Back to Journals » Journal of Inflammation Research » Volume 16
The Association of the Neutrophil-to-Lymphocyte Ratio, Platelet-to-Lymphocyte Ratio, Lymphocyte-to-Monocyte Ratio and Systemic Inflammation Response Index with Short-Term Functional Outcome in Patients with Acute Ischemic Stroke
Authors Zhang YX , Shen ZY, Jia YC, Guo X, Guo XS, Xing Y, Tian SJ
Received 11 May 2023
Accepted for publication 15 August 2023
Published 23 August 2023 Volume 2023:16 Pages 3619—3630
DOI https://doi.org/10.2147/JIR.S418106
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Tara Strutt
Ya Xin Zhang,1– 3,* Zhi Yuan Shen,1– 3,* Yi Cun Jia,1– 3 Xin Guo,1– 3 Xiao Su Guo,1– 3 Yuan Xing,1– 3 Shu Juan Tian1– 3
1Department of Neurology, The First Hospital of Hebei Medical University, Shijiazhuang, Hebei, People’s Republic of China; 2Department of Neurology, Hebei Hospital, Xuanwu Hospital, Capital Medical University, Shijiazhuang, Hebei, People’s Republic of China; 3Neuromedical Technology Innovation Center of Hebei Province, Shijiazhuang, Hebei, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Shu Juan Tian; Yuan Xing, Department of Neurology, The First hospital of Hebei Medical University, Shijiazhuang, Hebei, 050030, People’s Republic of China, Email [email protected]; [email protected]
Background and Purpose: The aim of this study was to explore the relationship between functional prognosis and the neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), lymphocyte-to-monocyte ratio (LMR) and systemic inflammatory response index (SIRI) in patients with acute ischemic stroke (AIS) at discharge.
Methods: A total of 861 patients with AIS were enrolled between January 2019 and December 2021. Blood cell counts were collected on admission. Logistic regression analysis was performed to assess the relationship between NLR, PLR, LMR, SIRI and adverse functional outcomes (modified Rankin scale score of 3– 6) at discharge. We also used receiver operating characteristic (ROC) curves to estimate the overall ability of NLR, PLR, LMR and SIRI to judge short-term functional outcomes. Associations between NLR, PLR, LMR, and SIRI with length of hospital stay were analyzed by Spearman correlation test.
Results: A total of 194 patients (22.5%) had poor functional outcomes at discharge. Multivariate logistic regression analysis showed that NLR (odds ratio [OR], 1.060; 95% confidence interval [CI] 1.004– 1.120, P=0.037), PLR (OR, 1.003; 95% CI 1.000– 1.005, P=0.018), LMR (OR, 0.872; 95% CI 0.774– 0.981, P=0.023) and SIRI (OR, 1.099; 95% CI 1.020– 1.184, P=0.013) were independent factors for poor functional outcome. The odds ratios of the highest versus lowest quartiles of NLR, PLR and SIRI were 2.495 (95% CI 1.394– 4.466), 1.959 (95% CI 1.138– 3.373) and 1.866 (95% CI 1.106– 3.146), respectively. The odds ratio of the lowest versus highest quartile of LMR was 2.300 (95% CI 1.331– 3.975). The areas under the curve (AUCs) of the NLR, PLR, LMR, and SIRI to discriminate poor functional prognosis were 0.644, 0.587, 0.628, and 0.651, respectively. NLR, LMR, and SIRI were related with the length of hospital stay (P< 0.05).
Conclusion: NLR, PLR, LMR, and SIRI were associated with functional outcome at discharge in AIS patients. NLR, LMR and SIRI were related to hospitalization days in patients with AIS.
Keywords: acute ischemic stroke, short-term functional outcome, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, lymphocyte-to-monocyte ratio, systemic inflammatory response index
Introduction
Stroke is the second leading cause of death and a major cause of disability worldwide. China bears the largest burden of stroke in the world and is the country with the highest risk of stroke. The incidence of stroke in China is about 343.4/100 000 person-years.1–3 Patients with acute ischemic stroke (AIS) often experience early neurological deterioration and poor functional outcome during hospitalization, which further prolongs the length of hospital stay and has a long-term impact on the patient’s quality of life.4 Identifying patients with a high risk of short-term functional prognosis as soon as possible can help doctors provide intensive treatment for patients and alleviate their adverse functional outcomes during hospitalization. Therefore, it is essential to identify rapidly available and reliable markers to optimize risk stratification for functional outcome in acute ischemic stroke.
Many studies have shown that inflammation is a major factor affecting the pathophysiology and prognosis of AIS.5–7 The inflammatory cascade after cerebral ischemia is a dynamic process in which various cells interact in the ischemic region, which can cause secondary brain injury.8 Inflammatory markers (including neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), lymphocyte to monocyte ratio (LMR), and systemic inflammatory response index (SIRI)) are excellent predictors of functional outcome in AIS. These indexes are readily available, and previous studies have shown that NLR, PLR, LMR, and SIRI correlate with stroke severity on admission, 3-month functional outcomes, and mortality.9–13 In addition, a retrospective study found that NLR, PLR, and LMR were associated with early neurological deterioration after intravenous thrombolysis in patients with AIS, NLR and PLR predicted early neurological deterioration after thrombolysis, and NLR was an independent factor of early neurological improvement after thrombolysis.14 However, few studies have investigated the relationship between inflammatory markers and clinical outcomes at discharge in patients with acute ischemic stroke. The length of hospital stay is an important indicator of disease severity in AIS, but few studies have linked the length of hospital stay with inflammatory markers.
To better evaluate the early risk predictive value of inflammatory composite indicators (including NLR, PLR, LMR and SIRI), we not only explore the relationship between inflammatory composite indicators and the poor functional outcome of patients with AIS at discharge, but also analyzed the association between inflammatory composite indicators and hospitalization days.
Method
Study Participants
We screened stroke patients who presented to the First Hospital of Hebei Medical University from January 2019 to December 2021. According to the inclusion and exclusion criteria, 861 patients with AIS were finally included. Finally, 861 AIS patients were eligible for this analysis (Figure 1).
 |
Figure 1 Flow chart of the present study participants. |
Inclusion criteria:
- Eighteen years or older.
- The patient was admitted within 7 days of onset.
- AIS was diagnosed by CT or MRI.
Exclusion criteria:
- Severe inflammatory diseases or infectious diseases.
- Patients with insufficient laboratory data and discharge modified Rankin scale (mRS) score data.
Data Collection and Outcome Assessment
Experienced doctors collected the participants’ demographic characteristics (age, sex, smoking and drinking), clinical characteristics (systolic blood pressure, diastolic blood pressure and NIHSS score at admission), TOAST classification, medical history (hypertension, diabetes, stroke, atrial fibrillation and coronary heart disease), past medication history (lipid-lowering drugs, hypoglycemic drugs, antihypertensive drugs and antiplatelet drugs) and time from onset to admission. Stroke severity was assessed by the National Institutes of Health Stroke Scale (NIHSS). All patients who participated in the study had routine blood tests on admission to measure neutrophil, lymphocyte, platelet, and monocyte counts. After fasting for at least 8 hours within 24 hours of admission, fasting blood samples were collected to measure total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C) and blood glucose (GLU).
Inflammatory indicators (including NLR, PLR, LMR, and SIRI) were calculated. The NLR was calculated as the neutrophil count/lymphocyte count. PLR was calculated as the platelet count/lymphocyte count. LMR was calculated as the lymphocyte count/monocyte count. SIRI was calculated as the neutrophil count * monocyte count/lymphocyte count. Short-term functional outcomes were assessed using the mRS scores at discharge. The mRS scores ranged from 0 to 6, with a score of 0 indicating no symptoms, a score of 3 indicating moderate disability, a score of 4 indicating moderate to severe disability, a score of 5 indicating severe disability, and a score of 6 indicating death. The primary outcome was poor functional outcome, defined as having an mRS score of 3–6 at discharge.
Statistical Analysis
The patients were divided into two groups based on mRS scores at discharge, with good functional outcomes (mRS: 0–2) and poor functional outcomes (mRS: 3–6). Continuous variables are represented by the mean (standard deviation, SD) or median (interquartile interval, IQR), and categorical variables are expressed as frequency (%). We tested for normality for continuous variables using the Kolmogorov‒Smirnov test. Differences in the baseline characteristics between the groups were analyzed by t tests for normally distributed variables, Mann‒Whitney tests for nonnormally distributed variables, and chi-square tests for categorical variables. Associations between NLR, PLR, LMR, and SIRI with short-term functional outcome and length of hospital stay were analyzed by Spearman correlation test. Univariate logistic regression and multivariate logistic regression analyses were performed to assess the association between the NLR, PLR, LMR, SIRI and clinical outcomes, generating odds ratios (ORs) and 95% confidence interval (95% CI). In the multivariate logistic regression analysis, we constructed two models: Model 1 was adjusted for age, sex, length of hospital stay, NIHSS score at admission, TOAST classification, and stroke history; Model 2 was further adjusted for TG, smoking, drinking, medical history (hypertension, diabetes, atrial fibrillation), thrombolysis and glucose-lowering drugs. We also divided the participants into four groups according to the quartiles of NLR, PLR, LMR, and SIRI, and logistic regression models were used to calculate the ORs and 95% CI for the high quartile versus the lowest quartile or the low quartile versus the highest quartile. A receiver operating characteristic (ROC) curve was used to test the overall ability of the NLR, PLR, LMR and SIRI to predict the short-term functional outcome of patients with AIS. The Youden Index was calculated as the sensitivity +specificity–1. All statistical analyses were performed using IBM SPSS Statistics version 25 and GraphPad Prism 9.0. A two-tailed value of P < 0.05 was considered significant.
Result
Baseline Characteristics
The study included 861 patients (552 males and 309 females) with an average age of 63.4 ± 12.1 years. According to the mRS score at the time of discharge, the patients were divided into a good functional outcome group (mRS: 0–2) and a poor functional outcome group (mRS: 3–6). The baseline characteristics of the participants are shown in Table 1. The participants with poor functional outcomes were older (66 versus 64 years; P=0.019) and less likely to be male (57.2 versus 66.1%; P=0.027) and had higher NIHSS scores on admission (6 versus 2; P<0.001), longer hospitalization days (12 versus 11; P<0.001), and a higher prevalence of stroke (37.6% versus 27.9%; P=0.010). Those with poor functional outcomes were more likely to have lower levels of triglycerides (1.20 versus 1.33; P=0.017). The baseline characteristics according to the quartiles of NLR, PLR, LMR and SIRI are detailed in Additional files: Tables S1–S4, respectively.
 |
Table 1 Demographics and Clinical Characteristics of the Subgroup According to Functional Outcome at Discharge |
Inflammatory Composite Index and Unfavorable Functional Outcome
Table 1 shows that the patients with poor functional outcomes had a higher NLR (3.34 versus 2.50; P < 0.001), higher PLR (150.45 versus 129.38; P < 0.001), higher SIRI (1.60 versus 1.02; P < 0.001) and lower LMR (3.34 versus 4.00; P < 0.001).
Table 2 presents the results of the univariate logistic regression analysis of poor functional prognosis. The univariate logistic regression analysis showed that age, sex, NIHSS score at admission, length of stay, TOAST classification, stroke history, NLR, PLR, LMR and SIRI were correlated with the prognosis of adverse function (P < 0.05).
 |
Table 2 Univariate Logistic Regression Analysis for Risk Factors with Poor Functional Prognosis |
Table 3 shows the results of the multivariate logistic regression analysis for poor functional outcome. After adjusting for age, sex, NIHSS score at admission, length of hospital stay, TOAST classification and stroke history, NLR (ORs, 1.062; 95% CI 1.006–1.121, P = 0.028), PLR (ORs, 1.003; 95% CI 1.001–1.005, P=0.014), LMR (ORs, 0.863; 95% CI 0.768–0.970, P=0.013) and SIRI (ORs, 1.101; 95% CI 1.022–1.186, P=0.012) were determined to be independent factors for poor functional outcome. Model 2 was further adjusted for TG, smoking, drinking, medical history (hypertension, diabetes, atrial fibrillation), thrombolysis and glucose-lowering drugs. NLR (ORs, 1.060; 95% CI 1.004–1.120, P=0.037), PLR (ORs, 1.003; 95% CI 1.000–1.005, P=0.018), LMR (ORs, 0.872; 95% CI 0.774–0.981, P=0.023) and SIRI (ORs, 1.099; 95% CI 1.020–1.184, P=0.013), which were still independent factors for poor functional outcome.
 |
Table 3 Multinomial Logistic Regression Models for Poor Function Outcome |
Table 4 shows the results of the multivariate logistic regression analysis based on the NLR, PLR, LMR, and SIRI quartile grouping. In Model 2, the ORs for poor functional outcome at discharge increased with the NLR, PLR, and SIRI quartiles and decreased with the LMR quartiles. The ORs of unfavorable functional outcome at discharge with NLR ranged from 1.463 (95% CI 0.795–2.694) in quartile 2 to 2.495 (95% CI 1.394–4.466) in quartile 4. The ORs of unfavorable functional outcome at discharge with PLR ranged from 1.421 (95% CI 0.811–2.491) in quartile 2 to 1.959 (95% CI 1.138–3.373) in quartile 4. The ORs of unfavorable functional outcome at discharge with SIRI ranged from 0.682 (95% CI 0.372–1.251) in quartile 2 to 1.866 (95% CI 1.106–3.146) in quartile 4. The ORs of unfavorable functional outcome at discharge with LMR ranged from 1.509 (95% CI 0.850–2.679) in quartile 3 to 2.300 (95% CI 1.331–3.975) in quartile 1.
 |
Table 4 Multivariate Logistic Regression Analysis According to Quartiles of NLR, PLR, LMR, and SIRI |
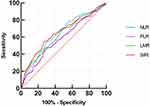
ROC curves were plotted to evaluate the efficiency of NLR, PLR, LMR, and SIRI in predicting the outcome of poor function in patients with AIS, as shown in Figure 2 and Table 5. We observed that the area under the curve (AUC) of the NLR, PLR, LMR, and SIRI was 0.644 (95% CI, 0.599–0.688). 0.587 (95% CI, 0.540–0.633), 0.628 (95% CI, 0.582–0.674), and 0.651 (95% CI, 0.605–0.697), respectively. The optimal diagnostic cutoff point of NLR was 3.16, the sensitivity was 55.2%, and the specificity was 69.3%. The optimal diagnostic cutoff point of PLR was 162.92, the sensitivity was 46.9%, and the specificity was 70.6%. The optimal diagnostic cutoff point of LMR was 3.95, the sensitivity was 52.6% and the specificity was 68.0%. The optimal diagnostic cutoff point of SIRI was 1.55, the sensitivity was 54.1%, and the specificity was 73.6%.
 |
Table 5 The Ability of NLR, PLR, LMR and SIRI to Predict Poor Functional Prognosis at Discharge |
Table 6 shows that the NLR, PLR and SIRI were positively correlated with poor functional outcome (P<0.001), and the LMR was negatively correlated with poor functional outcome (P<0.001). The NLR and SIRI were positively correlated with the length of hospital stay (P<0.05), the LMR was negatively correlated with the length of hospital stay (P<0.05), and the PLR was not significantly correlated with the length of hospital stay (P=0.063).
 |
Table 6 Association of NLR, PLR, LMR, and SIRI with Poor Functional Outcomes and Hospital Stay |
Discussion
In this study, we comprehensively investigated the relationship between the NLR, PLR, LMR, and SIRI and functional outcome at discharge in a large sample of adult patients with AIS. Our study, involving 861 patients, showed that the NLR, PLR, LMR, and SIRI were correlated with functional outcome at discharge in patients with acute ischemic stroke. Multivariate logistic regression analysis showed that the NLR, PLR, LMR and SIRI remained independent factors for poor functional prognosis after adjusting for confounding factors. The higher the NLR, PLR, SIRI or lower the LMR, the more likely the occurrence of poor functional outcome. In addition, the area under the ROC curve of NLR, LMR and SIRI in predicting the prognosis of acute ischemic stroke is roughly similar. Therefore, this study suggests that the NLR, PLR, LMR and SIRI have important clinical value in identifying early unfavorable outcomes in patients with AIS. In addition, we also found that the NLR, LMR and SIRI were correlated with the length of hospital stay by Spearman correlation analysis.
Studies have indicated that inflammatory mechanisms play an important role in the onset and progression of AIS. After cerebral ischemia, damaged brain cells produce a large number of inflammatory cytokines, chemokines, reactive oxygen species (ROS) and other neurotoxic substances that mediate the destruction of the blood‒brain barrier and the occurrence of inflammatory cascade reactions. At the same time, it guides immune inflammatory cells to enter the brain tissue, which further mediates secondary neuronal damage and aggravates neurological dysfunction.15 Neutrophils are the first blood-derived immune cells to invade ischemic brain tissue. After reaching the ischemic area, neutrophils release proinflammatory mediators, proteases, ROS and extracellular matrix metalloproteinases (MMPs), causing secondary damage to the ischemic tissue.16 Lymphocytes are thought to have neuroprotective and neurofunctional effects.6 Peripheral monocytes can serve as a source of MMP-9 and aggravate brain damage.17 Increased monocyte count has been shown to be an independent predictor of worse outcome after stroke.18,19 When acute ischemic stroke occurs, excessive activation and accumulation of platelets can lead to thrombosis and vascular obstruction, which in turn leads to vascular events.20 NLR, PLR, LMR, and SIRI are four inflammatory complex markers of different combinations of inflammatory parameters and thus may provide more information about immune activity in the pathogenesis of ischemic stroke. More importantly, these four composite markers of inflammation can be calculated from blood cell counts, so they are relatively easy to obtain.
Previous studies have shown that a high NLR on admission may be a useful marker for predicting functional outcome at discharge in mild AIS patients receiving intravenous thrombolysis.21 Kim et al found that the higher the NLR, the worse the short-term functional prognosis of AIS patients.22 In addition, a study of 346 patients with AIS showed that the higher the NLR, the higher the incidence of adverse outcomes at discharge.23 We included 861 AIS patients, and our findings suggested that the NLR was an independent risk factor for poor functional outcome at discharge, which was consistent with previous findings.
Altintas et al found that elevated PLR was associated with infarct volume and the occurrence of poor prognosis in AIS patients.24 A previous study showed that PLR predicted 90-day clinical outcomes in patients with AIS.11 Higher PLR before endovascular thrombectomy (EVT) was significantly associated with poor functional outcome at 90 days in AIS patients who successfully recovered after EVT.25 A recent study found that PLR at 24 hours after thrombolysis was a marker of 3-month functional outcome in AIS patients.26 To our knowledge, our study is the first to report the association of PLR with functional outcomes at discharge in AIS patients, and the finding is similar to the 90-day functional outcomes of previous studies.
Mao et al found that LMR was an independent predictor of progressive infarction in AIS patients.27 Li et al demonstrated that the LMR 48 h after intravenous thrombolysis can be used as a biomarker of the functional outcome of discharged patients with AIS.28 Our findings suggest that a low LMR increases the incidence of poor functional outcomes in AIS patients at discharge. This may be the first time that a correlation has been proposed between LMR at admission and functional outcome at discharge in patients with AIS. These findings may provide useful population-based information for researchers studying the PLR, LMR, and stroke short-term risk prediction, not only for research but also for clinical and public health applications.
A previous study showed that SIRI was a promising low-grade inflammatory factor for predicting stroke outcome. SIRI was also found to be superior to the NLR, PLR and LMR in predicting mortality in stroke patients.29 This is similar to our finding that SIRI exhibited better ability in predicting poor functional outcomes at discharge in AIS patients than NLR, PLR and LMR. Recently, a study showed that a higher SIRI was an independent risk factor for death in AIS patients.10 This suggests that SIRI is closely related to the prognosis of AIS patients. Huang’s results indicated that SIRI was not associated with poor outcomes in AIS patients at discharge.9 This is not consistent with the results of our study. We included more patients than Huang’s study, and through the comparison of baseline characteristics of patients, we found that the proportion of patients with diabetes in our included patients was slightly higher than that in Huang’s study population (28.5% vs 22.6%). Previous studies have shown that hyperglycemia may worsen the acute outcome of ischemic stroke,30 and perhaps for these reasons, different findings have been reported. Further studies with a larger sample size are needed to investigate the association between SIRI and functional outcomes at discharge in AIS patients.
A study has shown that an increase in the NLR can prolong the hospital stay of AIS patients.23 Our study had similar results, with the NLR positively correlated with the length of hospital stay in AIS patients. At the same time, we also reported that the LMR was negatively correlated with the length of hospital stay, and SIRI was positively correlated with the length of hospital stay.
In summary, our study enriches the current epidemiological evidence on the relationship between the NLR, PLR, LMR, SIRI and short-term clinical outcomes in patients with ischemic stroke, supporting the hypothesis that the NLR, PLR, and SIRI are positively correlated with poor functional outcome and that the LMR is negatively correlated with poor functional outcome. Our findings suggest that the NLR, PLR, LMR, and SIRI are important for optimizing the risk stratification of short-term ischemic stroke outcomes and guiding clinical decision making.
The NLR, PLR, LMR, and SIRI were also associated with poststroke complications. Some studies have shown that the NLR, PLR, and LMR can predict hemorrhagic transformation (HT) after ischemic stroke.31–33 High NLR and PLR were associated with symptomatic internal carotid artery stenosis.34 According to Hu et al, elevated levels of NLR and PLR are predictive of postischemic stroke depression.35 High PLR was a relevant factor for stroke-related pneumonia in AIS patients.36
These four inflammatory composite markers are relatively readily available biomarkers and are applied in clinical practice for the early identification of ischemic stroke patients with a higher risk of poor stroke outcome, and the findings suggest that close monitoring and management of inflammatory markers may be necessary. Furthermore, our study showed that NLR, LMR, and SIRI had similar predictive ability for short-term functional outcomes of ischemic stroke, with the best predictive ability of SIRI. The NLR, LMR, and SIRI were correlated with hospitalization days in ischemic stroke patients. This can also provide a reference for clinicians in the selection of indicators and the need for intensive treatment for patients.
There are several potential limitations to our study. First, we excluded patients without NLR, PLR, LMR, and SIRI data, which may have limited the generalizability of our findings. Second, this study only looked at the effect of inflammatory markers at admission on outcomes at discharge, whereas reports suggested that inflammation markers may change significantly during hospitalization. Future studies are needed to dynamically detect inflammatory composite markers, not just inflammatory markers at admission. Third, more than one neurologist assessed short-term functional outcomes, which may lead to bias in the assessment of mRS, even though the neurologists have received standardized training. Finally, all enrolled participants were Chinese patients, so the association between these inflammatory composite measures and short-term functional outcomes needs to be tested in populations from other countries.
Conclusion
Our findings indicated that increased levels of NLR, PLR, and SIRI in patients with AIS were associated with an increased risk of poor functional outcome at discharge, and decreased levels of LMR were associated with an increased risk of poor functional outcome at discharge. An increase in the NLR or SIRI or a decrease in the LMR may further prolong the length of hospital stay. The NLR, PLR, LMR, and SIRI may have the ability to predict short-term functional outcomes.
Abbreviations
AIS, acute ischemic stroke; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; LMR, lymphocyte-to-monocyte ratio; SIRI, systemic inflammatory response index; mRS, modified Rankin scale score; NIHSS, National Institutes of Health Stroke Scale; SBP, systolic blood pressure; DBP, diastolic blood pressure; TC, total cholesterol; TG, triglyceride; HDL, high-density lipoprotein; LDL, low-density lipoprotein; GLU, plasma glucose; ROC receiver operating characteristic; AUC, areas under the curve.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics Approval and Informed Consent
This study was approved by the Ethics Committee of the First Hospital of Hebei Medical University (approval number: 20200908). The fully deidentified data on the patients enrolled in the current study and its retrospective study design enabled this study to be conducted under waiver of informed consent by the local institutional review board. Our study also complied with the Declaration of Helsinki.
Consent for Publication
All the authors agree to publish.
Acknowledgments
We express our gratitude to all the researchers and patients who participated in this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by Hebei Natural Science Foundation (H2020206632) and National Key Research and Development Program of China (2022YFC3600503).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Katan M, Luft A. Global burden of stroke. Semin Neurol. 2018;38(2):208–211. doi:10.1055/s-0038-1649503
2. Wang W, Jiang B, Sun H, et al. Prevalence, incidence, and mortality of stroke in China. Circulation. 2017;135(8):759–771. doi:10.1161/CIRCULATIONAHA.116.025250
3. Tu W, Zhao Z, P Y, et al. Estimated burden of stroke in China in 2020. JAMA Network Open. 2023;6(3):e231455. doi:10.1001/jamanetworkopen.2023.1455
4. Helleberg BH, Ellekjaer H, Indredavik B. Outcomes after early neurological deterioration and transitory deterioration in acute ischemic stroke patients. Cerebrovasc Dis. 2016;42(5–6):378–386. doi:10.1159/000447130
5. Shi K, Tian DC, Li ZG, et al. Global brain inflammation in stroke. Lancet Neurol. 2019;18(11):1058–1066. doi:10.1016/S1474-4422(19)30078-X
6. Macrez R, Ali C, Toutirais O, et al. Stroke and the immune system: from pathophysiology to new therapeutic strategies. Lancet Neurol. 2011;10(5):471–480. doi:10.1016/S1474-4422(11)70066-7
7. Tuttolomondo A, Raimondo DI, Disciacca D, et al. Inflammatory cytokines in acute ischemic stroke. Curr Pharm Des. 2008;14(33):3574–3589. doi:10.2174/138161208786848739
8. Anrather J, Iadecola C. Inflammation and stroke: an overview. Neurotherapeutics. 2016;13(4):661–670. doi:10.1007/s13311-016-0483-x
9. Huang L. Increased systemic immune-inflammation index predicts disease severity and functional outcome in acute ischemic stroke patients. Neurologist. 2023;28(1):32–38. doi:10.1097/NRL.0000000000000464
10. Dang H, Mao W, Wang S, et al. Systemic inflammation response index as a prognostic predictor in patients with acute ischemic stroke: a propensity score matching analysis. Front Neurol. 2022;13:1049241. doi:10.3389/fneur.2022.1049241
11. Chen C, Gu L, Chen L, et al. Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio as potential predictors of prognosis in acute ischemic stroke. Front Neurol. 2020;11:525621. doi:10.3389/fneur.2020.525621
12. Xue J, Huang W, Chen X, et al. Neutrophil-to-lymphocyte ratio is a prognostic marker in acute ischemic stroke. J Stroke Cerebrovasc Dis. 2017;26(3):650–657. doi:10.1016/j.jstrokecerebrovasdis.2016.11.010
13. Ren H, Liu X, Wang L, et al. Lymphocyte-to-monocyte ratio: a novel predictor of the prognosis of acute ischemic stroke. J Stroke Cerebrovasc Dis. 2017;26(11):2595–2602. doi:10.1016/j.jstrokecerebrovasdis.2017.06.019
14. Gong P, Liu Y, Gong Y, et al. The association of neutrophil to lymphocyte ratio, platelet to lymphocyte ratio, and lymphocyte to monocyte ratio with post-thrombolysis early neurological outcomes in patients with acute ischemic stroke. J Neuroinflammation. 2021;18(1):51. doi:10.1186/s12974-021-02090-6
15. Yang K, Zeng L, Ge A, et al. A systematic review of the research progress of non-coding RNA in neuroinflammation and immune regulation in cerebral infarction/ischemia-reperfusion injury. Front Immunol. 2022;13:930171. doi:10.3389/fimmu.2022.930171
16. Pluta R, Januszewski S, Czuczwar SJ. Neuroinflammation in post-ischemic neurodegeneration of the brain: friend, foe, or both? Int J Mol Sci. 2021;22(9):4405. doi:10.3390/ijms22094405
17. Yamamoto Y, Osanai T, Nishizaki F, et al. Matrix metalloprotein-9 activation under cell-to-cell interaction between endothelial cells and monocytes: possible role of hypoxia and tumor necrosis factor-alpha. Heart Vessels. 2012;27(6):624–633. doi:10.1007/s00380-011-0214-5
18. Dong X, Nao J, Gao Y. Peripheral monocyte count predicts outcomes in patients with acute ischemic stroke treated with rtPA thrombolysis. Neurotox Res. 2020;37(2):469–477. doi:10.1007/s12640-019-00103-0
19. Liberale L, Montecucco F, Bonaventura A, et al. Monocyte count at onset predicts poststroke outcomes during a 90-day follow-up. Eur J Clin Invest. 2017;47(10):702–710. doi:10.1111/eci.12795
20. Franks ZG, Campbell RA, Weyrich AS, et al. Platelet-leukocyte interactions link inflammatory and thromboembolic events in ischemic stroke. Ann N Y Acad Sci. 2010;1207(1):11–17. doi:10.1111/j.1749-6632.2010.05733.x
21. Liu YL, Wu ZQ, Qu JF, et al. High neutrophil-to-lymphocyte ratio is a predictor of poor short-term outcome in patients with mild acute ischemic stroke receiving intravenous thrombolysis. Brain Behav. 2020;10(12):e01857. doi:10.1002/brb3.1857
22. Kim MS, Heo MY, Joo HJ, et al. Neutrophil-to-lymphocyte ratio as a predictor of short-term functional outcomes in acute ischemic stroke patients. Int J Environ Res Public Health. 2023;20(2):898.
23. Zhao L, Dai Q, Chen X, et al. Neutrophil-to-lymphocyte ratio predicts length of stay and acute hospital cost in patients with acute ischemic stroke. J Stroke Cerebrovasc Dis. 2016;25(4):739–744. doi:10.1016/j.jstrokecerebrovasdis.2015.11.012
24. Altintas O, Altintas MO, Tasal A, et al. The relationship of platelet-to-lymphocyte ratio with clinical outcome and final infarct core in acute ischemic stroke patients who have undergone endovascular therapy. Neurol Res. 2016;38(9):759–765. doi:10.1080/01616412.2016.1215030
25. Ma J, Guo W, Xu J, et al. Association of platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio with outcomes in stroke patients achieving successful recanalization by endovascular thrombectomy. Front Neurol. 2022;13:1039060. doi:10.3389/fneur.2022.1039060
26. Sun YY, Wang MQ, Wang Y, et al. Platelet-to-lymphocyte ratio at 24h after thrombolysis is a prognostic marker in acute ischemic stroke patients. Front Immunol. 2022;13:1000626. doi:10.3389/fimmu.2022.1000626
27. Mao X, Yu Q, Liao Y, et al. Lymphocyte-to-monocyte ratio is independently associated with progressive infarction in patients with acute ischemic stroke. Biomed Res Int. 2022;2022:2290524. doi:10.1155/2022/2290524
28. Li G, Hao Y, Wang C, et al. Association between neutrophil-to-lymphocyte ratio/lymphocyte-to-monocyte ratio and in-hospital clinical outcomes in ischemic stroke treated with intravenous thrombolysis. J Inflamm Res. 2022;15:5567–5578. doi:10.2147/JIR.S382876
29. Zhang Y, Xing Z, Zhou K, et al. The predictive role of systemic inflammation response index (SIRI) in the prognosis of stroke patients. Clin Interv Aging. 2021;16:1997–2007. doi:10.2147/CIA.S339221
30. Miao M, Bi Y, Hao L, et al. Triglyceride-glucose index and short-term functional outcome and in-hospital mortality in patients with ischemic stroke. Nutr Metab Cardiovasc Dis. 2023;33(2):399–407. doi:10.1016/j.numecd.2022.11.004
31. Yang Y, Xie D, Zhang Y. Increased platelet-to-lymphocyte ratio is an independent predictor of hemorrhagic transformation and in-hospital mortality among acute ischemic stroke with large-artery atherosclerosis patients. Int J Gen Med. 2021;14:7545–7555. doi:10.2147/IJGM.S329398
32. Zhang WB, Zeng YY, Wang F, et al. A high neutrophil-to-lymphocyte ratio predicts hemorrhagic transformation of large atherosclerotic infarction in patients with acute ischemic stroke. Aging. 2020;12(3):2428–2439. doi:10.18632/aging.102752
33. Song Q, Pan R, Jin Y, et al. Lymphocyte-to-monocyte ratio and risk of hemorrhagic transformation in patients with acute ischemic stroke. Neurol Sci. 2020;41(9):2511–2520. doi:10.1007/s10072-020-04355-z
34. Massiot N, Lareyre F, Voury-Pons A, et al. High neutrophil to lymphocyte ratio and platelet to lymphocyte ratio are associated with symptomatic internal carotid artery stenosis. J Stroke Cerebrovasc Dis. 2019;28(1):76–83. doi:10.1016/j.jstrokecerebrovasdis.2018.09.001
35. Hu J, Zhou W, Zhou Z, et al. Elevated neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios predict post-stroke depression with acute ischemic stroke. Exp Ther Med. 2020;19(4):2497–2504. doi:10.3892/etm.2020.8514
36. Li W, He C. Association of platelet-to-lymphocyte ratio with stroke-associated pneumonia in acute ischemic stroke. J Healthc Eng. 2022;2022:1033332. doi:10.1155/2022/1033332
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.