults over 40 years of age in the US The findings highlighted the importance of antioxidant vitamins and carotenoids in respiratory health, while the data showed no significant correlation between vitamin D (25-OHD) and the incidence of COPD.
Keywords: chronic obstructive pulmonary disease, morbidity, serum vitamins, serum carotenoids, anti-oxidation
Introduction
In 2019, chronic obstructive pulmonary disease (COPD) ranked as the third most prevalent cause of global mortality,1 90% of deaths occur in low- and middle-income countries, the main economic burden of chronic diseases in the future.2 Notably, it also presents as a stand-alone risk factor for lung cancer.3
COPD is mainly affected by genetic and environmental factors and usually manifests as persistent and progressive airflow obstruction. It is characterized by repeated coughing, expectoration, and dyspnea.4 Contemporary therapeutic strategies encompass inhalers, prolonged oxygen therapy, cessation of smoking, and pulmonary rehabilitation.1 However, recent years have seen minimal advancements in impeding the progression of COPD or decreasing its mortality rate.5 COPD’s pathophysiological underpinnings are complex; four basic mechanisms have been confirmed: oxidative stress, inflammation, protease/antiprotease system imbalance, and apoptosis. Each mechanism has different contributions to the occurrence and development of COPD, among which oxidative stress is the most critical factor because it can enhance the other three mechanisms besides direct injury.6 In light of this, vitamins and carotenoids attract research interest because they may have potent antioxidant and anti-inflammatory properties.7
The fact that lung function naturally deteriorates with age, rendering older individuals more vulnerable to environmental exposures, is well-established.8 Recent research predominantly centers around the preventive potential of nutrients or phytochemicals, particularly those with antioxidant or anti-inflammatory properties, in adult and elderly populations. While previous studies have explored the potential influence of antioxidant vitamins and carotenoids on the progression of respiratory diseases, their findings needed to be more consistent.9 Many such studies were potentially biased by their reliance on self-reported dietary or supplement intake rather than objective measures of serum vitamin levels.10,11 Therefore, the focus has shifted towards examining the role of specific serum levels in COPD.12
To examine the association between serum levels of vitamins C, E, D, alpha-carotene, trans-beta-carotene, and cis-beta-carotene and the incidence of COPD in American adults over 40, we conducted a comprehensive and nationally representative pooled analysis of data from the 2017–2018 NHANES cycle. This research, involving data from over 3487 participants, represents the most extensive investigation of its kind to date.
Materials and Methods
Survey Description
NHANES is a robust program administered by the National Center for Health Statistics (NCHS), an arm of the Centers for Disease Control and Prevention, committed to the ongoing assessment of the overall health status of the American populace. The survey employs a multi-stage probability sampling design to collect data biennially over two-year cycles, aiming to deliver population-level insights.
We derived cross-sectional data from NHANES, an exhaustive investigation executed by NCHS using a multi-stage probability design, to gauge the nutritional and health status across the United States.13 The study protocols of NHANES received the green light from the NCHS’s Research Ethics Review Board, securing adherence to the ethical principles laid out in the 1975 Declaration of Helsinki. Written informed consent was obtained from all participants. Comprehensive NHANES study designs and data are open to public scrutiny at www.cdc.gov/nchs/nhanes/. It is of significant note that the formulation of this report aligns with the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines for cross-sectional studies.14
The Enrollment of Participants
Our study incorporated 3487 participants who underwent serological testing as part of the NHANES cycle from 2017–2018. The 2017–2018 cycle was chosen due to its comprehensive collection of serum vitamins and carotene data about COPD. Participants included in our research were those with complete COPD and serological data. In the initial recruitment of 9254 participants, we excluded those who were younger than 40 years old (n = 5372), who did not know whether they had COPD (n = 8), and whose serum vitamin and carotene levels were unknown (n = 387). Following these exclusions, 3487 eligible subjects over 40 were included in our study (Figure 1).
 |
Figure 1 Flowchart of the sample selection from NHANES 2017–2018. |
Assessment of Serum Vitamins and Carotenoids
Serum concentrations of vitamin C (mg/dL), vitamin D (25-OHD) (nmol/L), vitamin E (α-tocopherol) (ug/dL), alpha-carotene (ug/dL), trans-beta-carotene (ug/dL), and cis-beta-carotene (ug/dL) were determined using a modified high-performance liquid chromatography method with photodiode array detection. For further analysis, subjects were divided into thirds based on serum content in our sensitivity analysis. In our study, serum content was considered an exposure variable.
Assessment of COPD
We used questionnaire results from the NHANES database about the question, “Has a doctor or other health professional ever told you that you had COPD?” under the medical condition section, which was included as the outcome variable.
Covariates
After reviewing the literature, the potential covariates that may confuse the relationship between serum levels and COPD are included in our adjustment models. We considered demographic and health condition factors, including gender, age, race, educational attainment, body mass index, income status, smoking status, drinking status, diabetes status, and moderate physical activity. It is worth noting that various factors, such as stress exposure to ultraviolet and infrared radiation, can deplete carotenoid levels in the body.15 For COPD, potential risk factors include smoking, air pollution, fume exposure, and occupational hazards.16
Statistical Analysis
All statistical analyses were conducted duly considering the complex sample design, using the appropriate NHANES sampling weights per CDC guidelines.13 Weighted chi-square tests were employed to evaluate differences in categorical data. Given the sophisticated multi-stage probability sampling technique of NHANES, inferential statistics were used to represent the large, nationally representative sample. In this context, continuous variables were articulated as means with standard errors (SE) using linear regression analyses, while categorical characteristics were expressed as percentages through logistic regression analyses.
We utilized weighted multivariable regression models across various models to explore the associations between serum vitamins, carotenoids, and COPD. Model 1 was not adjusted for covariates, while Model 2 incorporated adjustments for race, age, and sex. Model 3 factored in gender, age, race, education level, BMI, socioeconomic status, smoking status, drinking status, diabetes status, and moderate physical activity. Additional sensitivity analyses were undertaken for robustness. We used a generalized additive model (GAM) and smooth curve fits to handle nonlinearity.
Furthermore, to conduct a subgroup analysis of the relationships between blood vitamins, carotene, and COPD, we employed stratified multivariable logistic regression models with stratified covariates, including gender, age, smoking status, drinking status, diabetes status, and moderate physical activity. These stratified variables were also considered as potential effect modifiers. For variables with already collected data, missing values were inputted using the median for continuous variables or the mode for categorical variables.
All analyses were performed using R version 4.1.3 (http://www.R-project.org, The R Foundation) and Empower software (www.empowerstats.com; X&Y Solutions, Inc., Boston, MA). A two-sided P-value less than 0.05 was considered statistically significant.
Results
Baseline Characteristics
From the 9254 participants in the 2017–2018 NHANES cycle, 3487 individuals met the study’s inclusion criteria. Among these, males accounted for 48.84% (weighted percentage), and females comprised 51.16%, with an overall COPD prevalence of 7.31%. Differences in age, race, education, income status, diabetes, smoking, and serum concentrations of vitamin C, vitamin E (α-tocopherol), α-carotene, trans-β-carotene, and cis-β-carotene were found to be statistically significant (P < 0.05) when comparing groups of participants with and without COPD (Table 1).
 |
Table 1 Baseline Characteristics of Study Population According to COPD Weighted |
Serum Vitamins and Carotenoids are Associated with the Incidence of COPD
Table 2 delineates the association between serum levels of vitamins, carotenoids, and the incidence of COPD. Our findings revealed that the levels of serum vitamin C, vitamin E (α-tocopherol), α-carotene, trans-β-carotene, and cis-β-carotene were significantly associated with the incidence of COPD in both the crude and adjusted models (p<0.0001, p<0.0001, p=0.0001, p<0.0001, p<0.0001). Interestingly, lower levels of these substances corresponded with a higher incidence of COPD.
 |
Table 2 The Relationship Between Adjusted Serum Vitamins, Carotenoids and COPD |
In the fully adjusted model, the odds ratio (OR) for the incidence of COPD decreased with higher serum vitamin C levels. Specifically, relative to the lowest vitamin C level (tertile1), the adjusted ORs were 0.73 and 0.50 for tertile2 and tertile3 respectively (tertile2: OR=0.73, 95% CI 0.51–1.05; tertile3: OR=0.50, 95% CI 0.34–0.75; p for trend=0.0005).
Similarly, when compared to the lowest vitamin E (α-tocopherol) level (tertile1), the adjusted ORs for tertile2 and tertile3 were 0.62 and 0.35 respectively (tertile2: OR=0.62, 95% CI 0.43–0.89; tertile3: OR=0.35, 95% CI 0.23–0.53; p for trend<0.0001).
In terms of α-carotene, relative to the lowest level (tertile1), the adjusted ORs for tertile2 and tertile3 were 0.55 and 0.51, respectively (tertile2: OR=0.55, 95% CI 0.38–0.80; tertile3: OR=0.51, 95% CI 0.31–0.82; p for trend=0.0054).
With regards to trans-β-carotene, the adjusted ORs for tertile2 and tertile3, relative to the lowest level (tertile1), were 0.49 and 0.54 respectively (tertile2: OR=0.49, 95% CI 0.34–0.71; tertile3: OR=0.54, 95% CI 0.35–0.82; p for trend=0.0066). When compared to the lowest cis-β-carotene level (tertile1), the adjusted ORs for individuals in tertile2 and tertile3 were 0.56 and 0.51 respectively (tertile2: OR=0.56, 95% CI 0.37–0.85; tertile3: OR=0.51, 95% CI 0.31–0.86; p for trend=0.0049).
In our study, the incidence of COPD was not significantly correlated with serum vitamin D (25OHD2+25OHD3) level (p=0.8663). In the trichotomous subgroup, the adjusted ORs were 0.81 and 0.91 for tertile2 and tertile3, relative to the lowest level of serum 25(OH)D (tertile1) (tertile2: OR= 0.81, 95% CI 0.54–1.19; tertile3: OR= 0.91,95% CI 0.62–1.34; p for trend=1.0000).
The Nonlinear Correlation Between Serum Vitamins, Carotenoids, and COPD
Our study found a statistically significant negative association between serum levels of vitamins (except vitamin D), carotenoids, and the likelihood of developing COPD. The smooth curve shows the nonlinear correlation between serum vitamins, carotenoids, and COPD (Figure 2).
Subgroup Analysis
We conducted a subgroup analysis to evaluate the consistency of the association between serum vitamins, carotenoids, and the incidence of COPD across the general population and identify potentially different population subsets. Interaction tests were stratified by age, sex, diabetes, and smoking status. Our results indicated that these associations were not uniform.
Table 3 shows significant interactions for gender and age in the association plot between serum vitamin E and COPD incidence (interaction P all < 0.05). There were no statistically significant interaction tests for diabetes, smoking status, drinking status, or moderate physical activity (interaction P all > 0.05). Positive correlations were observed across all genders and in patients aged 50–59, 60–69, and ≥70.
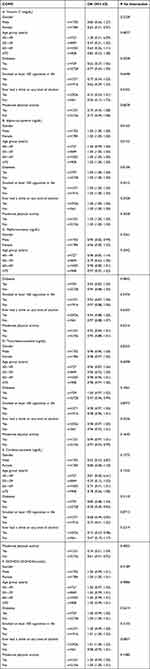 |
Table 3 Subgroup Analysis for the Association Between Serum Vitamins, Carotenoids, and COPD |
Discussion
With the rapid aging of the population, the global health burden of COPD is increasing. According to our cross-sectional analysis of 3487 participants, we observed that age, race, vitamin C, vitamin E (α-tocopherol), α-carotene, trans-β-carotene, and cis-β-carotene were statistically significant with the incidence of COPD. However, in the fully adjusted model of controlling variables such as age, sex, race, smoking status, drinking status, diabetes, and physical activity, there is a negative and nonlinear correlation between serum vitamins, carotenoids, and the incidence of COPD, which is similar in different population backgrounds. However, no discernible link was detected concerning vitamin D.
A matched case-control study in the past collected 52 patients with chronic respiratory diseases (CRD) and 52 healthy controls, and found that the plasma vitamin C concentration of patients with CRD was significantly lower than that of the control group (P=0.005).17 At the same time, a national study from South Korea showed that higher vitamin C intake can significantly reduce the risk of COPD by 76.7% compared with lower vitamin C intake, regardless of smoking history.18 This is consistent with our research. In the research on α-tocopherol, the research from Spanish shelters suggested that the elderly COPD patients ate less antioxidant diet than the healthy group, and the subjects with α-tocopherol<14.1mol/L (50th percentile) had 6.43 times the risk of COPD than those with α-tocopherol≥14.1mol/L (OR=6.43,95% CI 1.17–35.24, P<0.05).19 In addition, another comparative study of women’s health from the United States revealed that women who took 600 IU vitamin E had a 10% lower risk of COPD (HR 0.90; 95% CI 0.81–0.99, p=0.029).20 Although our models are different, the results are similar. However, in the subgroup analysis of vitamin E, our results show that the correlation between vitamin E and COPD is age-dependent and gender-dependent. The association is different between men and women and between different ages, which may be related to factors such as hormone level and vitamin E utilization level. Still, relevant researches have not confirmed it and further studies are needed. Van’s research showed that low levels of vitamin A (the main expression form of carotenoids) may increase the susceptibility to the development of emphysema induced by cigarette smoke.21 From 631 elderly women with moderate and severe disabilities in American communities, it was found that the higher serum concentration of α -carotene β -carotene was positively correlated with FEV1 and FVC (P< 0.05).22 Similarly, in the study of young people with coronary artery risk development, the change of vitamin A precursor carotenoid concentration in 15 years was related to the slow decline of the maximum FVC and FEV (P ≤ 0.04).23 However, an analysis from NHANES pointed out that the incidence of respiratory diseases has no apparent correlation with serum vitamin A levels. Still, the mortality rate may be related to it.24 We think this may be caused by the wide variety of diseases included in the study. It is not difficult to find that many previous studies focused on special populations (especially women) and carried out a wide range of statistical analyses centered on systemic diseases, leading to the unconvincing relevance. As far as we know, our research is the first comprehensive study on the correlation between the levels of vitamins and carotenoids and the risk of COPD. It is a more intuitive and scientific inquiry. Based on serology, which reveals explicitly the great clinical significance that such serological indicators may bring to this disease.
The mechanism that high levels of vitamin C, vitamin E, α-carotene, and β-carotene in serum are related to the lower risk of COPD is complicated, and there is no clear conclusion. The characteristics of COPD include airway inflammation, airway remodeling, and emphysema. Cigarette smoke is the leading risk factor, which can not only trigger pulmonary infiltration of neutrophils, enhance the production of cytokines, and activate pro-inflammatory factors but also contain many oxidants.25 Many studies show that oxidative stress in the lung has a significant influence, and infiltrated inflammatory cells can also produce a lot of reactive oxygen species, which reduces endogenous antioxidant function.26 Hanson suggested that the role of vitamin C in scavenging superoxide radicals and reducing antioxidant damage implies a protective effect on lung tissue.27 Experimental research by Koike also indicated that sufficient vitamin C could potentially treat and prevent emphysema in mice by reducing oxidative stress, stimulating collagen synthesis, and enhancing vascular endothelial growth factor (VEGF).28 It counteracts endogenous and exogenous oxidants by enhancing epithelial barrier function and influencing innate and adaptive immune cells. These mechanisms explain our research results.
At the same time, in our study, α -tocopherol with the broadest distribution, the highest activity, and the highest concentration in serum was used to study the effect of vitamin E. It primarily exerts its potent antioxidant effects through chain-breaking activities, membrane repair, and free extreme scavenging actions. A bioinformatics analysis by Zhao revealed that vitamin E alleviates cigarette smoke (CS)-induced inflammation, apoptosis, and reactive oxygen species (ROS) production by inhibiting the EGFR/MAPK axis, which in turn blocks COX2-mediated p-STAT3 nuclear translocation.29 Our research results also support α-tocopherol to exert its antioxidant and anti-inflammatory properties.
Carotenoids primarily act by inhibiting the NF-кB, MAPK, JAK/STAT-3, and PI3K/AKT pathways and activating the NRF2/HO-1 pathway.30 Meanwhile, A notable instance is the ability of β-carotene to quench singlet oxygen. Besides, bioactive compounds can also induce epigenetic changes, like histone deacetylase (HDAC) modification, thus inhibiting the production of pro-inflammatory cytokines.31 Therefore, its antioxidant capacity may explain its association with COPD.
Numerous recent revelations have shed light on myriad new locations for vitamin D and its receptor, VDR, across various organ systems, thereby accentuating their potential role in modulating inflammation and bolstering immunity.32,33 It can regulate a series of genes related to cell proliferation and differentiation, cell control, apoptosis, and host defense mechanisms. Although it’s widely recognized that vitamin D deficiency frequently plagues patients suffering from COPD, the relationship between serum vitamin D concentrations and COPD and the underlying mechanical interplay continues to be ambiguous.34 An array of differing viewpoints characterizes the discourse in this area. For instance, research by Camargo suggested that COPD patients who are severely deficient in vitamin D might witness benefits from a regular monthly regimen of vitamin D supplementation.35 In contrast, a 2013 meta-analysis indicated that only a subset (four sevenths) of the scrutinized observational studies could establish a link between dietary vitamin D intake and pulmonary function.27 Adding further to this, pertinent randomized controlled trials have shown that augmenting serum vitamin D levels failed to attenuate the incidence of acute exacerbations in COPD patients who are deficient in vitamin D.36
Surprisingly, our investigation failed to establish a significant link between COPD incidence and serum vitamin D (25OHD2+25OHD3) concentrations. One possible explanation for this finding could be the selective impact of vitamin D on pulmonary function in susceptible populations.37 Furthermore, it’s been demonstrated that the associations between serum 25(OH)D levels and COPD are contingent on variables such as skin color, air pollution,38 and smoking habits,39 our research cannot eliminate the influence of these variables. This is a new challenge for whether to supplement vitamin D as a treatment option.
Our research has several advantages. To ensure the accuracy of the results, we used appropriate weight and confounding factor analysis in the analysis process. Secondly, the study quoted much representative data from NHANES, covering a variety of vitamins and carotenoids, and proved a nonlinear negative correlation among them, which was improved by smooth curve fitting. However, limitations are inevitable. First, because this study is a cross-sectional analysis, it is based on correlation analysis, and exploring the causal relationship of effects is impossible. Secondly, the covariates we include may not be completely accurate, which may affect the accuracy of the results. The mechanism of the serological level, the risk of COPD, and the application value of indicators still need further study.
Conclusion
Our cross-sectional analysis revealed associations between specific serum vitamins and carotenoids with the risk of COPD, suggesting the potential of these micronutrients in mitigating or slowing down the evolution and progression of COPD. Compared to intake studies, serum levels are more intuitive in revealing associations between variables. Therefore, recommendations for dietary supplement intake need to be approached with prudence, and the need for more prospective research and meticulously planned randomized controlled trials is evident.
Data Sharing Statement
The study utilized publicly available datasets. These statistics can be accessed at www.cdc.gov/nchs/nhanes/.
Ethics Statement
Due to the de-identification of participants in the NHANES database, all participants in NHANES have written and signed the informed consent, consistent with and deemed by the National Center for Health Statistics Institutional Review Board (NCHSIRB) (Protocol #2018-01 Protocol #2011-17), the IRB of the study hospital (Affiliated Hospital of Hangzhou Normal University) waived both IRB review and informed consent by the participants for the present study.
Acknowledgments
We thank the NHANES database for generously sharing a large amount of data. At the same time, we are also grateful to Yanling Xu for helping our team in this research.
Disclosure
The authors declare no conflict of interest.
References
1. Labaki WW, Rosenberg SR. Chronic obstructive pulmonary disease. Ann Intern Med. 2020;173(3):ITC17–ITC32. doi:10.7326/AITC202008040
2. Agustí A, Celli BR, Criner GJ, et al. Global initiative for chronic obstructive lung disease 2023 report: GOLD executive summary. Eur Respir J. 2023;61(4):2300239. doi:10.1183/13993003.00239-2023
3. Papi A, Casoni G, Caramori G, et al. COPD increases the risk of squamous histological subtype in smokers who develop non-small cell lung carcinoma. Thorax. 2004;59(8):679–681. doi:10.1136/thx.2003.018291
4. Upadhyay P, Wu CW, Pham A, et al. Animal models and mechanisms of tobacco smoke-induced chronic obstructive pulmonary disease (COPD). J Toxicol Environ Health B Crit Rev. 2023;26(5):275–305. doi:10.1080/10937404.2023.2208886
5. Rabe KF, Watz H. Chronic obstructive pulmonary disease. Lancet. 2017;389(10082):1931–1940. doi:10.1016/S0140-6736(17)31222-9
6. Czarnecka-Chrebelska KH, Mukherjee D, Maryanchik SV, Rudzinska-Radecka M. Biological and genetic mechanisms of COPD, its diagnosis, treatment, and relationship with lung cancer. Biomedicines. 2023;11(2):448. doi:10.3390/biomedicines11020448
7. Zhai T, Li S, Hu W, Li D, Leng S. Potential micronutrients and phytochemicals against the pathogenesis of chronic obstructive pulmonary disease and lung cancer. Nutrients. 2018;10(7):813. doi:10.3390/nu10070813
8. Reyfman PA, Washko GR, Dransfield MT, Spira A, Han MK, Kalhan R. Defining impaired respiratory health. A paradigm shift for pulmonary medicine. Am J Respir Crit Care Med. 2018;198(4):440–446. doi:10.1164/rccm.201801-0120PP
9. Kelly FJ. Vitamins and respiratory disease: antioxidant micronutrients in pulmonary health and disease. Proc Nutr Soc. 2005;64(4):510–526. doi:10.1079/pns2005457
10. Grandjean AC. Dietary intake data collection: challenges and limitations. Nutr Rev. 2012;70(Suppl 2):S101–S104. doi:10.1111/j.1753-4887.2012.00545.x
11. Keranis E, Makris D, Rodopoulou P, et al. Impact of dietary shift to higher-antioxidant foods in COPD: a randomised trial. Eur Respir J. 2010;36(4):774–780. doi:10.1183/09031936.00113809
12. Moreno-Macias H, Romieu I. Effects of antioxidant supplements and nutrients on patients with asthma and allergies. J Allergy Clin Immunol. 2014;133(5):
13. Johnson CL, Paulose-Ram R, Ogden CL, et al. National health and nutrition examination survey: analytic guidelines, 1999–2010. Vital Health Stat. 2013;161:1–24.
14. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. doi:10.1016/S0140-6736(07)61602-X
15. Rivera-Madrid R, Carballo-Uicab VM, Cárdenas-Conejo Y, Aguilar-Espinosa M, Siva R. 1 - Overview of carotenoids and beneficial effects on human health. In: Galanakis CM, editor. Carotenoids: Properties, Processing and Applications. Academic Press; 2020:1–40. doi:10.1016/B978-0-12-817067-0.00001-4
16. Holtjer JCS, Bloemsma LD, Beijers RJHCG, et al. Identifying risk factors for COPD and adult-onset asthma: an umbrella review. Eur Respir Rev. 2023;32(168):230009. doi:10.1183/16000617.0009-2023
17. Abuhajar SM, Taleb MH, Ellulu MS. Vitamin C deficiency and risk of metabolic complications among adults with chronic respiratory diseases: a case-control study. Clin Nutr ESPEN. 2021;43:448–455. doi:10.1016/j.clnesp.2021.03.007
18. Park HJ, Byun MK, Kim HJ, et al. Dietary vitamin C intake protects against COPD: the Korea national health and nutrition examination survey in 2012. Int J Chron Obstruct Pulmon Dis. 2016;11:2721–2728. doi:10.2147/COPD.S119448
19. Rodríguez-Rodríguez E, Ortega RM, Andrés P, et al. Antioxidant status in a group of institutionalised elderly people with chronic obstructive pulmonary disease. Br J Nutr. 2016;115(10):1740–1747. doi:10.1017/S0007114516000878
20. Agler AH, Kurth T, Gaziano JM, Buring JE, Cassano PA. Randomised vitamin E supplementation and risk of chronic lung disease in the Women’s Health Study. Thorax. 2011;66(4):320–325. doi:10.1136/thx.2010.155028
21. van Eijl S, Mortaz E, Versluis C, Nijkamp FP, Folkerts G, Bloksma N. A low vitamin A status increases the susceptibility to cigarette smoke-induced lung emphysema in C57BL/6J mice. J Physiol Pharmacol. 2011;62(2):175–182.
22. Thyagarajan B, Meyer A, Smith LJ, et al. Serum carotenoid concentrations predict lung function evolution in young adults: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Am J Clin Nutr. 2011;94(5):1211–1218. doi:10.3945/ajcn.111.019067
23. Semba RD, Chang SS, Sun K, Talegawkar S, Ferrucci L, Fried LP. Serum carotenoids and pulmonary function in older community-dwelling women. J Nutr Health Aging. 2012;16(4):291–296. doi:10.1007/s12603-012-0034-z
24. Salo PM, Mendy A, Wilkerson J, et al. Serum antioxidant vitamins and respiratory morbidity and mortality: a pooled analysis. Respir Res. 2022;23(1):150. doi:10.1186/s12931-022-02059-w
25. Liu G, Jarnicki AG, Paudel KR, et al. Adverse roles of mast cell chymase-1 in COPD. Eur Respir J. 2022;60(6):2101431. doi:10.1183/13993003.01431-2021
26. Hu G, Zhang X, Chen J, Peto R, Campbell TC, Cassano PA. Dietary vitamin C intake and lung function in rural China. Am J Epidemiol. 1998;148(6):594–599. doi:10.1093/oxfordjournals.aje.a009685
27. Hanson C, Rutten EPA, Wouters EFM, Rennard S. Diet and vitamin D as risk factors for lung impairment and COPD. Transl Res. 2013;162(4):219–236. doi:10.1016/j.trsl.2013.04.004
28. Koike K, Ishigami A, Sato Y, et al. Vitamin C prevents cigarette smoke-induced pulmonary emphysema in mice and provides pulmonary restoration. Am J Respir Cell Mol Biol. 2014;50(2):347–357. doi:10.1165/rcmb.2013-0121OC
29. Zhao H, Gong J, Li L, et al. Vitamin E relieves chronic obstructive pulmonary disease by inhibiting COX2-mediated p-STAT3 nuclear translocation through the EGFR/MAPK signaling pathway. Lab Invest. 2022;102(3):272–280. doi:10.1038/s41374-021-00652-z
30. Manochkumar J, Singh A, Efferth T, Ramamoorthy S. Untapping the protective role of carotenoids against respiratory diseases. Phytomedicine. 2022;104:154286. doi:10.1016/j.phymed.2022.154286
31. Gozzi-Silva SC, Teixeira FME, Duarte AJDS, et al. Immunomodulatory role of nutrients: how can pulmonary dysfunctions improve? Front Nutr. 2021;8:674258. doi:10.3389/fnut.2021.674258
32. Sun J, Zhang YG. Vitamin D receptor influences intestinal barriers in health and disease. Cells. 2022;11(7):1129. doi:10.3390/cells11071129
33. Santa K. Grape phytochemicals and vitamin D in the alleviation of lung disorders. Endocr Metab Immune Disord Drug Targets. 2022;22(13):1276–1292. doi:10.2174/1871530322666220407002936
34. Mullin MLL, Milne S. Vitamin D deficiency in chronic obstructive pulmonary disease. Curr Opin Pulm Med. 2023;29(2):96–103. doi:10.1097/MCP.0000000000000935
35. Camargo CA, Toop L, Sluyter J, et al. Effect of monthly vitamin D supplementation on preventing exacerbations of asthma or chronic obstructive pulmonary disease in older adults: post hoc analysis of a randomized controlled trial. Nutrients. 2021;13(2):521. doi:10.3390/nu13020521
36. Rafiq R, Aleva FE, Schrumpf JA, et al. Vitamin D supplementation in chronic obstructive pulmonary disease patients with low serum vitamin D: a randomized controlled trial. Am J Clin Nutr. 2022;116(2):491–499. doi:10.1093/ajcn/nqac083
37. Semba RD, Chang SS, Sun K, Cappola AR, Ferrucci L, Fried LP. Serum 25-hydroxyvitamin D and pulmonary function in older disabled community-dwelling women. J Gerontol a Biol Sci Med Sci. 2012;67(6):683–689. doi:10.1093/gerona/glr213
38. Mi S, Ad B, Ja K. Higher serum vitamin D levels are associated with decreased odds of obstructive lung disease in the general population: an NHANES analysis (2007–2008 to 2009–2010). BMJ Open Respir Res. 2020;7(1). doi:10.1136/bmjresp-2020-000798
39. Heulens N, Korf H, Cielen N, et al. Vitamin D deficiency exacerbates COPD-like characteristics in the lungs of cigarette smoke-exposed mice. Respir Res. 2015;16(1):110. doi:10.1186/s12931-015-0271-x
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

