Back to Journals » Neuropsychiatric Disease and Treatment » Volume 19
The Association of Nocturnal Seizures and Interictal Cardiac/Central Autonomic Function in Frontal Lobe Epilepsy: Heart Rate Variability and Central Autonomic Network Analysis
Authors Kim W , Lee H, Lee KW, Yang E, Kim S
Received 6 July 2023
Accepted for publication 27 September 2023
Published 3 October 2023 Volume 2023:19 Pages 2081—2091
DOI https://doi.org/10.2147/NDT.S426263
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Taro Kishi
Woojun Kim,1 Hyunjo Lee,2 Kyung Won Lee,3 Eunjin Yang,3 Seonghoon Kim3
1Department of Neurology, Seoul St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Republic of Korea; 2Department of Neurology, Ulsan University Hospital, College of Medicine, University of Ulsan, Ulsan, Republic of Korea; 3Department of Neurology, Uijeongbu St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Republic of Korea
Correspondence: Seonghoon Kim, Department of Neurology, The Catholic University of Korea, College of Medicine, Uijeongbu St. Mary’s Hospital, 271 Cheonbo-ro, Uijeongbu-si, Gyeonggi-do, Seoul, Republic of Korea, Tel +82-31-820-3114, Fax +82-31-821-3661, Email [email protected]
Purpose: Patients with epilepsy frequently experience autonomic dysfunction, closely related to sudden unexplained death in epilepsy (SUDEP). SUDEP occurs most often at night or during sleep, and frequent nocturnal seizures are an established risk factor. This study investigated the influence of nocturnal seizures on autonomic dysfunction in epilepsy.
Patients and Methods: This retrospective study enrolled frontal lobe epilepsy (FLE) patients who performed 24-hour EEG monitoring. All participants were divided into nocturnal FLE (NFLE, > 90% of seizures occurring during sleep) or diurnal FLE (DFLE) groups. EEG and ECG signals were simultaneously obtained during waking and sleep stages. EEG current density source and connectivity analysis of the autonomic network were performed. ECG was analyzed across time and frequency domains heart rate variability (HRV) analysis method was used. The obtained parameters were compared between the NFLE and DFLE groups.
Results: Fifteen NFLE and 16 DFLE patients were enrolled with no significant difference in age, sex, disease duration, seizure frequency, or the number of anti-seizure medications between the two groups. During sleep, a decrease in HRV parameters and an increase of the beta-1 (13– 22 Hz) current source density power in the bilateral paracentral lobule (BA4,5,6), precuneus (BA7), and cingulate (BA31) were observed in the NFLE group compared to DFLE group. The NFLE group also showed hyperconnectivity in the central autonomic (12 edges distributed over 10 nodes), sympathetic (2 edges distributed over 3 nodes), and parasympathetic (4 edges distributed over 6 nodes) beta-1 frequency band networks during sleep. During wakefulness, central and cardiac autonomic variables were not significantly different between the NFLE and DFLE groups.
Conclusion: Interictal cardiac and central autonomic dysfunction occurred simultaneously and can be attributed to the brain-heart autonomic axis. Our findings suggest that nocturnal seizures may contribute to interictal autonomic dysfunction during sleep in people with epilepsy.
Keywords: nocturnal seizure, heart rate variability, central autonomic network, sleep, autonomic dysfunction
Introduction
Patients with epilepsy frequently experience autonomic symptoms during seizures, including changes in heart rate, respiration, and gastrointestinal, urogenital, and pupillary functions.1,2 Autonomic dysfunction is a risk factor for mortality and morbidity in people with epilepsy and is closely related to Sudden Unexpected Death in Epilepsy (SUDEP).3 SUDEP is a prevalent cause of death in epilepsy, accounting for up to 17% of all deaths in epilepsy.4 Although the pathophysiology of SUDEP is still uncertain, autonomic dysfunction is presumed to play an important role. The autonomic nervous system modulates cardiorespiratory function, and its dysfunctions are associated with sudden death in cardiac disease.5–7 Repetitive seizures in people with epilepsy may promote cardiorespiratory dysregulation and subsequent fatal cardiac arrhythmia and respiratory failure.8
The cardiac autonomic nervous system is modulated by the central autonomic network (CAN), which integrates afferent cardiac autonomic information. The CAN is composed of various forebrain and brainstem structures, and each CAN structure has a feedback circuit via close interconnection.9 Autonomic dysfunctions develop during the ictal period as a result of ictal propagation to CAN structures.1 Previous studies reported that central autonomic dysfunction in epilepsy is related to dysregulations of cardiac and respiratory autonomic nerve systems.10,11 Cardiac autonomic dysfunction could be evaluated using heart rate variability (HRV), which is a fluctuation of time intervals between adjacent heartbeats that is used as a marker of the autonomic nerve system, cardiac mortality, and sudden cardiac death.12,13 In people with epilepsy, significant HRV changes appear in temporal lobe epilepsy (TLE) and drug-resistant epilepsy. Furthermore, HRV is related to seizure severity and serves as a biomarker for increased SUDEP risk.14,15 These findings imply that cardiac and central autonomic dysfunction occur in people with epilepsy, although a definitive link remains elusive.
Previous studies have reported that people with epilepsy may develop autonomic imbalances during sleep.16–18 In addition, it is known that more than 40% of SUDEP is related to sleep, and frequent nocturnal seizures are recognized as its risk factor.19–21 It could be supposed that sleep and frequent nocturnal seizures promote autonomic dysfunction in epilepsy. Nocturnal frontal lobe epilepsy (NFLE) is characterized by seizures that mostly occur during sleep and is thought to be associated with a higher risk of SUDEP as well as autonomic dysfunction during sleep.22 Patients with frontal lobe epilepsy (FLE) have faster heart rates and lower parasympathetic drive compared to normal control during interictal periods, and NFLE patients show significant HRV changes during sleep compared to normal control.23,24 However, most research on HRV has been conducted in TLE patients, and studies on other types of epilepsies, including FLE, are rare.7
The aim of this study was to investigate the influence of nocturnal seizures on the cardiac and central autonomic systems in FLE patients. The differences in HRV and CAN during sleep and waking stages between diurnal FLE (DFLE) and NFLE groups were studied.
Materials and Methods
Study Design and Subjects
This retrospective study was carried out in a single epilepsy center (Uijeongbu St Mary’s Hospital, the Republic of Korea). The study protocol received approval from the Institutional Review Board of the Catholic University of Korea (Approval No. UC19RISI0146), which determined that informed consent was not required as the researchers did not collect any personally identifiable information while examining patient records. This study was conducted according to the principles of the Declaration of Helsinki. Participants confirmed with FLE after 24-hour EEG monitoring from 2013 to 2019 were enrolled. The diagnosis of FLE was based on previous routine or video EEG findings and seizure semiology. FLE was confirmed as interictal discharges exclusively in the frontal lobe during the 24-hour EEG, with no interictal epileptiform discharges observed in other regions. Seizure patterns, frequencies, and demographic information were collected through seizure diaries and clinical chart reviews. All FLE patients were classified into either DFLE or NFLE groups. Patients who experienced more than 90% of seizures during sleep were classified as NFLE, whereas the remaining patients were classified as DFLE.25,26 The exclusion criteria were as follows: other neurologic, psychiatric, or cardiac disorders, abnormal brain imaging, hypertension, diabetes, renal failure, abnormal ECG findings, clinical/electrical ictal event during 24-hour EEG monitoring, or clinical seizure within 24 hours prior to the EEG monitoring.
ECG Acquisition and Analysis
The ECG signals were recorded simultaneously with waking and sleep EEG during 24-hour EEG monitoring. Artifact-free ECG data over 30 minutes were obtained during the stage 2 sleep and waking stages. The ECG signals were analyzed using Kubios HRV software (version 2.2, http://www.kubios.com) and MATLAB (MathWorks, Inc, Natick, MA, USA).27 The QRS complex was identified automatically and confirmed manually. Subsequently, time- and frequency-domain HRV parameters were obtained. The time-domain parameters included the mean of the R wave-to-R wave interval (meanRR), standard deviation of heart rate (SDHR), standard deviation of all Normal-to-Normal intervals (SDNN), standard deviation of the averages of NN intervals in all 5-minute segments of the entire recording (SDANN), SDNN index (SDNNI), the root mean square of the difference between successive normal intervals (RMSSD), number of successive NN interval pairs that differ more than 50ms (NN50), NN50 divided by the total number of NN intervals (PNN50), the integral of the NN interval histogram divided by the height of the histogram (HRV triangular index), and the baseline width of the NN interval histogram (TINN). The frequency-domain parameters included total power, very low frequency (VLF, ≤ 0.04 Hz), low frequency (LF, 0.04–0.15 Hz), and high frequency (HF, 0.15–0.4 Hz) band power, and the ratio between LF and HF band power (LF/HF).
EEG Acquisition and Data Preparation
During 24-hour EEG monitoring, the EEG signal was obtained using a routine 10–20 scalp electrode system (GRASS technology; West Warwick, RI, USA). The sampling rate was 200 Hz, and 19 electrodes (Fp1, Fp2, F3, F4, C3, C4, P3, P4, F7, F8, T3, T4, T5, T6, O1, O2, Fz, Cz, and Pz) were used. The ground and reference electrodes were placed at FPz and FCz, respectively. All participants showed interictal discharges limited to the frontal lobe without recorded EEG seizures. The stage 2 sleep EEGs were obtained from the data between 04:00 and 08:00 a.m. Two neurologists (SH. Kim, and HJ. Lee) independently reviewed the waking and sleep EEG signals. The waking and sleep stages were confirmed by the American Academy of Sleep Medicine (AASM) scoring criteria.28
EEG Current Source Density Analysis
Waking and sleep EEG data were visually reviewed, and the epoching procedures were performed using the Insight II software package (Persyst Development Corporation, Prescott, AZ, USA). Two-second epochs without ictal discharge or artifacts were selected in each participant’s waking and sleep EEG signals. All epoched EEG data were analyzed using standardized low-resolution brain electromagnetic tomography (sLORETA) software (http://www.uzh.ch/keyinst/loreta).29 sLORETA utilized a three-spherical head model based on the Montreal Neurological Institute 152 template with the 3D solution space restricted to the cortical gray matter as determined by the probabilistic Talairach atlas. It revealed an area in solution space that consisted of 6239 voxels at 5 mm spatial resolution.30,31 Electrode coordinates were made for the calculation of the transform matrix, which was created by registration and interpolation of 19 scalp electrodes to the average head model. After then, cross spectra were calculated, and the corresponding 3D cortical distribution of the electric neuronal generators was computed for each participant and each frequency band. The difference in current source density between NFLE and DFLE in each waking and sleep stage was investigated, and the following EEG frequency domains were analyzed: delta (1–4 Hz), theta (4–8 Hz), alpha-1 (8–10.5 Hz), alpha-2 (10.5–13 Hz), beta-1 (13–22 Hz), and beta-2 (22–30 Hz).29
Connectivity Analysis of the Central Autonomic Network
The connectivity of the CAN between pairs of regions of interest (ROIs) was analyzed using sLORETA software. Autonomic function-related brain regions were defined as 13-central autonomic, 9-central sympathetic, and 8-central parasympathetic ROIs (Supplementary Tables 1–3).32 For analysis, each ROI was defined by “ROI maker 1” with the “all voxels within a radius of (15 mm)” option. The functional connectivity for each pair of ROIs was calculated using the linear similarity method (lagged-linear connectivity). The obtained connectivity was compared between NFLE and DFLE groups in each waking and sleep stage. The final results were visualized using BrainNet viewer (https://helab.bnu.edu.cn/DOWNLOADS/BrainNetViewer/index.htm).33
Statistical Analysis
Demographic and characteristic variables were compared using the appropriate statistical methods, including the Mann–Whitney U-test or the chi-square test with Fisher’s correction. In each waking and sleep stage, the HRV parameters were compared between the NFLE and DFLE groups using the Analysis of Covariance (ANCOVA) method to adjust for age. The non-parametric ANCOVA were used when appropriate. The localization of current source density (CSD) power and connectivity between pairs of ROIs was performed using the sLORETA software statistics tool. Significant differences between the NFLE and DFLE groups were assessed in the six frequency bands using t-tests, and multiple comparison-corrected critical probability threshold values were calculated using the nonparametric randomization method based on empirical probability distributions.29,34 All statistical analyses were performed using the R software package (version 3.5.1) and the statistics tool of sLORETA software.
Results
Demographics and Seizure Characteristics
A total of 31 subjects with FLE were enrolled in this study, including 21 males with a median age of 24 years (range, 14–65 years). Fifteen participants were confirmed to have NFLE, including 12 males with a median age of 23 years (range, 14–54 years) and a seizure frequency of 2.7±3.8/month. Sixteen participants were diagnosed with DFLE, including 9 males with a median age of 30 years (range, 15–65 years) and a seizure frequency of 1.5±2.6/month (Figure 1). All subjects were taking anti-seizure medication (ASMs) during the study period. Demographics and seizure characteristics including age, sex, disease duration, seizure frequency, and the number of ASMs were not significantly different between the NFLE and DFLE groups (Table 1).
 |
Table 1 Demographics and Clinical Information |
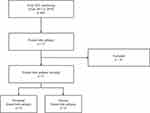 |
Figure 1 The flow chart of participants included in this study. |
Heart Rate Variability
During the sleep stage, there were significant differences in HRV parameters between the two groups. The time domain analysis showed that the SDHR (p<0.019), SDNN (p<0.044), and triangular index (p<0.033) were lower in the NFLE compared to the DFLE group. Similarly, frequency domain analysis showed lower LF (p<0.041), and total power (p<0.031) in the NFLE group compared to the DFLE group. However, during the waking stage, there were no significant differences in HRV parameters between the two groups (Table 2).
 |
Table 2 HRV Parameters During Wake and Sleep and Periods Between NFLE and DFLE Groups |
Current Source Density and the CAN
During the sleep stage, the NFLE group showed a significant increase in beta-1 CSD power (p<0.05) in several brain regions including the bilateral frontal paracentral lobule (BA4,5,6), precuneus (BA7), and cingulate (BA31) compared to the DFLE group (Figure 2). There was no significant difference in other frequency bands, and no decrease in CSD was observed in any frequency band analyses.
In the analysis of CAN connectivity during the sleep stage, the NFLE group showed CAN hyperconnectivity in the beta-1 frequency band (p<0.05, t=4.0971), involving 12 edges distributed over 10 nodes, with most connections in the Rt and mid anterior cingulate gyri (5 edges). The mid anterior cingulate gyrus_1, Rt anterior cingulate gyrus, Lt parahippocampal gyrus, Rt insula gyrus, Rt parahippocampal gyrus, Lt insula gyrus_1, Lt rectal gyrus, mid anterior cingulate gyrus_2, Rt supramarginal gyrus, and Lt insula gyrus_2 were the involved nodes (Figure 3).
In addition, the NFLE group showed hyperconnected central parasympathetic-associated and central sympathetic-associated networks in the beta-1 frequency band, involving 4 edges distributed over 6 nodes (p<0.05, t=4.0282) and 2 edges distributed over 3 nodes (p<0.05, t=3.9691), respectively. The Lt parahippocampal gyrus, Lt insula gyrus, Lt precentral gyrus, Rt superior temporal gyrus_1, Rt superior temporal gyrus_2, and Rt insula gyrus were the involved parasympathetic nodes. The involved sympathetic nodes were the mid anterior cingulate gyrus, Lt orbital gyrus, and Lt insular gyrus, and CAN hypoconnectivity was not observed during sleep.
During the waking stage, no significant differences were found in CSD or in central autonomic, sympathetic, or parasympathetic networks between the two groups.
Discussion
In this study, more severe cardiac and central autonomic abnormalities in the interictal period were observed in the NFLE group compared to the DFLE group. Cardiac and central autonomic dysfunctions occurred in a simultaneous manner and were more pronounced during sleep.
Interictal Autonomic Dysfunction
Autonomic dysfunction during the interictal period is well documented in people with epilepsy.2,35 Previous studies have reported significant differences in HRV parameters, such as SDNN, LF, HF, and LF/HF, between people with epilepsy and normal controls.36–38 In previous studies, TLE patients exhibited autonomic dysfunction and lower HRV parameters compared to age-matched normal controls.36,38 FLE patients also showed lower HRV parameters, which were correlated with the risk of SUDEP.39 Although autonomic dysfunctions in FLE have been reported, most studies investigated TLE, and the evidence in FLE patients has been limited.2,23 Our study showed significantly lower HRV parameters in the NFLE group compared to the DFLE group during the interictal period, consistent with previous studies. SDNN is widely used as the gold standard for assessing cardiac risk, and lower SDNN values are indicative of increased mortality and reduced mobility. SDNN reflects the combined influence of both the sympathetic and parasympathetic nervous systems.12 The triangular index is closely linked to arrhythmias, and a low triangular index in atrial fibrillation is associated with higher mortality rates.12,40 While SDHR is associated with mortality risk in heart disease, it is not commonly employed as a standalone measure.41 LF represents the frequency component ranging from 0.04 to 0.15 Hz. Total power encompasses energy across the ULF, VLF, LF, and HF frequency bands. LF reflects the combined function of both the sympathetic and parasympathetic nervous systems and reduced LF values have been associated with sudden cardiac death.12,42 Our results suggest that cardiac autonomic dysfunction could occur, even during the interictal period, in NFLE patients.
The involvement of CAN structures in seizure may result in severe cardiac arrhythmia, which could be associated with SUDEP.9 Abnormalities in the medial thalamus and superior temporal gyrus, which are structures of the CAN, have been observed in postmortem studies of SUDEP.43 These structural abnormalities were observed even before SUDEP events, indicating that CAN dysfunction could occur during the interictal period in people with epilepsy.44 We employed EEG source and connectivity analysis to assess the CAN. These techniques provide insights into the activation of specific brain regions or networks and are commonly utilized to investigate various neurological diseases.29,45,46 In our study, changes in central autonomic, sympathetic, and parasympathetic network activity were seen during the interictal period in NFLE patients. Our findings also showed that the parasympathetic network was more extensively activated than the sympathetic network. Previous studies reported increased sympathetic tone and decreased HRV during sleep in people with epilepsy.18,47 However, our study focused on preganglionic CAN activity rather than the autonomic output of the CAN. The results of our HRV analysis are consistent with previous studies. As a result, our structural and network analysis findings indicate that the dysregulation of the CAN occurred even during the interictal period in NFLE.
In our study, the NFLE group showed significant activation of the bilateral paracentral lobule, precuneus, and cingulate during the interictal period.48 The paracentral lobule and cingulate are involved in baroreflex function.49 The precuneus has a sympathetic vasoconstrictive wakefulness drive to resting muscles, and the precuneus and paracentral lobule have extensive connections with the prefrontal and insular areas.50,51 Our findings suggest that cardiac and central autonomic dysfunction develop simultaneously during the interictal period in people with epilepsy.
Sleep, Nocturnal Seizure, and Autonomic Dysfunction in Epilepsy
Sleep promotes synaptic renormalization and downscaling, which is related to autonomic system homeostasis. During normal non-REM sleep, parasympathetic drive increases while sympathetic drive decreases, resulting in BP dipping, respiratory depression, and an increase in HRV parameters. Meanwhile, during REM sleep, there are fluctuations in blood pressure and respiration, and the autonomic state is similar to that during wakefulness.52 People with epilepsy, exhibit interictal autonomic dysfunction and HRV changes that are more pronounced during sleep.7 HRV parameters decrease during sleep in patients with FLE and intractable epilepsy, indicating autonomic alterations.18,39 The current study found that central and cardiac autonomic alterations in NFLE patients were more pronounced than DFLE group only during sleep, implying that autonomic function is more vulnerable during sleep in people with epilepsy.
Autonomic changes are frequently observed in people with epilepsy. During the ictal or peri-ictal stage, these changes are resulted from the propagation of the epileptic activity into the CAN. The exact mechanism underlying interictal autonomic dysfunction remains unclear. However, it is hypothesized that repetitive seizures can potentially induce alterations in the CAN, thereby contributing to interictal autonomic dysfunction.1,53 Frequent seizure is a risk factor of SUDEP and is associated with autonomic dysfunction.3 HRV was observed to be lower in the refractory TLE group than in normal controls but not in those with well-controlled TLE.54 In our study, more severe autonomic dysfunction developed in the NFLE group than in the DFLE group, although there was no significant difference in total seizure frequency between the two groups. Moreover, the DFLE group did not show significant changes in autonomic alteration during waking compared to the NFLE group. Our findings suggest that nocturnal seizures may have a greater impact on interictal autonomic alteration. The exact mechanism by which nocturnal seizures cause autonomic dysfunction remains unclear, but there are several possible explanations. Some studies reported that epileptiform discharges cause arousal, cortical activation, and transient autonomic activation in people with epilepsy.18,55 Other studies have found that autonomic activation precedes phasic EEG changes, cortical activation, and arousal.56,57 Nocturnal seizures and interictal epileptiform discharges may interfere with sleep processes, potentially causing autonomic alteration.58,59 Therefore, frequent nocturnal seizures are likely to cause frequent arousal and autonomic fluctuations, resulting in chronic autonomic dysfunction. Our findings suggest that nocturnal seizures during sleep, which is a vulnerable stage for autonomic function, may have a greater influence on autonomic dysfunction in people with epilepsy.
Brain-Heart Autonomic Axis
In our study, concurrent cardiac and central autonomic dysfunction occurred during sleep. HRV is generated by heart-brain interactions and dynamic non-linear autonomic nervous system (ANS) processes.12 Stimulation of the left and right insula induces bradycardia and tachycardia, respectively.60 Sympathetic activation is associated with tachyarrhythmia during the ictal and peri-ictal stages of epilepsy, whereas parasympathetic activation is associated with bradyarrhythmia and dyspnea.53,61 Furthermore, alterations in the CAN have been reported in SUDEP and high-risk SUDEP patients.10,11,43 In a patient with Takotsubo syndrome, a catecholamine-induced reversible cardiomyopathy, as well as alterations in the CAN and limbic network, was seen.62,63 These findings imply that autonomic changes occur through the brain-heart autonomic axis during the interictal stage in people with epilepsy.
Our study has several limitations. Firstly, we applied strict enrollment criteria, resulting in a relatively small number of subjects. Secondly, the study groups were not compared to an appropriate normal control group. The current study aimed to investigate the influence of nocturnal seizures on autonomic function in epilepsy and involved only FLE patients with the same characteristics except for the frequency of nocturnal seizures. Thirdly, the type of ASM, which may have affected ANS, was not considered.
Conclusion
Our findings suggest that nocturnal seizure might be the most pronounced factor for interictal autonomic dysfunction during sleep in FLE patients. Interictal cardiac and central autonomic dysfunction occurred simultaneously and can be attributed to the brain-heart autonomic axis. Further studies including a larger sample size and comparison to a normal control group would provide additional insight. Studies on the mechanism between nocturnal seizure and autonomic dysfunction are also needed.
Statement of Ethics
There is no potential for individual researcher access to personal information, and there is no element of human rights violation. The Institutional Review Board of the Catholic University of Korea determined that consent is not required because the researchers are not collecting any personally identifiable information (such as patient name, hospital identification number, or social security number) when examining patients’ records.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
References
1. Baumgartner C, Lurger S, Leutmezer F. Autonomic symptoms during epileptic seizures. Epileptic Disord. 2001;3(3):103–116.
2. Sivathamboo S, Perucca P. Interictal autonomic dysfunction. Curr Opin Neurol. 2021;34(2):197–205. doi:10.1097/WCO.0000000000000906
3. Chen SF, Pan HY, Huang CR, et al. Autonomic dysfunction contributes to impairment of cerebral autoregulation in patients with epilepsy. J Pers Med. 2021;11(4). doi:10.3390/jpm11040313
4. Lotufo PA, Valiengo L, Bensenor IM, Brunoni AR. A systematic review and meta-analysis of heart rate variability in epilepsy and antiepileptic drugs. Epilepsia. 2012;53(2):272–282. doi:10.1111/j.1528-1167.2011.03361.x
5. Marin Collazo IV, Tatum WO. Sudden unexpected death in epilepsy (SUDEP): are all your patients informed? Neurologist. 2016;21(4):66–71. doi:10.1097/NRL.0000000000000083
6. Smith BN. Seizures, Epilepsy, and SUDEP: a change of heart? Epilepsy Curr. 2016;16(3):166–167. doi:10.5698/1535-7511-16.3.166
7. Myers KA, Sivathamboo S, Perucca P. Heart rate variability measurement in epilepsy: how can we move from research to clinical practice? Epilepsia. 2018;59(12):2169–2178. doi:10.1111/epi.14587
8. Surges R, Sander JW. Sudden unexpected death in epilepsy: mechanisms, prevalence, and prevention. Curr Opin Neurol. 2012;25(2):201–207. doi:10.1097/WCO.0b013e3283506714
9. Benarroch EE. The central autonomic network: functional organization, dysfunction, and perspective. Mayo Clin Proc. 1993;68(10):988–1001. doi:10.1016/S0025-6196(12)62272-1
10. La A, Rm H, G M, et al. Altered brain connectivity in sudden unexpected death in epilepsy (SUDEP) revealed using resting-state fMRI. Neuroimage Clin. 2019;24:102060. doi:10.1016/j.nicl.2019.102060
11. Allen LA, Harper RM, Kumar R, et al. Dysfunctional brain networking among autonomic regulatory structures in temporal lobe epilepsy patients at high risk of sudden unexpected death in epilepsy. Front Neurol. 2017;8:544. doi:10.3389/fneur.2017.00544
12. Shaffer F, Ginsberg JP. An overview of heart rate variability metrics and norms. Front Public Health. 2017;5:258. doi:10.3389/fpubh.2017.00258
13. Sessa F, Anna V, Messina G, et al. Heart rate variability as predictive factor for sudden cardiac death. Aging. 2018;10(2):166–177. doi:10.18632/aging.101386
14. DeGiorgio CM, Curtis A, Hertling D, Moseley BD. Sudden unexpected death in epilepsy: risk factors, biomarkers, and prevention. Acta Neurol Scand. 2019;139(3):220–230. doi:10.1111/ane.13049
15. Myers KA, Bello-Espinosa LE, Symonds JD, et al. Heart rate variability in epilepsy: a potential biomarker of sudden unexpected death in epilepsy risk. Epilepsia. 2018;59(7):1372–1380. doi:10.1111/epi.14438
16. Sivakumar SS, Namath AG, Tuxhorn IE, Lewis SJ, Galan RF. Decreased heart rate and enhanced sinus arrhythmia during interictal sleep demonstrate autonomic imbalance in generalized epilepsy. J Neurophysiol. 2016;115(4):1988–1999.
17. Hamdy RM, Abdel-Tawab H, Abd Elaziz OH, Sobhy El Attar R, Kotb FM. Evaluation of heart rate variability parameters during awake and sleep in refractory and controlled epileptic patients. Int J Gen Med. 2022;15:3865–3877. doi:10.2147/IJGM.S354895
18. Proserpio P, Giacomini T, Agostoni EC, Nobili L. Sleep-Related Epilepsy, Dysautonomia, and Sudden Nocturnal Death. In: Chokroverty S, Cortelli P, editors. Autonomic Nervous System and Sleep: Order and Disorder. Cham: Springer International Publishing; 2021:213–228.
19. Nobili L, Proserpio P, Rubboli G, Montano N, Didato G, Tassinari CA. Sudden unexpected death in epilepsy (SUDEP) and sleep. Sleep Med Rev. 2011;15(4):237–246. doi:10.1016/j.smrv.2010.07.006
20. Lamberts RJ, Thijs RD, Laffan A, Langan Y, Sander JW. Sudden unexpected death in epilepsy: people with nocturnal seizures may be at highest risk. Epilepsia. 2012;53(2):253–257. doi:10.1111/j.1528-1167.2011.03360.x
21. van der Lende M, Hesdorffer DC, Sander JW, Thijs RD. Nocturnal supervision and SUDEP risk at different epilepsy care settings. Neurology. 2018;91(16):e1508–e1518. doi:10.1212/WNL.0000000000006356
22. Tinuper P, Bisulli F. From nocturnal frontal lobe epilepsy to Sleep-Related Hypermotor Epilepsy: a 35-year diagnostic challenge. Seizure. 2017;44:87–92. doi:10.1016/j.seizure.2016.11.023
23. Harnod T, Yang CC, Hsin YL, Wang PJ, Shieh KR, Kuo TB. Heart rate variability in patients with frontal lobe epilepsy. Seizure. 2009;18(1):21–25. doi:10.1016/j.seizure.2008.05.013
24. Dorantes G, Mendez M, Alba A, Gonzalez JS, Parrino L, Milioli G. Heart rate variability in cyclic alternating pattern during sleep in healthy and Nocturnal Front Lobe Epilepsy patients. Conf Proc IEEE Eng Med Biol Soc. 2015;2015:5944–5947.
25. Mostacci B, Bisulli F, Vignatelli L, et al. Incidence of sudden unexpected death in nocturnal frontal lobe epilepsy: a cohort study. Sleep Med. 2015;16(2):232–236. doi:10.1016/j.sleep.2014.09.019
26. Nobili L, Proserpio P, Combi R, et al. Nocturnal frontal lobe epilepsy. Curr Neurol Neurosci Rep. 2014;14(2):424. doi:10.1007/s11910-013-0424-6
27. Tarvainen MP, Niskanen JP, Lipponen JA, Ranta-Aho PO, Karjalainen PA. Kubios HRV--heart rate variability analysis software. Comput Methods Programs Biomed. 2014;113(1):210–220. doi:10.1016/j.cmpb.2013.07.024
28. Berry R, Quan S, Abreu A. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, Version 2.6. Darien: American Academy of Sleep Medicine; 2020.
29. Pascual-Marqui RD. Standardized low-resolution brain electromagnetic tomography (sLORETA): technical details. Methods Find Exp Clin Pharmacol. 2002;24:5–12.
30. Lancaster JL, Woldorff MG, Parsons LM, et al. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10(3):120–131. doi:10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8
31. Mazziotta J, Toga A, Evans A, et al. A probabilistic atlas and reference system for the human brain: international consortium for brain mapping (ICBM). Philos Trans R Soc Lond B Biol Sci. 2001;356(1412):1293–1322. doi:10.1098/rstb.2001.0915
32. Beissner F, Meissner K, Bar KJ, Napadow V. The autonomic brain: an activation likelihood estimation meta-analysis for central processing of autonomic function. J Neurosci. 2013;33(25):10503–10511. doi:10.1523/JNEUROSCI.1103-13.2013
33. Xia M, Wang J, He Y. BrainNet Viewer: a network visualization tool for human brain connectomics. PLoS One. 2013;8(7):e68910. doi:10.1371/journal.pone.0068910
34. Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15(1):1–25. doi:10.1002/hbm.1058
35. Shaker KK, Al Mahdawi AM, Hamdan FB. Interictal autonomic dysfunction in patients with epilepsy. Egypt J Neurol Psychiatr Neurosurg. 2021;57(1):165. doi:10.1186/s41983-021-00422-0
36. Tomson T, Ericson M, Ihrman C, Lindblad LE. Heart rate variability in patients with epilepsy. Epilepsy Res. 1998;30(1):77–83. doi:10.1016/S0920-1211(97)00094-6
37. Tosun O, Karatoprak E. Analysis of tissue Doppler parameters and 24-hour heart rate variations in children with newly diagnosed untreated idiopathic epilepsy in interictal period. Epilepsy Behav. 2019;90:11–14. doi:10.1016/j.yebeh.2018.10.039
38. Massetani R, Strata G, Galli R, et al. Alteration of cardiac function in patients with temporal lobe epilepsy: different roles of EEG-ECG monitoring and spectral analysis of RR variability. Epilepsia. 1997;38(3):363–369. doi:10.1111/j.1528-1157.1997.tb01129.x
39. Do Nascimento Vinholes L, Sousa da Silva A, Marinho Tassi E, Correa Borges de Lacerda G. Heart rate variability in frontal lobe epilepsy: association with SUDEP risk. Acta Neurol Scand. 2021;143(1):62–70. doi:10.1111/ane.13330
40. Hammerle P, Eick C, Blum S, et al. Heart rate variability triangular index as a predictor of cardiovascular mortality in patients with atrial fibrillation. J Am Heart Assoc. 2020;9(15):e016075. doi:10.1161/JAHA.120.016075
41. Levin CJ, Swoap SJ. The impact of deep breathing and alternate nostril breathing on heart rate variability: a human physiology laboratory. Adv Physiol Educ. 2019;43(3):270–276. doi:10.1152/advan.00019.2019
42. La Rovere MT, Pinna GD, Maestri R, et al. Short-term heart rate variability strongly predicts sudden cardiac death in chronic heart failure patients. Circulation. 2003;107(4):565–570. doi:10.1161/01.CIR.0000047275.25795.17
43. Somani A, El-Hachami H, Patodia S, Sisodiya S, Thom M. Regional microglial populations in central autonomic brain regions in SUDEP. Epilepsia. 2021;62(6):1318–1328. doi:10.1111/epi.16904
44. Mueller SG, Nei M, Bateman LM, et al. Brainstem network disruption: a pathway to sudden unexplained death in epilepsy? Hum Brain Mapp. 2018;39(12):4820–4830. doi:10.1002/hbm.24325
45. Pascual-Marqui RD, Michel CM, Lehmann D. Low resolution electromagnetic tomography: a new method for localizing electrical activity in the brain. Int J Psychophysiol. 1994;18(1):49–65. doi:10.1016/0167-8760(84)90014-x
46. Xie W, Richards JE. Cortical Source Localization in EEG Frequency Analysis. In: Gable PA, Miller MW, Bernat EM, editors. The Oxford Handbook of EEG Frequency. Oxford University Press; 2022.
47. Barot N, Nei M. Autonomic aspects of sudden unexpected death in epilepsy (SUDEP). Clin Auton Res. 2019;29(2):151–160. doi:10.1007/s10286-018-0576-1
48. Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129(Pt 3):564–583. doi:10.1093/brain/awl004
49. Ding K, Tarumi T, Wang C, Vernino S, Zhang R, Zhu DC. Central autonomic network functional connectivity: correlation with baroreflex function and cardiovascular variability in older adults. Brain Struct Funct. 2020;225(5):1575–1585. doi:10.1007/s00429-020-02075-w
50. Macefield VG, Henderson LA. “Real-time” imaging of cortical and subcortical sites of cardiovascular control: concurrent recordings of sympathetic nerve activity and fMRI in awake subjects. J Neurophysiol. 2016;116(3):1199–1207. doi:10.1152/jn.00783.2015
51. James C, Macefield VG, Henderson LA. Real-time imaging of cortical and subcortical control of muscle sympathetic nerve activity in awake human subjects. Neuroimage. 2013;70:59–65. doi:10.1016/j.neuroimage.2012.12.047
52. Fink AM, Bronas UG, Calik MW. Autonomic regulation during sleep and wakefulness: a review with implications for defining the pathophysiology of neurological disorders. Clin Auton Res. 2018;28(6):509–518. doi:10.1007/s10286-018-0560-9
53. Moseley B, Bateman L, Millichap JJ, Wirrell E, Panayiotopoulos CP. Autonomic epileptic seizures, autonomic effects of seizures, and SUDEP. Epilepsy Behav. 2013;26(3):375–385. doi:10.1016/j.yebeh.2012.08.020
54. Suorsa E, Korpelainen JT, Ansakorpi H, et al. Heart rate dynamics in temporal lobe epilepsy-A long-term follow-up study. Epilepsy Res. 2011;93(1):80–83. doi:10.1016/j.eplepsyres.2010.10.005
55. Carreno M, Fernandez S. Sleep-Related Epilepsy. Curr Treat Options Neurol. 2016;18(5):23. doi:10.1007/s11940-016-0402-9
56. Tobaldini E, Nobili L, Strada S, Casali KR, Braghiroli A, Montano N. Heart rate variability in normal and pathological sleep. Front Physiol. 2013;4:294. doi:10.3389/fphys.2013.00294
57. Halasz P, Terzano M, Parrino L, Bodizs R. The nature of arousal in sleep. J Sleep Res. 2004;13(1):1–23. doi:10.1111/j.1365-2869.2004.00388.x
58. Moore JL, Carvalho DZ, St Louis EK, Bazil C. Sleep and epilepsy: a focused review of pathophysiology, clinical syndromes, co-morbidities, and therapy. Neurotherapeutics. 2021;18(1):170–180. doi:10.1007/s13311-021-01021-w
59. Kim S, Park JW. Chapter 19 - Sleep deprivation, headache, and Fos immunohistochemistry. In: Rajendram R, Patel VB, Preedy VR, Martin CR, editors. The Neurobiology, Physiology, and Psychology of Pain. Academic Press; 2022:203–215.
60. Oppenheimer SM, Gelb A, Girvin JP, Hachinski VC. Cardiovascular effects of human insular cortex stimulation. Neurology. 1992;42(9):1727–1732. doi:10.1212/WNL.42.9.1727
61. Brotherstone R, McLellan A. Parasympathetic alteration during sub-clinical seizures. Seizure. 2012;21(5):391–398. doi:10.1016/j.seizure.2012.03.011
62. Templin C, Hanggi J, Klein C, et al. Altered limbic and autonomic processing supports brain-heart axis in Takotsubo syndrome. Eur Heart J. 2019;40(15):1183–1187. doi:10.1093/eurheartj/ehz068
63. Pereira VH, Marques P, Magalhaes R, et al. Central autonomic nervous system response to autonomic challenges is altered in patients with a previous episode of Takotsubo cardiomyopathy. Eur Heart J Acute Cardiovasc Care. 2016;5(2):152–163. doi:10.1177/2048872615568968
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


